


Climate-driven diversity changes of Mediterranean echinoids over the last 6 Ma
ENRICO BORGHI and VITTORIO GARILLI
Borghi E. and Garilli, V. 2022. Climate-driven diversity changes of Mediterranean echinoids over the last 6 Ma. Acta Palaeontologica Polonica 67 (4): 781–805.
Echinoids represent an important component of the Cenozoic marine benthic communities. Their diversity in the Mediterranean area is reviewed within the Late Miocene–Recent, a period of remarkable paleogeographic and paleoclimate changes. Of the 37 genera that lived during the Late Miocene, only Holaster, Pliolampas, and Trachyaster did not survive the Messinian Mediterranean salinity crisis (MSC), indicating that this event was not as drastic as for other marine groups. The presence of Brissopsis within the uppermost Messinian testifies to the existence of fully marine conditions at least towards the end of the MSC. Severe drops in the echinoid diversity, involving the loss of 40% of the Pliocene genera, occurred during the Piacenzian, likely because of the onset of the Northern Hemisphere glaciation. Most of the echinoid extinctions correlate with the crisis of the Mediterranean bivalve assemblage recorded at about 3 Ma. The Early Pleistocene progressive cooling caused the disappearance of further thermophilous shallow-water genera (Clypeaster, Schizechinus, Echinolampas) and allowed the entrance of temperate taxa (Paracentrotus lividus, Placentinechinus davolii and Sphaerechinus granularis) from the Atlantic. Some deep-water taxa (Histocidaris sicula, Stirechinus scillae, Cidaris margaritifera), whose Recent relatives are currently restricted to tropical areas, are not found in the area after the Calabrian possibly because of the disappearance of the psychrosphere. The extant Mediterranean echinoid fauna mainly derives from the Late Miocene fauna, reduced after several climatic changes by about 43% at the genus level. The recent increase of the sea surface temperatures allowed the entrance of the Lessepsian Diadema setosum and confined the deep-water species of Holanthus to the coldest areas of the basin, making this genus endangered.
Key words: Echinoidea, biodiversity, biogeography, paleoclimate, Mediterranean bioevents, Miocene salinity crisis, late Cenozoic.
Enrico Borghi [enrico.borghi20@gmail.com], Società Reggiana di Scienze Naturali, Via A. Gramsci 109, 42024, Castelnovo Sotto (RE), Italy.
Vittorio Garilli [paleosofiavg@gmail.com] (corresponding author), PaleoSofia—Research and Educational Service, Via Gagini 19, 90133 Palermo, Italy.
Received 8 March 2022, accepted 15 July 2022, available online 7 December 2022.
Copyright © 2022 E. Borghi and V. Garilli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Since the Early Triassic echinoids are a relevant component of marine benthic invertebrate assemblages (Kroh and Smith 2010). Today they live in a wide latitudinal range, from polar to equatorial, at various depths, from intertidal bottoms to the deeper abyssal waters, though their highest diversity occurs at shelf depths (Kroh and Smith 2010; Mironov et al. 2015). According to the morphology of their test, the echinoids are subdivided into two main groups: regular echinoids, with a radial symmetry, and irregular echinoids, with a bilateral symmetry. In the present seas little more than 50% of all the echinoid species is represented by regulars, while in the Cenozoic fossil record the regular echinoid species represent only the 20% of all the known echinoids, possibly because of their lower potential to be fossilised (Kier 1977). Both groups are ecologically important, can fossilize rather easily thanks to the calcareous nature of their test, and play a relevant role in sedimentary and taphonomical processes. Regular echinoids belong to the marine epifauna and may be dominant algal grazers in shallow bottoms, where they can be also very active bioeroders (Bak 1990; Mokady et al. 1996; Belaústegui et al. 2017); irregular echinoids are mainly infaunal deposit feeders and important bioturbators that may intensely modify the sedimentary structures by deeply burrowing into the sediments (Hammond 1981; Queirós et al. 2013; Belaústegui et al. 2017).
Since their appearance in the early Paleozoic (Middle Ordovician according to Lefebvre et al. 2013), several evolutionary trends have been recognised among the echinoid group (Kier 1965). However, only after the Permian mass extinction the flourishing of the echinoid group started with the strong differentiation of their morphology (Saucède et al. 2007 with references; Kroh and Smith 2010; Kroh 2011). Particularly, the diversification of many major lines of echinoids took place in the Jurassic, when they became a major constituent of the shallow-water benthos and the irregular echinoids appeared for the first time (Barras 2007; Smith and Kroh 2011). From that time on, a general increase of their biodiversification occurred, above all triggered by changes in their lifestyle (Jeffery and Emlet 2003; Kroh and Smith 2010; Hopkins and Smith 2015). A good example of diversification in their mode of life is represented by the different kinds of reproduction they have acquired during their evolution, with strategies ranging from a complete lack of parental care (broadcasting) to internal and external brooding of the eggs, embryos, and juveniles (Gillespie and McClintock 2007). Climate and paleogeographic changes drove the diversification of the Mediterranean echinoid group (e.g., Néraudeau et al. 1999, 2001, 2003; Kroh 2007). Significant cases of the interaction between climate changes and the evolution of the echinoid diversity in the late Cenozoic of the Atlanto-Mediterranean area have been reported: the disappearances of genera Amphiope Agassiz, 1840, Clypeaster Lamarck, 1801, Hypsoclypus Pomel, 1869, and Echinoneus Leske, 1778 (Néraudeau et al. 2001), and the Mediterranean appearance of Paracentrotus Mortensen, 1903, Sphaerechinus Desor, 1856, and of Placentinechinus Borghi and Garilli, 2016. However, apart from the review by Néraudeau et al. (2001) concerning only the irregulars, the biogeography and biostratigraphy of the echinoids were generally discussed singularly, without providing a synoptical view of how the whole Mediterranean assemblage changed in the late Neogene–Quaternary. Furthermore, the lack of a complete biostratigraphic framework prevented the identification of the late Cenozoic climate changes that determined the diversity of the current Mediterranean echinoid assemblage whose composition and distribution were studied by Mortensen (1913), Kohler (1927), Péquignat (1964) and Tortonese (1965, 1977).
The Mediterranean and Paratethys Miocene echinoid fauna were respectively overviewed by Roman (1989a) and Kroh (2005, 2007). These faunas mainly derived from the Paleogene assemblage, mostly endemic to Tethys. About 70, mainly tropical, genera lived during that time in the shallow waters of the Mediterranean domain (Roman 1989a). They were mostly represented by irregular echinoids, above all clypeastroids, cassiduloids and spatangoids. Extensive studies on deep-water associations have been provided only from the Serravallian (Middle Miocene) of Cyprus (Smith and Gale 2009) and the upper Burdigalian (Lower Miocene) to Serravallian (Middle Miocene) of northern Italy (Stefanini 1908, 1909; Borghi 2020). Although the number of the genera and species recorded in the literature (about 70 and 600, respectively) may be regarded as overestimated (Roman and Soudet 1990), the echinoid diversity in the Miocene of the Mediterranean was clearly much greater than the Recent one (Roman 1989a).
In the last two decades significant developments have been achieved in the knowledge of the Mediterranean fossil and Recent echinoids, above all thanks to the new findings and progress in the systematics of this group (e.g., Kroh and Smith 2010; Smith and Kroh 2011; Mongiardino Koch et al. 2018, 2022; Kroh and Mooi 2021; Mongiardino Koch and Thompson 2021). During this period, however, no relevant progress has been achieved on the knowledge of the late Cenozoic Mediterranean echinoid diversity, apart from the deep-water echinoids (Borghi et al. 2014). Based on the new data acquired by own studies and recent literature we present a review of the diversity of the Mediterranean regular and irregular echinoids from the late Miocene (Messinian) to the present day, a period of geological events and climate fluctuations that have marked important steps for the paleobiogeographic evolution of the Mediterranean area. We will also discuss the paleoclimatic and paleogeographical significance of the echinoid assemblages reviewed, for which we will provide an exhaustive stratigraphic frame. Paleobiological responses to climate changes as detected for the echinoid assemblages reviewed will be compared with those recorded for other marine groups with special regard to molluscs, for which Monegatti and Raffi (2001) proposed an eco-biostratigraphic zonation of the Mediterranean Pliocene and Early Pleistocene.
Institutional abbreviations.—MCS, Museo Paleontologico “Il Mare Antico”, Salsomaggiore Terme, Italy; MG, Museo Geologico “G. Cortesi”, Castell’Arquato, Italy; MTPL, Museo Regionale di Scienze Naturali, Torino, Italy; MGUS, Museo de Geologia de la Universidad de Sevilla, Spain; MZUF, Museo di Storia Naturale “La Specola”, Firenze, Italy.
Other abbreviations.—MPMU, Mediterranean Pliocene molluscan unit; MSC, Messinian salinity crisis; SST(s), sea surface temperature(s).
Paleogeographic and paleoclimatic setting
Since the Middle Miocene, after the more-or-less final interruption of the communication with the Indo-Pacific area (Rögl 1999; Harzhauser et al. 2002, 2007; Popov et al. 2004), the Mediterranean province became a sort of Atlantic “pocket” characterised by a tropical fauna (Por 2009). This condition persisted untill the Late Miocene, when, during the Messinian, the Mediterranean area underwent severe paleobiogeographic changes involving desiccation processes and the precipitation of a large quantity of salts on the sea bottom, the geological event known as the Messinian Salinity Crisis (MSC) (Ruggieri 1967; Hsu et al. 1973; Isaji et al. 2019 and references therein). It is still debated which main factor drove the MSC, which started at 5.971 Ma (Manzi et al. 2013). Most likely the combination of climate and paleogeographic factors, such as the dry conditions with a water loss by evaporation exceeding the volume of inflow, a tectonic uplift at the Gibraltar area (Garcia-Castellanos and Villaseñor 2011) or a global sea-level fall, caused the interruption of the Atlanto-Mediterranean communication or its relevant reduction (for an essential overview see Kastens 1992; Flecker et al. 2002; Flecker and Ellam 2006; Rouchy and Caruso 2006; Ryan 2009; Roveri et al. 2014; Ohneiser et al. 2015). In any case, it is generally assumed that the MSC caused deep changes in the Atlanto-Mediterranean faunal relationships, with a drop of the Mediterranean marine biodiversity (e.g., Landau et al. 2003; Coll et al. 2010; Monegatti and Raffi 2010; Agiadi et al. 2020) that also involved echinoids (Néraudeau et al. 1999, 2001).
After the MSC, with the normalization of the water interchange with the east Atlantic at the beginning of the Pliocene at about 5.3 Ma, the Mediterranean basin progressively assumed its present geographical setting and underwent an intricate climatic evolution. The Pliocene period (Zanclean–Piacenzian) was almost globally characterised by a much warmer climate, particularly during the so-called Pliocene climatic optimum (e.g., Fedorov et al. 2013). During this period, also known as “warm Pliocene”, a strictly tropical regime settled in Mediterranean (e.g., Haywood and Valdes 2004; Dowsett and Caballero-Gill 2010; Dowsett et al. 2013; De Shepper et al. 2014; Jiménez-Moreno et al. 2019). Approximately from the beginning of the Piacenzian (Late Pliocene), at the onset and the intensification of Northern Hemisphere glaciation (Mudelsee and Raymo 2005), to the Early Pleistocene (Gelasian), the Mediterranean progressively experienced a climatic regime characterised by higher-amplitude variability, with main cooling events at about 3.0, 2.5, and 2.1 Ma. These events determined changes in the depositional settings (Lickorish and Butler 1996; Roveri and Taviani 2003; Massari and Chiocci 2006) and in the molluscan assemblages, which were deprived of several tropical taxa (Monegatti and Raffi 2001). Biodiversity declines of the thermophilous molluscan assemblages were reported also from the eastern Atlantic, particularly in Iberian deposits straddling the Strait of Gibraltar (Landau et al. 2007). In general, throughout the Pleistocene (Gelasian–Calabrian–Chibanian and a still unnamed upper stage previously named “Tyrrhenian”), particularly since the Calabrian, the Mediterranean experienced alternances of warmer and colder phases, respectively characterised by lower (Garilli 2011) and higher seasonality (Raffi 1986; Klotz et al. 2006). A frequence of 41 kyr controlled the climate variations in the late Piacenzian–Calabrian, while a frequence of 100 kyr governed the more pronounced alternances of cold and warm phases during the Middle–Late Pleistocene “Ice Ages” (e.g., Raymo 1994; Zachos et al. 2001; Raymo and Nisancioglu 2003; Head and Gibbard 2005; Dowsett and Caballero-Gill 2010). It is remarkable that a paleooceanographical regime similar to the current one established during the Gelasian (early Lower Pleistocene) in the Mediterranean region (Serrano 2020). Changes in the paleogeography of the Mediterranean shore environments occurred in the Pleistocene due to climate changes, especially during the Last Glacial Maximum, when the sea level globally fell 120–130 metres below the present one (e.g., Lambeck et al. 2002, 2014).
All this intricate late Neogene–Quaternary history caused important shifts in the faunal assemblages of the Atlanto-Mediterranean domain (e.g., Malatesta and Zarlenga 1986; Loubere and Moss 1986; Cronin et al. 1993, 1999; Head 1998; Monegatti and Raffi 2001; Taviani 2002; Wood 2009; Garilli 2011; Vertino et al. 2014), and echinoids are not an exception (Roman and Soudet 1990; Kroh 2007; Néraudeau et al. 1999, 2001; Borghi et al. 2014).
Historical background
Changes in diversity of Mediterranean regular and irregular echinoids were investigated over the interval Messinian to present day, in more detail up to the Calabrian. The post Calabrian fossil record is patchy and largely incomplete, with exceptions regarding the upper Pleistocene deposits of Favignana Island (Borghi et al. 2006) and Milazzo (Borghi et al. 2014), in Sicily (southern Italy), which provided a few shallow-water species.
Results presented in this paper are based on field observations by the first author (EB), on a bibliographic data set on the distribution of late Neogene–Quaternary echinoids and on recent advancements of their systematics. Bibliographic data were collected mainly from Lachkhem and Roman (1995) and Néraudeau and Masrour (2008) for the Messinian echinoids of north Africa; from Montenat and Roman (1970), Roman and Soudet (1990), Néraudeau et al. (1999), Lacour and Néraudeau (2000), Saint-Martin et al. (2000) for the Messinian to Pleistocene echinoids of Spain; from Néraudeau et al. (1998), Borghi et al. (2006, 2014), Borghi and Garilli (2017) for the Pliocene and Pleistocene echinoids of Italy; from Challis (1980) and Cappelletti (2008), for the Messinian echinoids of the Maltese islands; from Marcopoulou-Diacantoni (1967, 1972, 1974, 1977) and Heimann and Marcopoulou-Diacantoni (1977), for the Late Miocene to Pleistocene echinoids of Eastern Mediterranean. Néraudeau et al. (2001) provided an overview of irregular echinoids from the whole Mediterranean area. A list of the discussed echinoid genera and their respective species with localities, age and references is reported in SOM: table S1 (Supplementary Online Material available at http://app.pan.pl/SOM/app67-Borghi_Garilli_SOM.pdf). The foraminifer and nannofossil biozonations cited in this paper are from Lirer et al. (2019) and Backman et al. (2012), respectively.
A problem of determining complete paleobiogeographic and biostratigraphic frameworks for echinoids may rise from the uncertainty sometimes affecting the systematics of some fossil specimens (Roman and Soudet 1990). The taxonomy of several fossil genera is still poorly defined. This is the case with the genera Clypeaster, Echinolampas Gray, 1825 and Trachypatagus Pomel, 1869. Many Late Miocene–Recent (chrono)species attributed to Echinolampas were often distinguished only on the base of their different stratigraphy (Roman 1965). Biometric and statistical analyses by Néraudeau et al. (1998) showed that it was not possible to distinguish the Miocene Echinolampas hemisphaerica (Lamarck, 1816), the Pliocene–Pleistocene Echinolampas hoffmanni Desor in Agassiz and Desor, 1847b, and the Recent Echinolampas rangii Des Moulins, 1870. They concluded that a single morphological species of Echinolampas survived the Messinian crisis in the Mediterranean, where it lived until the Pleistocene, and is still living in the eastern Atlantic. There are several cases of echinoid species that survived the Late Miocene salinity crisis and reappeared in the Mediterranean with the same morphology. In some cases, different names were attributed to these taxa (e.g., Roman and Soudet 1990; Néraudeau et al. 1998). Conversely, we prefer to regard chronotaxa that are morphologically close as belonging to one morphospecies, as proposed by Néraudeau et al. (2001), and to base our investigation on the genus level. For this purpose, we took into consideration only well supported citations, both from the taxonomic and the stratigraphic point of view. A further problem of systematics concerns the Amblypygus Agassiz, 1840. Roman and Saint-Martin (1987) ascribed the holotype of Amblypygus lorioli Simonelli, 1889, known from the Mediterranean Pliocene, to the Echinoneus. However, this species differs from those in Echinoneus by having perforate primary tubercles, transversely elongated outer pores and the periproct much closer to the peristome than to the posterior margin of the test (Smith and Kroh 2011; Ali 2017). For these reasons this species is here maintained in the Amblypygus.
Doubtful records and taxa identified solely at the family (or higher) level were not considered.
If not otherwise noticed, we followed the systematic proposed by Smith and Kroh (2011). Conolampas Agassiz, 1883, is considered as a junior synonym of Hypsoclypus Pomel, 1869; Aliaster Valdinucci, 1974, is included in Opissaster Pomel, 1883, and Granopatagus Lambert, 1915, is maintained as a valid genus, distinct from Spatangus Gray, 1825. Following Kroh and Mooi (2021), Gracilechinus Fell and Pawson, 1966, is accepted as a valid genus including the species Gracilechinus acutus Lamarck, 1816. Also, it is here accepted that the specimens of Arbaciella Mortensen, 1910, reported for the Mediterranean Sea are juveniles of Arbacia lixula (Linnaeus, 1758), as recently proved by studies based on DNA analyses (Kroh et al. 2011; Wangensteen 2013). Arbacina Pomel, 1869, is here maintained temporarily as separated from Genocidaris Agassiz, 1869 (study in progress by the first author).
Methods
Key sections used for echinoid biostratigraphy.—Some sections (Fig. 1) in northern Apennines and other parts of Italy were used to provide a stratigraphic frame as precise as possible for some echinoid bioevents occurred during the Mediterranean Pliocene to Early Pleistocene. These key sites are mostly from Emilia Romagna, where this time interval is well documented within several marine upper slope to inner shelf successions for which a detailed stratigraphy frame is known (Capozzi and Picotti 2003; Ceregato et al. 2007; Cau et al. 2015; Crippa et al. 2019). Some other deposits in Italy are also relevant for the characterization of the echinoid Mediterranean stratigraphy, particularly their disappearance events (Fig. 1): the yellow sandy sediments generally known as “Sabbie di Asti” in Piedmont (north Italy), late Zanclean–earliest Piacenzian in age (biozone MPL4a according to Violanti 2005); similar, inner shelf deposits in SW Piedmont, probably of Piacenzian age not younger than the base of the biozone MPL5a (Violanti 2005); the prevalently arenite/calcarenite deposit of the lower unit of the Pianosa Formation (Pianosa Island), dated to the early Piacenzian, upper part of the MPL4 biozone (Foresi et al. 2007); the upper Amphistegina-rich level and related calcarenite body in central Italy (Anzio, Latium), attributed to the early–middle Gelasian, middle–upper part of the MPL5b biozone, possibly within a 2.15–2.3 Ma time interval (Di Bella et al. 2005; see Fig. 1); the calcarenite of Sant’Andrea in Puglia (southern Italy), attributable to the Gelasian lower CNPL6 biozone (Borghi and Garilli 2017 and references therein).
A synoptic, quantitative frame of the Mediterranean echinoids throughout the late Cenozoic is shown in Table 1.
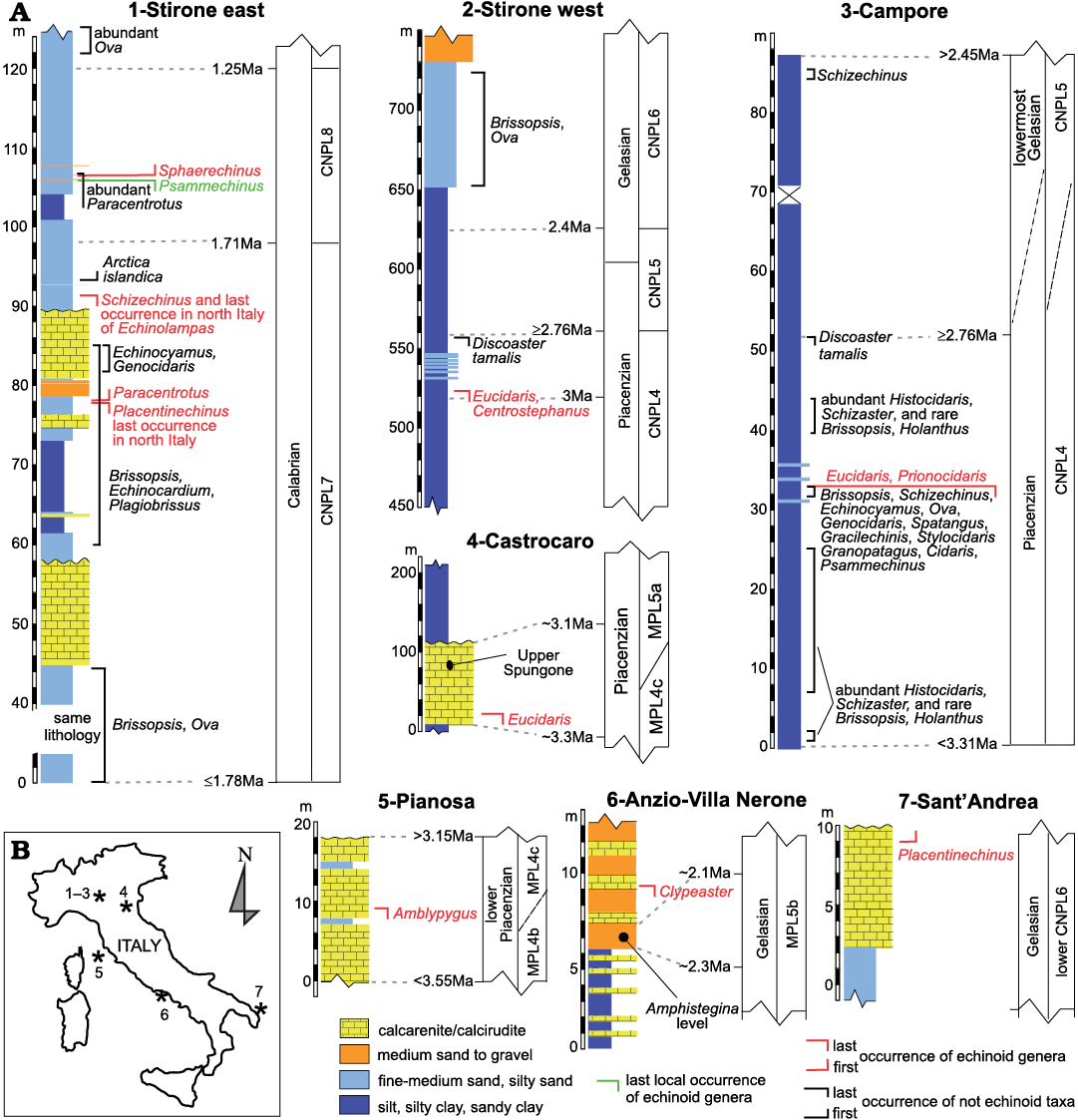
Fig. 1. Stratigraphy (A) and location (B) of some echinoid key sites from Italy, showing some of the most significant appearances, disappearances and other occurrences of Mediterranean late Cenozoic echinoids. Lithology and stratigraphy are reconstructed from Borghi and Garilli (2017) and Crippa et al. (2019) for Stirone east; Cau et al. (2015) for Stirone west; Ceregato et al. (2007) for Campore; Capozzi and Picotti (2003) for Castrocaro; Di Bella et al. (2005) for Anzio; Borghi and Garilli (2017) for Sant’Andrea. The nannofossil biozones (CNPL) and the age of Discoaster tamalis last occurrence are after Backman et al. (2012). The foraminifer biozonation (MPL) is according to Lirer et al. (2019).
Table 1. Quantitative data of Mediterranean echinoid genera from Messinian to Recent. n, number of genera; D, number of disappeared genera; A, number of appeared genera; MSC, Miocene salinity crisis; sNHG, setting of Northern Hemisphere glaciation. For each geological period appearances and disappearances are in relation to the previous one.
|
Echinoid group |
Miocene (Messinian) |
Pliocene |
Pleistocene |
Recent |
|||
|
Pre MSC |
MSC |
Pre sNHG |
Post sNHG |
Gelasian |
Calabrian |
||
|
Regularia |
|||||||
|
n |
13 |
0 |
15 |
10 |
11 |
13 |
11 |
|
D |
– |
13 |
0 |
5 |
0 |
0 |
5 |
|
A |
– |
0 |
15 |
0 |
1 |
2 |
3 |
|
Irregularia |
|||||||
|
n |
24 |
1 |
22 |
12 |
12 |
11 |
10 |
|
D |
– |
23 |
0 |
10 |
0 |
1 |
2 |
|
A |
– |
0 |
21 |
0 |
0 |
0 |
1 |
|
n total |
37 |
1 |
37 |
22 |
23 |
24 |
21 |
|
D total |
– |
36 |
0 |
15 |
0 |
1 |
7 |
|
A total |
– |
0 |
36 |
0 |
1 |
2 |
4 |
The paleoclimatic/biogeographic approach.—The geographical distribution of many echinoids is strongly correlated with SSTs. This is especially true for shallow-water species (Fell 1954), which are the most represented among the here studied genera (Fig. 2 and SOM: table S2), while deeper-water taxa (e.g., Cidaris Leske, 1778, and Brissopsis Agassiz, 1840) are less affected by SSTs and occur widely across climate zone boundaries (Pereira 2008) characterised by remarkably different SSTs (SOM: table S3). In fact, sea temperatures are generally invariable down to depths of little more than 100 m at the mid latitudes and down to few tens of meters deeper in tropical seas (Webb 2017). For this reason the strictly deep-water echinoid assemblage is discussed separately. For a better understanding of the climatic significance of the still extant echinoid genera discussed we also used a biogeographic framework (Fig. 3). The biogeography of these taxa has been reconstructed using data from Tortonese (1965, 1977), OBIS (Ocean Biodiversity Information System 2021) and Kroh and Mooi (2021). We did not consider single records of extant echinoids outside the range and the climatic zone considered as typical for the taxon. The ocean climate regions of the biogeographic framework used are mainly based on the average annual SSTs as reported by Webb (2017). According to this data set, we defined the following zones: tropical-equatorial zone (hereafter simply called tropical) with temperatures generally over 23°C; subtropical-temperate zone with temperatures usually ranging from 9 up to 23°C; subpolar zone with temperatures lower than 9°C. In OBIS (2021) the first two climatic zones respectively correspond to temperature ranges of 10–20°C and 20–30°C.
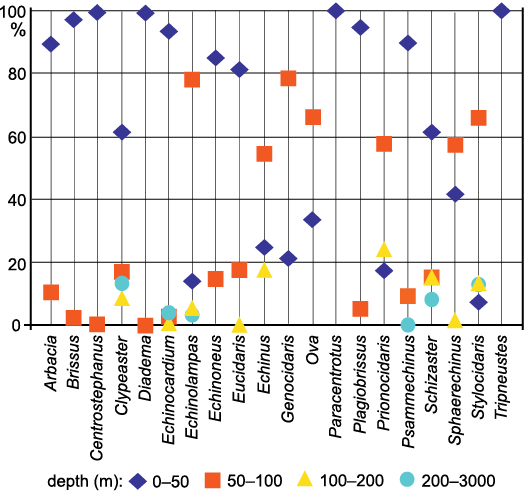
Fig. 2. Current distribution by depth of shelf-preferring/exclusive echinoid genera that still live or lived in Mediterranean during the late Cenozoic. Data are expressed in percentage of the centennial bathymetric records collected by OBIS (https://obis.org).
Many genera found in the Mediterranean upper Cenozoic deposits are still living outside this basin (Pereira 2008). Assuming that from an ecological point of view they did not change considerably, their current climatic distribution can be useful to define the climatic requirements of their fossil relatives. This actualistic approach was successfully applied to the Lower–Middle Miocene of the Central Paratethys (Kroh 2007) and Portugal (Pereira 2008) as well as to earlier echinoids (Carter 2003). Data concerning the distribution and ecological requirements of extant forms are from Mortensen (1928, 1935, 1940, 1943, 1948a, b, 1951), Tortonese (1965), Clark and Rowe (1971), Phelan (1970), Picton (1993), Smith and Kroh (2011). As for the still living echinoids average annual SSTs have been provided based on reports by OBIS (2021), and of personal and literature data (SOM: tables S3, S4 for an overview). For these taxa the SST and biogeographical data sets compiled have been combined to better define their climatic requirements and group them into climate classes.
As for the present time, it should be considered that the climate of the Mediterranean area (mostly the typical dry-summer and wet-winter Mediterranean climate) is rather variable (Köppen 1931), being subtropical in the south and east, and warm temperate to temperate in most of the westernmost and in the northernmost parts. This climatic framework is well applied to the marine benthos (e.g., Pérès 1967). In this study we consider Mediterranean extant echinoids as belonging to a group requiring subtropical-warm temperate conditions and prefer to regard as strictly temperate those taxa living outside the Mediterranean at similar or higher European latitudes. The climatic significance of Mediterranean extinct species has been determined by means of the paleoclimate as reconstructed for the stratigraphic range where they lived.
The biostratigraphy of those taxa that went extinct in the Mediterranean domain has been also used for the determination of their respective climatic requirements. We considered as strictly tropical those genera that lived in that area during the Miocene–Pliocene and/or the warm Late Pleistocene (the marine isotopic stage 5); genera that survived the Pliocene–Pleistocene climatic deteriorations were considered as taxa tolerating a wide thermal range. The climatic requirement of genera still living outside the Mediterranean was defined also by means of their biogeography.
Climatic groups within
the Mediterranean echinoids
and their stratigraphy
According to their ecological requirements, particularly their average annual SSTs, and (paleo)biogeography, the echinoid genera occurring in the Mediterranean Late Miocene to Recent were included in the hereafter reported five climatic groups. Only the still living species of genera Brissopsis and Echinocyamus Van Phelsum, 1774, should be considered as “outsider” taxa, whose climatic requirements do not match those of the identified climatic groups, as their biogeography is not remarkably affected by temperature (Fell 1954; Kroh 2005; Pereira 2008). Their representatives are well distributed in tropical-subtropical environments as well as in temperate waters (generally in slope environments) and are recorded even in shallow waters of cold regions (Fig. 3). Also their Mediterranean stratigraphic history is broad, having being recorded almost continuously throughout the late Cenozoic, above all with the widespread species Brissopsis atlantica Mortensen, 1907, from the Messinian (Lacour and Néraudeau 2000) and the Calabrian (Borghi 1997), Brissopsis lyrifera (Forbes, 1841), from the Messinian to Recent (Checchia Rispoli 1919, 1923; Tortonese 1965; Lachkhem and Roman 1995; Borghi 1997; Lacour and Néraudeau 2000; Néraudeau et al. 2001), and Echinocyamus pusillus (Müller, 1776). This last species is known from the Messinian of Melilla (Lachkhem and Roman 1995) and Alicante, Spain (Montenat and Roman 1970), the Zanclean–Piacenzian of Piedmont (Airaghi 1901), the Piacenzian of Tuscany (Simonelli 1889) and Spain (Roman and Soudet 1990), the Gelasian of Anzio (Checchia Rispoli 1923), the Calabrian of Emilia (Borghi 1993a) and Sicily (Borghi et al. 2006), and from the Upper Pleistocene of Milazzo, Sicily (Borghi et al. 2014). Outside the Mediterranean the species has a less wide stratigraphic distribution having been reported from the Upper Miocene to Pliocene of the Azores (Madeira et al. 2011). It is still common in the Recent Mediterranean (Tortonese 1965) and in the northeastern Atlantic (Madeira et al. 2019). Brissopsis and Echinocyamus are therefore genera with a wide climatic distribution.
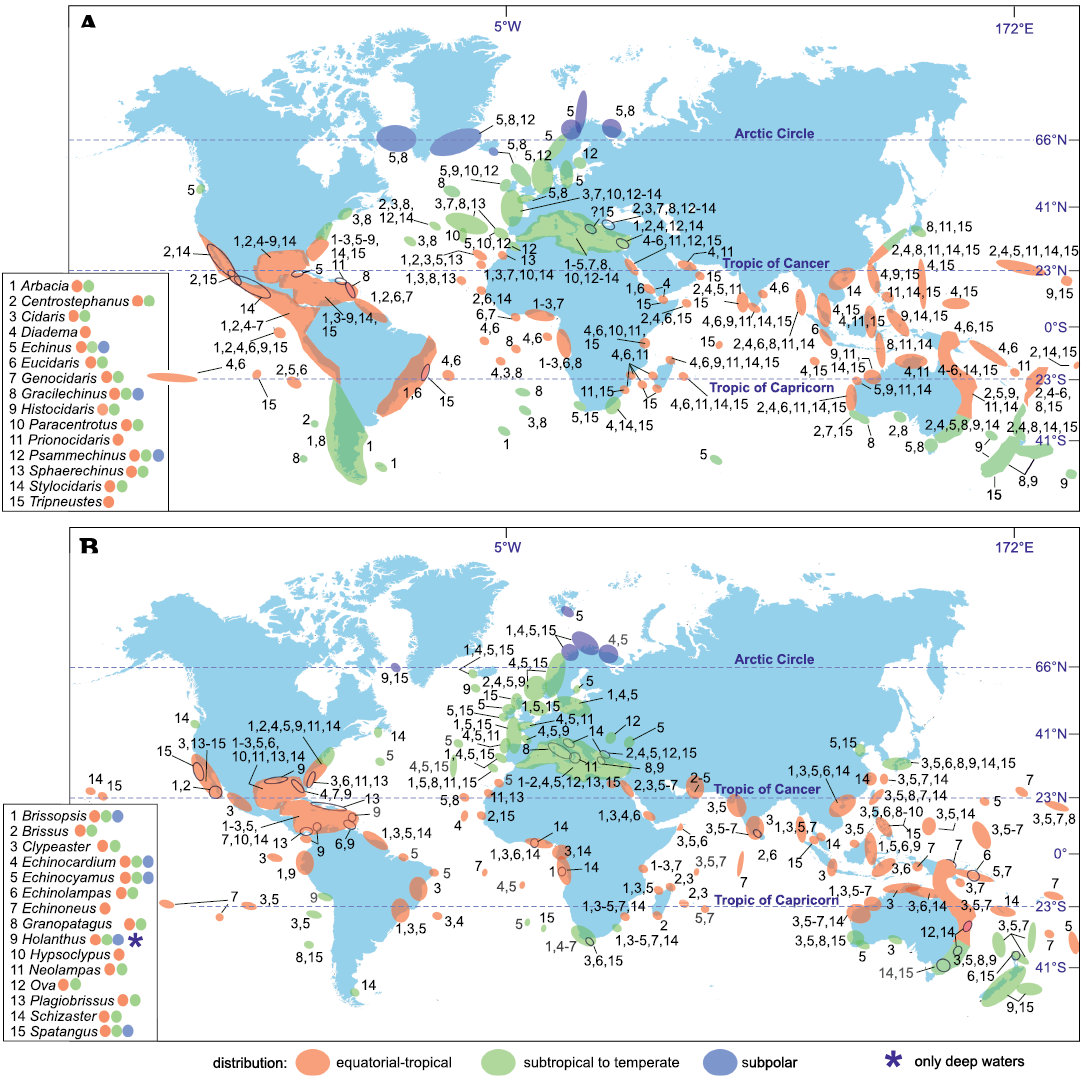
Fig. 3. Biogeography of regular (A) and irregular (B) echinoid genera which occurred in the Mediterranean during the Late Miocene–Holocene.
Strictly tropical genera.—This group includes genera the species of which are still living in tropical (very rarely in subtropical) seas or that lived in warm geological periods, generally not younger than the warm middle Piacenzian.
The Recent species of Diadema Gray, 1825, Eucidaris Pomel, 1883, Prionocidaris Agassiz, 1863, Tripneustes Agassiz, 1841, Hypsoclypus, and Echinoneus are widespread in tropical seas (Fig. 3), generally in shallow to moderate depth (see Smith and Kroh 2011), and the respective fossil representatives should be regarded as taxa with tropical affinity. Recently Hypsoclypus and Echinoneus species have been reported from the Mediterranean warm middle Piacenzian in shelf paleoenvironments: Hypsoclypus pouyannei Pomel, 1887, associated with Clypeaster sp. from Tuscany, Central Italy (Borghi and Ciappelli 2014); Echinoneus cyclostomus Leske, 1778, from Cabo Cope, Spain (Diego Garcia Ramos, personal communication 2012). Today the species of Hypsoclypus usually inhabit environments deeper than those reported for its fossil Mediterranean representatives.
The extinct Amphiope, Pliolampas Pomel, 1888, and Schizobrissus Pomel, 1869, should be considered as tropical taxa as they are closely related respectively to Echinodiscus Leske, 1778, Studeria Duncan, 1889, and Meoma Gray, 1851, the specie of which live today in strictly tropical seas (Pereira 2008). Also the species of some other extinct shallow-water genera, such as Amblypygus, Opissaster, Peribrissus Pomel, 1869, Trachyaster Pomel, 1869, Trachypatagus, and Sardospatangus Stara, Charbonnier, and Borghi, 2018, share the same climatic significance because of their paleogeographic distribution and stratigraphy. The species of these genera are limited to the warm Eocene–Pliocene of the Mediterranean area or to other regions characterised by similar climate (e.g., Simonelli 1889; Mortensen 1928, 1935, 1940, 1943, 1948a, b, 1951; Roman and Saint-Martin 1987; Lachkhem and Roman 1995; Néraudeau et al. 2001; Smith and Kroh 2011; Borghi and Ciappelli 2014; Mancosu and Nebelsick 2016; Stara et al. 2018). Species of Opissaster, Peribrissus, Pliolampas, Schizobrissus, Sardospatangus, Trachyaster, and Trachypatagus were present in the pre-evaporitic Messinian of the Mediterranean (Roman and Soudet 1990; Lachkhem and Roman 1995; Néraudeau et al. 2001; Stara et al. 2018). Only Opissaster, Peribrissus, Schizobrissus, Sardospatangus, and Trachypatagus have been cited also from the Mediterranean Pliocene, although their occurrence was rare during that period. Earlier citations of these five genera from the “Pliocene” of Algeria were provided by Pomel (1869, 1887) and Cotteau et al. (1891). Subsequently, a species of Trachypatagus was reported also from the Pliocene of Spain (Roman and Soudet 1990), and a representative of Schizobrissus from the Pliocene of North Africa (Lachkhem and Roman 1995; Néraudeau et al. 2001).
Eucidaris was frequent in the Late Miocene (Néraudeau et al. 1999) and Pliocene, where the species Eucidaris desmoulinsi (Sismonda, 1842) has been recognised in the Zanclean of Tuscany and Sicily, and in many Piacenzian sites of Emilia, Piedmont (Borghi 1999) and Tuscany (Simonelli 1889, cited as Cidaris tessurata).
The only Pliocene species of Amphiope from the Mediterranean, Amphiope tipasensis (Aymé and Roman, 1954), was described from the “middle Astian” of Tipaza (Algiers), Piacenzian in the modern stratigraphy (see MNHN at http://coldb.mnhn.fr/catalognumber/mnhn/f/r06930 also for pictures).
The last Mediterranean occurrence of Amblypygus is that of the holotype of Amblypygus lorioli, from the lower Piacenzian (Colantoni and Borsetti 1973; Foresi et al. 2007) of Pianosa Island in north-central Italy.
Tripneustes was widespread during the Lower and Middle Miocene in the Mediterranean and Paratethys seas (Philippe 1998; Kroh 2005). It is known from the Tortonian and Messinian of North Africa (Lachkhem and Roman 1995) and Spain (Roman and Soudet 1990; Bajo and Borghi 2009; Fig. 4F). Two species, Tripneustes gahardensis (Seunes, 1896) and Tripneustes planus (Agassiz in Agassiz and Desor, 1847a), have been recorded also from the Spanish Zanclean (Roman and Soudet 1990; Lachkhem and Roman 1995).
According to Stara et al. (2018), the earliest records of the extinct Sardospatangus are from the Aquitanian (Lower Miocene) of Sardinia, with Sardospatangus thieryi (Lambert, 1909) and Sardospatangus arburensis Stara, Charbonnier, and Borghi, 2018. Later occurrences regard Sardospatangus pustulosus (Wright, 1855), in the Aquitanian–Burdigalian (Lower Miocene) of Malta, Sardospatangus subconicus (Mazzetti, 1882), Sardospatangus destefanii (Stefanini, 1908), and Sardospatangus fabianii (Lambert in Lambert and Thiéry, 1924), in the upper Burdigalian–early Langhian (upper Lower–lower Upper Miocene) of Emilia (North Italy), and Sardospatangus saheliensis (Pomel, 1887), in the Upper Miocene of North Africa. Only Sardospatangus saheliensis and Sardospatangus rovasendai (Airaghi, 1901) (Fig. 4C), have been found in the Piacenzian of the Mediterranean area (Stara et al. 2018), respectively from the inner shelf deposits of Almeria (Roman and Soudet 1990) and Piedmont.
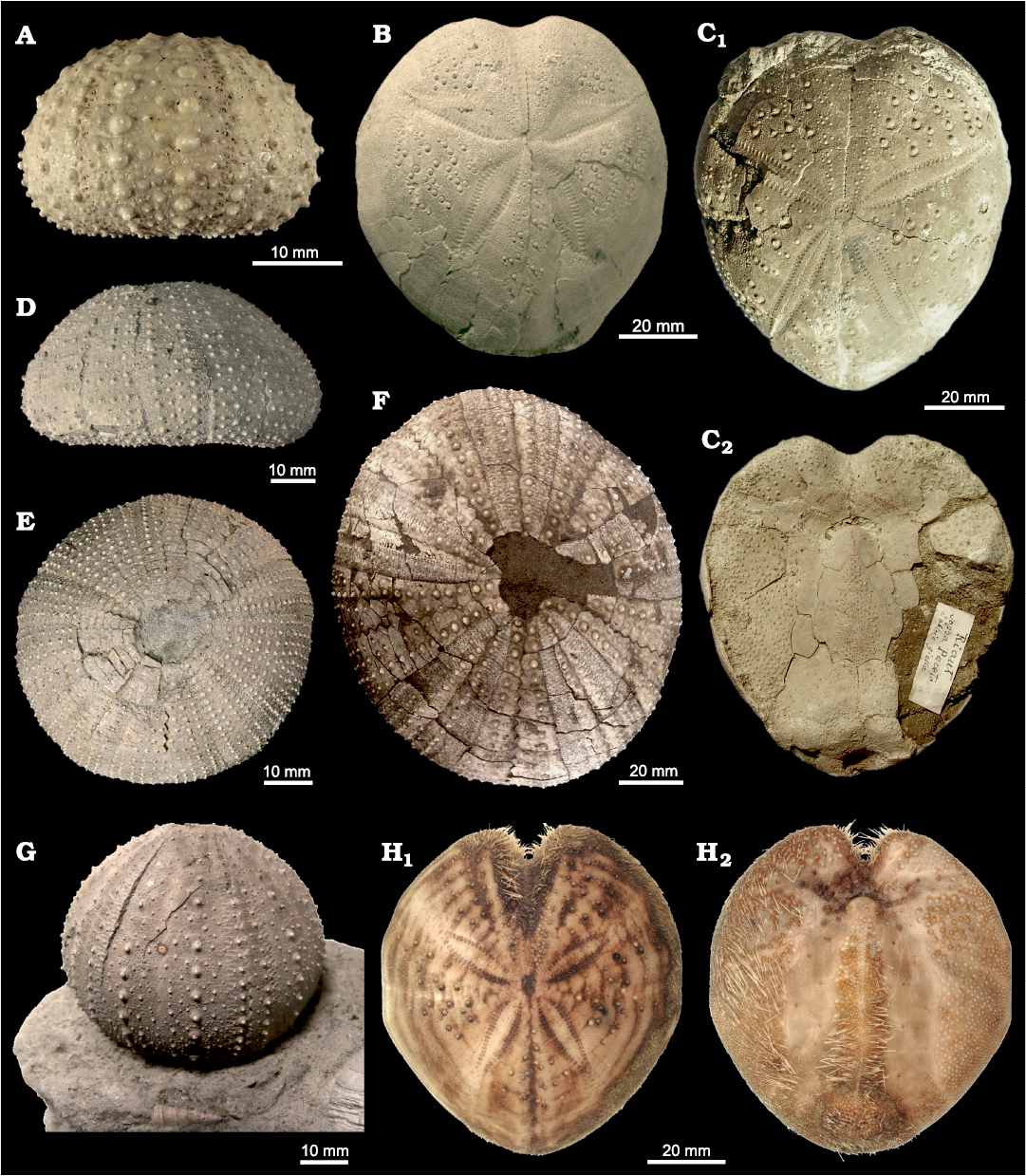
Fig. 4. Rare echinoids from the Mediterranean late Cenozoic to Recent. A. Stirechinus scillae Desor, 1856, MG 1034.10, Early Pleistocene, Contrada Coilare (Messina, Sicily), in lateral view. B. Spatangus purpureus (Müller, 1776), MG 1038.01, Calabrian, Castell’Arquato, Italy, in aboral view. C. Sardospatangus rovasendai (Airaghi, 1901), MTPL.1246, Piacenzian of Pecetto Piemontese, Italy, in aboral (C1) and oral (C2) views. D. Schizechinus serialis Pomel, 1887, MCS Ss.17, Calabrian, Stirone River, Parma, Italy, in lateral view. E. Schizechinus serialis Pomel, 1887, MG Sz.15, Calabrian, Castell’Arquato, Italy, in aboral view. F. Tripneustes gahardensis (Seunes, 1896), MGUS 2040, late Tortonian–early Messinian, Espera, Spain, in aboral view. G. Gracilechinus acutus (Lamarck, 1816), MCS Ga.12 (ex Bussolati collection), Calabrian, Stirone River, Parma, Italy, in lateral view. H. Granopatagus subinermis (Pomel, 1887), MZUF.1359, Recent, off Livorno, Italy, in oral (H1) and aboral (H2) views.
Tropical-subtropical genera.—This group concerns genera that generally lived or live in tropical to subtropical settings, including the Mediterranean Sea, but may be rarely found in temperate conditions.
After Roman (1989a) and Pereira (2008) the following genera, although generally found in tropical (paleo)environments, have also some subtropical representatives (Fig. 3) or lived in paleoclimate setting that could be regarded as subtropical: Stylocidaris Mortensen, 1909, Centrostephanus Peters, 1855, Arbacia Gray, 1835, Genocidaris, Clypeaster, Echinolampas, Granopatagus, Ova Gray, 1825, Brissus Gray, 1825, Plagiobrissus Pomel, 1883. Prionocidaris is typical of tropical areas and its unique record from the shallow cold waters of Antarctica (OBIS 2021) should be regarded as accidental. Also Neolampas Agassiz, 1869, should be included into this group as it only rarely occurs in temperate localities (Mortensen 1927) and most records are from tropical and subtropical areas (see Fig. 3). We prefer to regard Schizaster Agassiz, 1836, species of which live outside the Mediterranean mainly in warm climatic conditions, as belonging to the group of the deep-sea echinoids hereafter described. In fact, in the Mediterranean area species of this genus were limited to deep environments throughout the late Cenozoic.
The extinct Schizechinus Pomel, 1869, is also placed into this group because of its wide biostratigraphy, including the late Upper Miocene (Roman and Soudet 1990), the Pliocene (Checchia Rispoli 1917; Lachkhem and Roman 1995) and the earliest Pleistocene (Checchia-Rispoli 1916; Roman and Soudet 1990; Borghi et al. 2018). The last Mediterranean occurrence of this genus, concerning the species Schizechinus serialis Pomel, 1887 (Fig. 4D, E), is that recorded in the lower Calabrian of northern Italy (Borghi et al. 2018), in a layer preceding the entrance of the boreal guest Arctica islandica (Linnaeus, 1767). The disappearance of this genus roughly correlates to the first Mediterranean occurrence of the temperate Sphaerechinus, with Sphaerechinus granularis (Lamarck, 1816), which likely occupied the same ecological niche of Schizechinus. As a matter of fact, Sphaerechinus found favourable conditions in the late Calabrian of Southern Italy, at Favignana Island, where it is very common (Borghi et al. 2006).
Also, Arbacina, another extinct genus, has wide stratigraphy: the Messinian of Spain (Roman and Soudet 1990; Bajo et al. 2008), the Pliocene of Altavilla Milicia, Sicily (Checchia Rispoli 1916), the Gelasian of Anzio (Checchia Rispoli 1923), the last known occurrence from the Calabrian of Sicily (Checchia Rispoli 1907; Borghi and Garilli 2017).
Genocidaris shows a distribution similar to that of Arbacina: Messinian of North Africa (Néraudeau et al. 1999), Zanclean to Calabrian of Emilia (Borghi, 1995b), Upper Pleistocene of Capo Milazzo, Sicily (Borghi et al. 2014). The species Genocidaris maculata Agassiz, 1869 is still present in the Mediterranean (Tortonese, 1965).
Plagiobrissus inhabited the Messinian Mediterranean with two species: Plagiobrissus imbricatus (Wright, 1855), from the Maltese Islands, and Plagiobrissus costae (Gasco, 1876), from Melilla, in North Africa (Lachkhem and Roman 1995), and Spain (Bajo et al. 2008). Plagiobrissus costae, recorded also from the Zanclean to Piacenzian of Almeria, Spain (Roman and Soudet 1990), and the Calabrian of Emilia (Borghi, 1993c), is still living in the Mediterranean (Tortonese, 1965). Plagiobrissus has always been rather rare in the Mediterranean, from the Late Miocene to Recent.
The genus Ova was represented by Ova saheliensis (Pomel, 1887), cited from the Messinian of North Africa (Lachkhem and Roman 1985), and the Pliocene of Spain (Néraudeau et al. 2001), and by Ova canalifera (Lamarck, 1816), known from the Pliocene–Pleistocene of Italy (Borghi 2021b). Ova is still present in the Mediterranean with the endemic species Ova canalifera.
Centrostephanus, with its species Centrostephanus longispinus (Philippi, 1845), has been commonly cited from the Mediterranean upper Cenozoic: in the late Upper Miocene (Roman and Soudet 1990), in the Pliocene (Seguenza 1880; Checchia Rispoli 1917; Tortonese 1965) and in the Recent (Tortonese 1965). Also, the report from the Pliocene of North Italy (Airaghi 1901) of Diadema, indeed a very recent Mediterranean acquisition (Por 2009; Bronstein et al. 2017), should be attributed to Centrostephanus. In fact, the identification of these two genera based on disarticulated remains is still problematic (see Kroh 2005) despite Donovan (2018) attempted to distinguish them based on the transverse section of spines. Also the loose spines collected by the first author from the Messinian of Vigoleno and the Piacenzian of Campore (North Italy) belong to Centrostephanus.
Arbacia is represented in the Pliocene (presumably upper Piacenzian) of Cabo de Gata (Spain) by rare specimens of a still undetermined species, which are morphologically close to some congeneric ones known from the Pliocene of the Caribbean area (Philippe Nicolleau and Leonardo Hernandez, personal communication 2020). Arbacia lixula was reported by Tortonese (1965) for the “Panchina di Livorno” (Tuscany), which formed in the Late (warm) Pleistocene (Bartoletti et al. 1986; Mazzanti 2016). Consistently, Wangensteen (2013) regarded this species as a thermophilous taxon that likely colonised the Mediterranean during the last interglacial. It still lives in the Mediterranean.
One of the most representative species of Echinolampas, Echinolampas hoffmanni, has been reported from the Piacenzian to the Calabrian of Italy (Simonelli 1889; Checchia Rispoli 1907, 1917; Borghi 1994b; Borghi and Garilli 2017). Interestingly, the genus Echinolampas disappears in the basal layers of the Calabrian succession in northern Italy (Borghi 1994b), while it is still abundant in the Calabrian of southern Italy deposited in warmer waters (Checchia Rispoli 1907; Borghi and Garilli 2017), where it never occurs together with boreal guest. Echinolampas persists in Western Africa till today (Kroh and Mooi 2021), with the species Echinolampas rangii.
The Mediterranean records of Brissus concern only the extant species Brissus unicolor (Leske, 1778), known from the Piacenzian of Pianosa (Simonelli 1889; for the stratigraphy see Colantoni and Borsetti 1973 and Foresi et al. 2007), the Calabrian of Sicily (Borghi et al. 2006) and the Late Pleistocene-aged (last interglacial) “Panchina di Livorno” (Checchia Rispoli 1907). This species also lives in the tropical-subtropical Atlantic, at the Antilles, Azores, Canary, and Capo Verde Islands (Koehler 1927).
Prionocidaris has been recorded from the Messinian of Sardinia (Cotteau 1895; Lambert 1907). It was recently recognised also in the Pliocene of Campore (northern Italy), where loose spines attributable to Prionocidaris avenionensis (Des Moulins, 1837) have been collected by one of the authors (EB). These spines were found in lenticular bioclastic horizons (see Fig. 1) bearing a shallow-water reworked assemblage, embedded within the poorly stratified clayey siltstone at 31–37 m of the section described by Ceregato et al. (2007). This finding represents the last Mediterranean occurrence of this genus, at the approximate age of 3 Ma, in the mid-Piacenzian.
Clypeaster was well diversified in the Late Miocene (Néraudeau et al. 1998). Its species were frequent also in the Pliocene of Tuscany (Desor in Agassiz and Desor 1847a; Simonelli 1889), Spain (Rose and Wood 1999) and North Africa (Michelin 1863). From these localities only two species, Clypeaster altus (Leske, 1778) and Clypeaster aegyptiacus Michelin, 1863, are currently recognised as valid (Esu and Kotsakis 1981; Néraudeau et al. 1998). The last Mediterranean occurrence of this genus is recorded in the Gelasian calcarenite of Anzio (Checchia Rispoli 1923; for stratigraphy see Carboni and Di Bella 1997) and should be placed in the MPL5b biozone (Fig. 1), in the around of the 2.1 Ma climate deterioration, marking the boundary between the ecobiozones MPMU3 and MPMU4 of Monegatti and Raffi (2001).
Granopatagus is still living in the Mediterranean Sea, where Granopatagus subinermis (Pomel, 1887) inhabits shelf waters (Tortonese 1965; Néraudeau et al. 1998), shallower than in the Pacific Ocean (Noordenburg 2008). Also, its Mediterranean fossil species preferred shallow waters (Néraudeau et al. 1998).
Subtropical-temperate genera.—This group consists of genera that today live predominantly in temperate regions but that include also subtropical species and very rarely tropical or subpolar species (Fig. 3): Echinus Linnaeus, 1758, Gracilechinus, Echinocardium Gray, 1825, Psammechinus Agassiz and Desor, 1846, and Spatangus. Cidaris should be included in this group though some of its species inhabit cold, or even tropical waters, also in slope environments (OBIS 2021). This genus is the most eurythermal of this group and will be also discussed hereafter, in the section dedicated to the group of the Mediterranean deep-water echinoids. However, following OBIS (2021) most of the records of this genus concerns SSTs of 15–20°C. Two Mediterranean fossil species, Cidaris remiger Ponzi, 1858, and Cidaris margaritifera (Meneghini, 1862) typically lived in deep waters, respectively during the Zanclean and the Zanclean–Calabrian (Meneghini 1862; Borghi 1999; Borghi et al. 2014). The eurybathic Cidaris cidaris (Linnaeus, 1758) is known from the Pliocene of Spain (Montenat and Roman 1970) and the Pliocene–Pleistocene of Italy (Simonelli 1889, cited as Dorocidaris papillata; Borghi et al. 2006, 2014).
Echinocardium and Spatangus are rarely reported also from sites with tropical climate, but most species are confined to higher latitudes, where they are widespread and abundant in shallow waters (Mortensen 1951), although the latter is more frequent in bathyal waters. This is the case with Echinocardium cordatum (Pennant, 1777) and Spatangus purpureus (Müller, 1776) (Fig. 4B), extant species that were already present in the Mediterranean Pliocene. Spatangus purpureus often coexisted sympatrically in the Pliocene–Pleistocene of the Mediterranean with the morphologically close Granopatagus subinermis (Fig. 4H), a thermophilous species (see above). Probably due to different climate conditions, the quantitative ratio between specimens of Spatangus purpureus and Granopatagus subinermis drastically changed during the Calabrian, being of 1:3 in the lower Calabrian of Emilia-Romagna, and more than 100:1 in the upper, colder, Calabrian of south Italy, (Borghi et al. 2006). In the present-day Mediterranean Granopatagus subinermis is still very rare (Koehler 1927). Several species of Echinocardium have been recorded from the Messinian (Néraudeau et al. 2001) and the Pliocene (Pomel 1887; Cotteau et al. 1891) of North Africa, while in the Gelasian the genus was quantitatively well represented only by Echinocardium cordatum, in sandy facies, and Echinocardium melii Checchia Rispoli, 1923, likely the ancestor of the extant Echinocardium mortenseni Thiéry, 1909, in silty-clay sediments (Borghi 1997). Echinocardium cordatum became common in the Calabrian (Checchia Rispoli 1907; Borghi 1997). Also, Echinocardium mediterraneum (Forbes, 1841) is present in the upper Calabrian of Sicily (Checchia Rispoli 1907).
Significant changes occurred from the Late Miocene to the Quaternary within a few genera of this group. Echinus entered in the Mediterranean with the species Echinus algirus Pomel, 1887, in the Late Miocene (Roman and Soudet 1990), while Gracilechinus acutus (Lamarck, 1816) colonised the Mediterranean during the Zanclean (Roman and Soudet 1990). There are only a few records of these genera in the Pliocene (Cotteau et al. 1891; Borghi 1993b). Gracilechinus became much more frequent in northern (Borghi 1993b; Fig. 4G) and southern Italy (Borghi et al. 2006) during the Calabrian, with the species Gracilechinus acutus, which today is still living in the Mediterranean and in North and East Atlantic from the Barents Sea to Sierra Leone. Rarely, species of Gracilechinus also inhabits the cold waters of southern Greenland and northeastern Canada (Fig. 3). Echinus algirus was replaced during the Pliocene by the Atlantic species Echinus melo Lamarck, 1816 today living in temperate and subtropical areas. A similar case was observed for the genus Psammechinus. Psammechinus astensis (Sismonda, 1842), a species close to the Late Miocene Psammechinus dubius (Agassiz, 1840), was common in the Mediterranean during the Pliocene (Airaghi 1901), Gelasian (Checchia Rispoli 1923) and Calabrian (Checchia Rispoli 1907; Borghi 2021a). It is commonly assumed that it was replaced by Psammechinus microtuberculatus Blainville, 1825, during the Calabrian in Sicily and southern Italy (Checchia Rispoli 1907). However, Psammechinus dubius, Psammechinus astensis, and Psammechinus microtuberculatus are so similar (Kroh 2005) that they could belong to the same chronospecies.
The post Messinian north-eastern Atlantic immigrants.—Some echinoids from the north-eastern Atlantic entered the Mediterranean during the Pleistocene: Placentinechinus, Sphaerechinus, and Paracentrotus.
The monospecific Placentinechinus likely originated from the group of marsupiate echinoids of Western Europe described by Roman (1983), Néraudeau et al. (2003), Dudicourt et al. (2005), and its species entered the Mediterranean at the beginning of the Gelasian. It was still present during the early Calabrian of northern and southern peninsular Italy, and during the late Calabrian of Sicily (Borghi and Garilli 2017).
Another monospecific genus, Sphaerechinus, lives today in the Mediterranean and in the East Atlantic (Fig. 3), from the English Channel to the Gulf of Guinea and Azores (Tortonese 1965). It has been recorded from the Calabrian of Monte Mario, in central Italy (Tortonese 1965) and in large numbers in the upper Calabrian of Sicily (Checchia Rispoli 1907; Borghi et al. 2006).
Paracentrotus is currently represented in the Mediterranean by Paracentrotus lividus (Lamarck, 1816), today living also in north eastern Atlantic, from Ireland to the Canary and Azores Islands (Tortonese 1965). This species is common along the western coast of Ireland and South-western England, often in shallow waters (Picton 1993). Although Roman and Soudet (1990) reported this taxon from the “Pliocene” of the Mediterranean, the first well-supported record from this basin is that of the lower Calabrian of northern Italy (Borghi 1995a). Also along the Atlantic coasts of Morocco it was found only in Pleistocene sediments (Néraudeau and Masrour 2008), as well as in the upper Calabrian of Sicily (Checchia Rispoli 1907; Borghi et al. 2006) and Calabria (Seguenza 1880). From a climatic point of view this group includes temperate to warm temperate taxa.
The deep-water assemblage.—There are two main subgroups in this category: one includes the strictly bathyal, the other consists of taxa that live in the deep waters but usually prefer shallower settings (eurybatic taxa).
Holaster Agassiz, 1836, a genus of the early strictly deep echinoids in the Mediterranean upper Cenozoic, was recently found in the upper Miocene bathyal deposits of north Italy, from the Marne di S. Agata Fossili Formation (Gianni Repetto, personal communication 2014, and study in progress by the authors of this paper, EB and VG) and the Marne del Termina Formation, near Parma (Dimitri Bertolaso, personal communication 2019). However, the paleontological evidence of Mediterranean autochthonous deep-water assemblages comes from the upper Pliocene–Pleistocene of southern Italy and the Pliocene of northern and central Italy (Borghi et al. 2014 and EB personal observations). In South Italy the deep assemblage from Capo Milazzo (Sicily), indicating muddy settings with psychrospheric conditions (Borghi et al. 2014), is characterised by a low diversity, being dominated by the Cidaris, Histocidaris Mortensen, 1903, Holanthus Lambert and Thiéry, 1925, Schizaster Agassiz, 1836, and Stirechinus Desor, 1856, each represented by only one species: Cidaris margaritifera, Histocidaris sicula Borghi, 1999, Holanthus ovatus, Schizaster braidensis Botto Micca, 1896, and Stirechinus scillae Desor, 1856 (Fig. 4A). The stratigraphic range of this assemblage is upper Piacenzian to Calabrian (Borghi et al. 2014 and references therein). The deep-water assemblage from the Argille Azzurre Formation, in North Italy, is partly comparable, including Cidaris margaritifera, Histocidaris rosaria, Holanthus ovatus, and Schizaster braidensis (Airaghi 1901; Borghi et al. 2014). Recently, also Mazettia Lambert and Thiéry in Lambert, 1915, a genus so far considered as extinguished during the Middle Miocene (Smith and Kroh 2011), has been found in the Zanclean of Emilia (Borghi et al. 2022). All these echinoids can be regarded as strictly bathyal and some of them show affinities with Recent taxa confined to the western Atlantic deep waters (Borghi et al. 2014), particularly in the tropics. Specifically, Cidaris margaritifera is close to Cidaris rugosa (Clark, 1907), living on soft bottoms of the western Atlantic (including the Caribbean area); Histocidaris rosaria (Bronn, 1831) is similar to Histocidaris nuttingi Mortensen, 1928, found from Cuba to Antigua on muddy bottoms at depth of 225–740 m (Donovan et al. 2005); Histocidaris sicula Borghi, 1999, resembles to Histocidaris sharreri Agassiz, 1880, living in the Caribbean at 200–740 m on muddy bottoms (Serafy 1979); Stirechinus scillae Desor, 1856, is close to Stirechinus tyloides (Clark, 1912), living in the Caribbean deep waters. Interestingly, Stirechinus was reported from the Upper Miocene of the upper Coralline Limestone Formation in Malta (Wright 1864), and of Capo San Marco in Sardinia (Mariani and Parona 1887). It is interesting that Holanthus ovatus is closely related to the extant Holanthus expergitus (Lovén, 1874), which is confined to the coldest area of the Mediterranean at 600–750 m (Tortonese 1977). Holanthus expergitus is a deep-sea species living in the Atlantic Ocean from Iceland to Azores and Cape Verde Islands, at depth of 400–3120 m (Mortensen 1927).
The echinoid group of Mediterranean eurybatic taxa includes: Cidaris cidaris, Stylocidaris affinis (Philippi, 1845), Brissopsis lyrifera, and Brissopsis atlantica. The morphologically close Cidaris cidaris and Stylocidaris affinis respectively live in Mediterranean and East Atlantic (from Norway to Cape Verde), at depth of 50–2000 m, and in Mediterranean, at depth of 30–1000 m depth (Tortonese 1965). The main diagnostic character for distinguishing these two species, consisting of the valves of the globiferous pedicellariae, allowed the detection of both species in the Calabrian of northern Italy (Borghi 1994a). Fossils of these two species were commonly misinterpreted and determined as Dorocidaris papillata (Leske, 1778) by early authors (e.g., Meneghini 1862; Simonelli 1889; Checchia Rispoli 1907, 1916). Brissopsis lyrifera and Brissopsis lyrifera atlantica were regarded as already well-differentiated species in the Messinian of the Sorbas Basin, Spain (Lacour and Néraudeau 2000). Both are common in the present-day north-eastern Atlantic and in Mediterranean, with higher numbers in muddy or sandy bathyal bottoms deeper than 200 m (Tortonese 1965; Picton 1993).
Five additional eurybatic taxa, still living in the deep Mediterranean, have been recorded also from the Pliocene–Pleistocene: Genocidaris maculata, Gracilechinus acutus, Echinus melo, Echinocyamus pusillus, and Spatangus purpureus. All of them are more frequent in shallow settings. Records from deep-water deposits are rather rare: Gracilechinus acutus and Spatangus purpureus from the Zanclean–Piacenzian Argille Azzurre Formation of Italy and in upper Piacenzian–Calabrian of Capo Milazzo (Borghi et al. 2014) and the surroundings of Messina (Checchia Rispoli 1916); Genocidaris maculata and Echinocyamus pusillus from the Calabrian of Lazzaro, in southern Italy (Dimitri Bertolaso, personal communication 2014).
Discussion
The identification of the climatic requirements of the echinoid genera that lived in the Mediterranean area during the late Cenozoic (summarised in Fig. 5), and the recognition of their appearance and disappearances within a detailed biostratigraphic scheme (Fig. 6) allowed the definition of the climatic changes that drove the most severe modifications of their diversity. From a quantitative point of view, in the Mediterranean domain the shallow-water echinoid diversity underwent three important changes (in the Late Miocene, during the Pliocene and the Early Pleistocene), which determined the current assemblage. Different causes likely triggered the diversity changes of the deep-water assemblages.
Diversity of the Late Miocene assemblages.—The Miocene was a period of great flourishing for the Mediterranean echinoids, although the number of genera and species cited in literature (respectively 70 and 400) is likely overestimated, mainly because of the still unresolved problems with synonymies (Roman 1989a), which especially trouble the number of species. The Miocene echinoid fauna was of clear tropical affinity, showing remarkable similarities with that today living in the Indo-West Pacific (Roman 1989a), and many differences from the Mediterranean Recent assemblage, with which shares only 15 genera (Fig. 5).
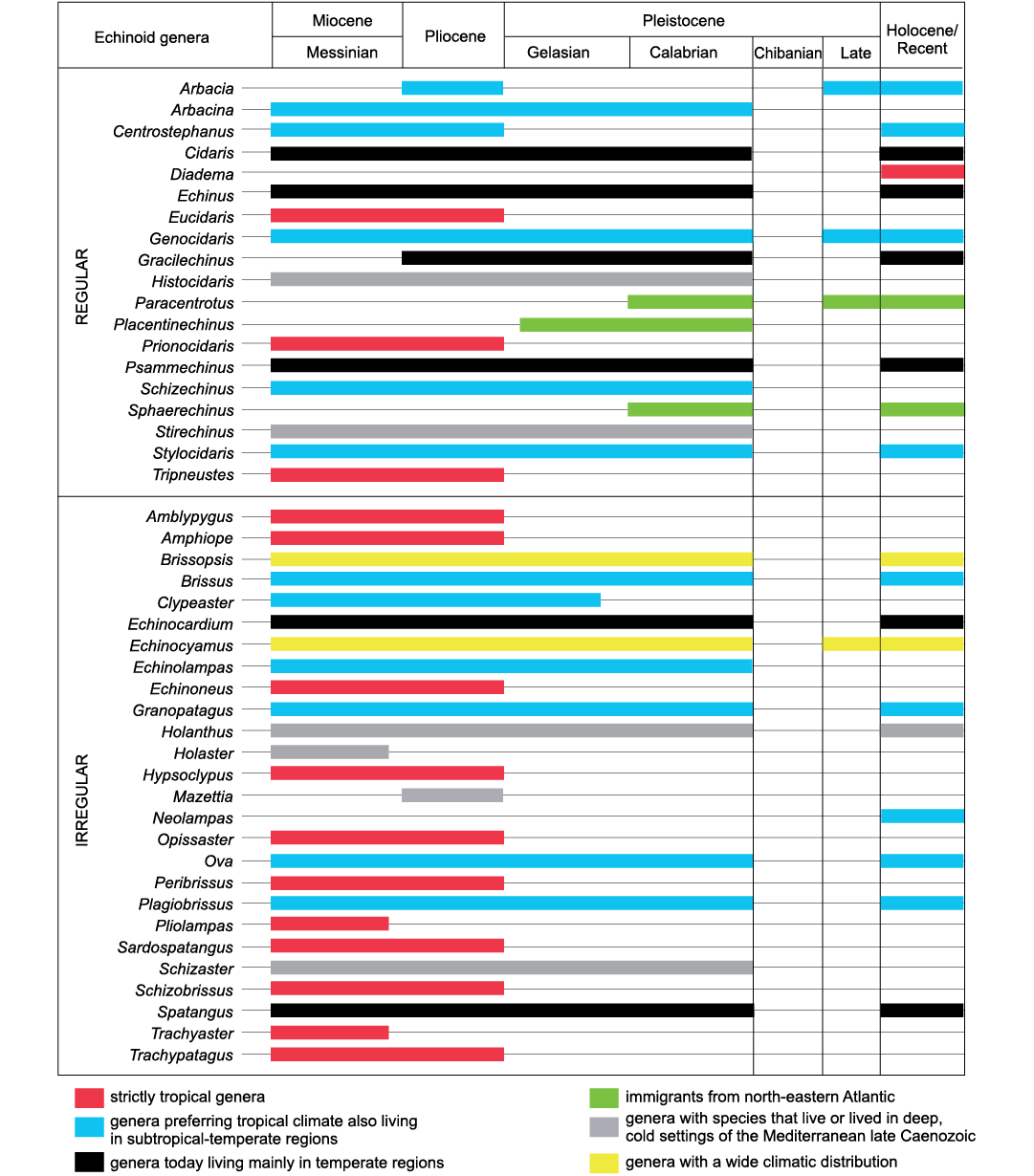
Fig. 5. Stratigraphic distribution and climatic preferences of regular and irregular echinoids recorded in the Miocene to Recent Mediterranean area.
During the pre-evaporitic Messinian (7.25–5.97 Ma), the gradual restriction of the Mediterranean, coupled with water stratification and intense temperature and salinity fluctuations, led to a drop in species richness affecting the whole marine fauna (Agiadi et al. 2020). A reduction was observed also in the echinoid fauna (Roman and Soudet 1990), with special regard to the decrease of the species within some previously well-developed genera, such as Amphiope, Clypeaster, and Echinolampas.
It was commonly assumed that the MSC drastically changed the marine fauna (see Néraudeau et al. 1999 for the irregular echinoid assemblage) that repopulated the Mediterranean Sea at the beginning of the Pliocene. However, results of our review showed a different scenario (Fig. 5). Only the species of Pliolampas, Trachyaster, and the deep-water Holaster, already decimated in the Late Miocene, globally extinguished during the Messinian. Some species certainly survived, at least during some phases of the evaporitic Messinian, possibly even in some refuge parts of the basin, as similarly reported by Landau et al. (2003) for some early Pliocene gastropods of southern Spain. This is the case with few taxa found in beds intercaled in the evaporites: Brissopsis lyrifera, in circalittoral marl of the Sorbas Basin, Spain (Lacour and Néraudeau 2000), and in marly lenses at the gypsum quarry of Vezzano, in North Italy (Dimitri Bertolaso, personal communication 2015; and EB personal observations). This paleobiogeographic setting is consistent with that described by Por (2009), who reported cases of marine life survived to the MSC, like those found in the Messinian of southern Spain in association with Porites reefs, among which some irregular tropical sea urchins, such as Amphiope, Hypsoclypus, Pliolampas, and Schizobrissus (Esteban 1979–1980; Néraudeau et al. 2001). However, most echinoid taxa likely re-entered the Mediterranean in Zanclean time, after the MSC. This paleobiogeographic dynamic could be especially applied to most of the echinoids, which are stenohaline (Russell 2013) and would not have tolerated the remarkable salinity variation that diffusely affected the Mediterranean during the MSC (e.g., Clauzon et al. 1996; Flecker et al. 2002; Roveri et al. 2014). All these biogeographic considerations indicate that the MSC had no drastic effects in the long terms on the composition of the Mediterranean echinoid fauna. Even from a quantitatively point of view, no relevant change affected the whole echinoid assemblage at the Miocene/Pliocene transition (Table 1).
Different patterns of Mediterranean diversity change have been recognised in the Late Miocene and in the Miocene/Pliocene transition. The fish fauna of the pre-evaporitic Messinian, for example, was taxonomically quite different from that of the Zanclean, as most of the Miocene species were replaced in the Pliocene, without a quantitative diversity drop. The calcareous nannoplankton met a similar fate, recording a moderate (about 8%) drop in diversity from the Tortonian to the Messinian (Late Miocene), and only a little more in the Early Pliocene. Overall, because of the relevant species replacement, the Tortonian and pre-evaporitic Messinian calcareous nannoplankton faunas were moderately different from the Zanclean ones (Agiadi et al. 2020). A further example concerns the Mediterranean coral assemblages, which were drastically modified by the disappearance of many taxa (especially zooxanthellatae) and of the shallow-water coral-reef province at the end of the Miocene but increased in the generic diversity within azooxanthellate and deep-water taxa at the end of the Pliocene and of the Pleistocene (Vertino et al. 2014).
The shallow-water Pliocene assemblages.—After the MSC, fully marine conditions re-established in the Mediterranean at the beginning of the Pliocene. Untill the middle Piacenzian this epoch was characterized by a tropical climate in the Mediterranean and, in general, climate conditions warmer than today characterised even the North Atlantic (e.g., Lauriat-Rage et al. 1992; Dowsett and Caballero-Gill 2010; Da Silva et al. 2010; Dowsett et al. 2013). Most of the pre-Messinian echinoids likely re-entered the Mediterranean from the Atlantic at the beginning of the Pliocene, leading to a quick recovery of the echinoid diversity, reaching the same number of genera as in the pre-Messinian crisis (Table 1). Surviving taxa include both shallow-water and deep-sea species. However, a clear reduction in the intra-generic diversity is observed, since most genera of tropical affinity, which were well developed and diversified in the Late Miocene, were represented by a sole species in the Early Pliocene. This was the case with Eucidaris, Amphiope, Hypsoclypus, and Trachypatagus, represented in the Pliocene only by Eucidaris desmoulinsi, Amphiope tipasensis from Algeria, Hypsoclypus pouyannei from Tuscany (Borghi and Ciappelli 2014), and Trachypatagus gouini Pomel, 1887, from Algeria. More than ten species of Clypeaster have been distinguished in the late Tortonian–early Messinian Mediterranean, while only two, Clypeaster altus and Clypeaster aegyptiacus, are currently recognised as valid in the Pliocene (Esu and Kotsakis 1981; Néraudeau et al. 1998). The same occurred with the genus Sardospatangus, recorded with only two species, Sardospatangus saheliensis from Almeria (Roman and Soudet 1990) and Sardospatangus rovasendai from Piedmont (Airaghi 1901). The Piedmont deposit near Pecetto, of probable Piacenzian age, records the last occurrence of this genus. Other, monospecific, thermophilous genera (Amblypygus, Echinoneus, Opissaster, Peribrissus, and Schizobrissus) were represented in the Mediterranean basal Pliocene only by very rare specimens. These genera became extinct (or, at least, vanished from the basin) during the Zanclean/Piacenzian transition (Fig. 6), when the Mediterranean climate underwent the early effects of the Northern Hemisphere glaciation. The scarcity of these taxa, as recorded at Zanclean time, probably was not caused by climatic deteriorations as the Pliocene, at least till about 3.6 Ma, generally benefited from well-established very warm conditions in the Mediterranean, especially during the so-called Pliocene climatic optimum. For the low and mid-latitudes of the warmest Pliocene, Haywood et al. (2000) calculated SSTs up to 5°C warmer and 400–1000 mm more precipitations than today at middle and high latitudes in Europe. Other factors possibly drove this decrease of diversity, for example reducing salinity. Interestingly, lower salinity than today was supposed for the (warm) interstadials of the Mediterranean Pleistocene (Garilli 2011). As a matter of fact, no evidence of Clypeaster, still common during the Piacenzian of the Tyrrhenian and Ionian areas, has been found in the shallow-water, highly fossiliferous deposits of the paleo-Adriatic basin. Possibly the hyperpicnal flows that dominated the Early Pleistocene depositional regime, producing turbidites in the northern paleo-Adriatic Sea (Pervesler et al. 2011), also occurred during the Piacenzian causing unfavorable conditions for this echinoid genus.
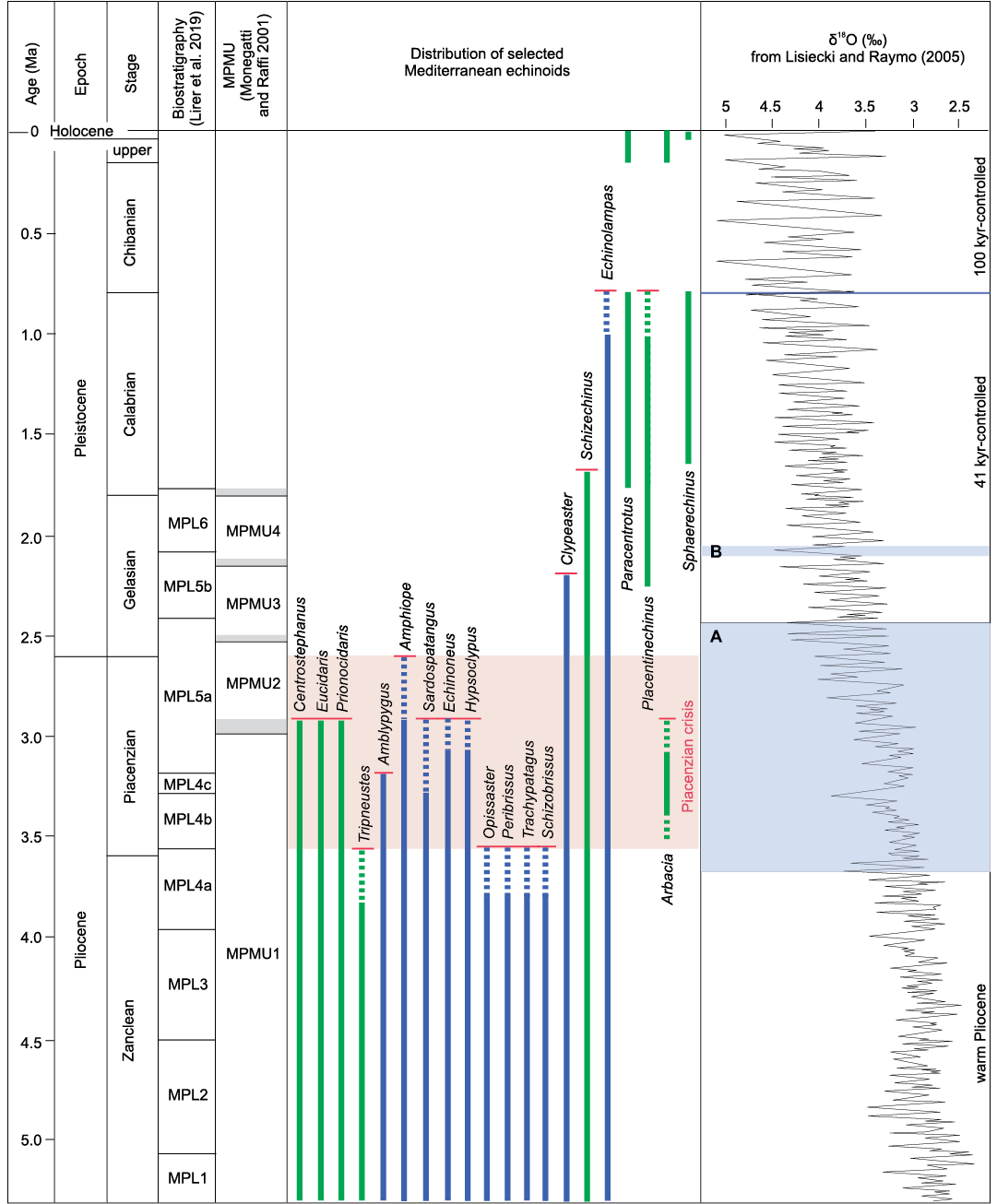
Fig. 6. Mediterranean biostratigraphy of some regular (green) and irregular (blue) echinoid genera showing appearances and disappearances during the Pliocene–Holocene interval. Shaded parts of the δ18O curve indicate the beginning and the gradual intensification of the Northern Hemisphere glaciation (A) according to Mudelsee and Raymo (2005), and the remarkable climate deterioration within the Gelasian at around 2.1 Ma (B). Chronostratigraphy is from Cohen et al. (2013).
A severe drop in the number of genera, however, occurred during the Piacenzian, mostly at about 3 Ma, with the extinction of fourteen tropical taxa, among which are nine genera of irregulars and the unique Pliocene representative of Arbacia, a still undetermined species (Fig. 6). A similar destiny concerned the Mediterranean Pliocene mollusc assemblage, characterised by several typically tropical taxa, which flourished till the mid-Piacenzian. From that time on a climatic deterioration, after the onset of the Northern Hemisphere glaciation, caused the extinction of many tropical molluscs and a consistent impoverishment of a few warm-water groups, such as Terebridae, Conidae, and Strombidae among gastropods, marking the transition between the molluscan ecobiozones MPMU1 and MPMU2 at about 3.0 Ma (Monegatti and Raffi 2001; Monegatti 2008). The onset of the Northern Hemisphere glaciation, particularly the climatic deterioration at about 3.0 Ma, was therefore decisive also for the Mediterranean echinoid diversity. During this Piacenzian crisis, the disappearance events of Mediterranean echinoids involved 39% of the whole Early Pliocene assemblage, 33% for regular echinoid genera, 43% for irregulars. It is relevant that three of the genera that disappeared from the Mediterranean during this Pliocene crisis (Echinoneus, Eucidaris, and Prionocidaris) are still living today in climatic settings with SSTs never lower than 20°C (OBIS 2021), while one genus, Tripneustes, is only very rarely reported from waters with SSTs of 15–20°C and is much more abundant in conditions with SSTs of 20–30°C (see SOM: table S3). The echinoid taxa that tolerate or require tropical conditions with SST range of 20–30°C show a significant drop in the Mediterranean area after the climate deterioration at 3.0 Ma (SOM: table S5 with associated plot).
Outside the Mediterranean, in the Late Pliocene of the Mondego Basin (Portugal), the Atlantic echinoid fauna was different from the coeval one that lived in Mediterranean. In fact, it consisted only of Echinocyamus and of undetermined Camarodonta (Pereira 2008). This low echinoid diversity is consistent with the temperature drop occurred after the Pliocene climatic optimum and with the onset of a climatic setting colder than that established in the Mediterranean during the same time. The Mondego Basin echinoid assemblage is comparable with that known from the Late Miocene (Messinian) to Pliocene of the Nantes-Rennes region (Bretagne, Atlantic coast of France), which is dominated by regular echinoids (particularly marsupiate forms), followed by irregulars such as Echinocyamus and spatangoids. This assemblage was regarded as characteristic of cooling water conditions by Roman (1989b) and Néraudeau et al. (2003). Nevertheless, the Mondego Basin is located at approximately one-fourth the distance between Algarve (southernmost Portugal) and Bretagne and, therefore, the prevailing climatic conditions should be closer to the subtropical conditions of the latest Neogene of the Mediterranean (Néraudeau et al. 2003) than to the colder conditions of Bretagne. Moreover, Da Silva (2002) highlighted some climatic similarities between the late Zanclean–early Piacenzian of the Mondego Basin and the present-day subtropical region of Canary Islands/Cape Blanc (western African coast), with a minimum winter SST of approximately 18–19ºC, and a maximum summer SST of about 22.5–23.5ºC.
The shallow-water Pleistocene assemblages.—With the beginning of the Pleistocene a new climatic regime settled down in the Mediterranean, influencing its biodiversity. Consequently, with the exception of the few thermophilous species that survived in some warmest part of the Mediterranean, in accordance with a refuge model (Garilli 2011; Dominici et al. 2020), many tropical molluscs disappeared or were severely depleted (Monegatti and Raffi 2001; Monegatti et al. 2002). Among these taxa several bivalve species became extinct in the very early Gelasian, earliest Pleistocene (top of MPMU2 of Monegatti and Raffi 2001), and tropical gastropod families, such as Terebridae and Conidae, underwent a drastic impoverishment. Also some thermophilous fish genera, Spratelloides, Stephanolepis, Sargocentron, Hemiramphus, and Etrumeus, known from the Piacenzian site of Marecchia (Italy) died out during the Gelasian (Sorbini 1988; Sorbini and Tyler 2004; Landini and Sorbini 2005). These taxa resettled the Mediterranean in the 1920’s and 1930’s ahead of most of the subsequent migrants from the Suez Canal (Por 2009).
The Mediterranean Gelasian echinoid fauna was characterised by genera that today prefer the temperate regions (Psammechinus, Brissopsis, Echinocardium, and Spatangus) and by tropical genera that include some subtropical-temperate representatives (Genocidaris, Echinocyamus, Echinolampas, Granopatagus, Ova, Plagiobrissus, and Schizechinus).
The moderately low diversity, the new arrival from the Atlantic of Placentinechinus and the persistence of the warm genus Clypeaster up to the late Gelasian, though with a drastic reduction, support the interpretation of the settlement of subtropical to warm temperate conditions in Mediterranean at least for a considerable part of the earliest Pleistocene. Significantly, the last Mediterranean citation of Clypeaster, recorded in Central Italy from the middle Gelasian upper part of the MPL5b biozone, in the around of 2.1 Ma (Carboni and Di Bella 1997), possibly correlates with the climate deterioration that led to the extinction of a few molluscs at the top of the MPMU3 zone (Monegatti and Raffi 2001; Monegatti 2008; see Fig. 6). Overall, the Gelasian echinoid assemblage of the Mediterranean resembles that known from the Late Miocene (Messinian)–Pliocene of the north-eastern Atlantic (Bretagne).
Changes occurred during the late Early Pleistocene (Calabrian) are well recorded in the quantitatively rich assemblages of northern and southern Italy (Borghi et al. 2006, 2014; Borghi and Garilli 2017). Schizechinus serialis and Psammechinus astensis extinguished during the Calabrian and were replaced respectively by the Atlantic species Sphaerechinus granularis and Psammechinus microtuberculatus, which occupied the same ecologic niches of the two extinct taxa. Another new arrival from the Atlantic was represented by Paracentrotus lividus (Lamarck, 1816). These new incomers were still rare in the northern Italy, in the lower Calabrian of Emilia-Romagna (Borghi 1995a), but very common in sediments from southern Italy, in the upper Calabrian of Sicily, which were deposited in colder waters (Borghi et al. 2006). In Emilia the last occurrence of Echinolampas has been recorded in the lower Calabrian (Borghi 1994b) a few meters below the first appearance of the boreal immigrant Arctica islandica, while at Favignana Island and in other Sicilian localities this genus was recorded also in the late Calabrian. Gracilechinus acutus shows a similar distribution pattern. This Atlantic species was, in fact, uncommon throughout the Early Pleistocene of Emilia-Romagna (Borghi 1993b), but frequent in the late Calabrian of Favignana. These discrepancies suggest that the Calabrian was throughout the Mediterranean a period of intense climatic oscillation, similarly to the present-day, its southern parts were sensibly warmer than the northern one.
Overall, up to 30% of the echinoid genera occurred in Mediterranean at the beginning of the Pleistocene extinguished during the Calabrian, recording a significant drop of biodiversity but less dramatic than that recorded in the Piacenzian.
The Mediterranean fossil record of the echinoids from the Middle Pleistocene is unknown, likely because of the scarcity of the fossiliferous marine deposits of this period, whereas reports from and Late Pleistocene and Holocene are rare and presumably incomplete. Only shallow-water echinoids have been collected from the Late (warm-water) Pleistocene-aged deposits of Sicily (Borghi et al. 2014): Genocidaris maculata, Echinocyanus pusillus, and Paracentrotus lividus. Arbacia lixula has been recorded from the coeval “Panchina di Livorno” (Tortonese 1965), Spatangus purpureus from the “Holocene” of Ostia (Stara et al. 2018). The low diversity of these assemblages was likely due to the depositional conditions, which prevented the preservation of other echinoids present in the Mediterranean during the interglacials. Wangensteen (2013) supposed that Arbacia lixula is a recent thermophilous colonizer, which spread throughout the Mediterranean during a warm period of the Pleistocene, probably during the last interglacial. However, the poor fossil record prevents to know exactly when the new taxa (Arbacia, Centrostephanus, Neolampas, and Plagiobrissus) entered (or re-entered) the Mediterranean.
The fossil deep-water assemblage.—The deep-water Mediterranean echinoid assemblage had a separate history from the shallow-water counterpart. The Pliocene–Pleistocene assemblage, consisting of six species belonging to five genera, shows a patchy record. Based on the deep marine deposits in north-eastern Sicily (Borghi et al. 2014), we know that these strictly bathyal echinoids (Cidaris margaritifera, Histocidaris rosaria, Histocidaris sicula, Stirechinus scillae, Holanthus ovatus, Schizaster braidensis) certainly lived in the Mediterranean until the Calabrian and showed strongest affinities with some deep-water western Atlantic species, particularly those of the Caribbean area (Borghi et al. 2014). They have been never reported from the 100-kyr-controlled Middle to Upper Pleistocene, which was characterised by severe glacial periods. It is, however, presumable that these bathyal echinoids re-entered the Mediterranean during those cold stages, similarly to some north-eastern Atlantic molluscs known as boreal guests (e.g., Malatesta and Zarlenga 1986; Raffi 1986; Taviani et al. 1991; Por 2009), The cold-water echinoids possibly vanished from the Mediterranean during the warm phase of the Late Pleistocene, likely because of the loss of psychrospheric conditions (Borghi et al. 2014). It seems that they are absent in the present-day deep Mediterranean, even in the white coral banks (Marco Taviani, personal communication 2011).
The present-day Mediterranean echinoids.—The present-day Mediterranean echinoid fauna is the result of a progressive impoverishment of the Miocene assemblage, mainly triggered by a complex climatic history. It consists of 26 species belonging to 21 genera (Fig. 5 and Table 1), mostly living in shelf environments, and shares 15 out of the 37 genera of the pre-Messinian Miocene assemblage. The Recent Mediterranean assemblage shows, therefore, a clear reduction in diversity in comparison with the pre-Messinian assemblage. This reduction is mainly due to the decrease of the irregular echinoids, which reduced from 24 to 10 genera.
As for the strictly bathyal Mediterranean echinoids, the Mediterranean Pliocene–Early Pleistocene assemblage lost five species, belonging to four genera, possibly because of the disappearance of the psychrospheric conditions. Actually, the Mediterranean displays homothermy, as the water temperature remains constant and relatively high at depths below 200–300 m (13–13.5°C in the west and 14–15°C in the east). The deep waters are very saline with values up to 38.5–39 psu. These conditions create a strongly stratified water body, which further enhances the oligotrophy of the Mediterranean (Emig and Geistdoerfer 2004), and remarkably restrict the diversity of its deep-sea echinoid assemblage. Up to now only twelve species of echinoids, belonging to eleven genera, are known to inhabit the deep waters of the Mediterranean below 250–300 m (Borghi et al. 2014: table 2). Differently from the cold stenothermal deep-water echinoids that lived in the Mediterranean during the Zanclean–Calabrian time, these present-day species, apart from Holanthus expergitus, are eurybathic, as they also live in shallower shelf settings. Most of them are also markedly eurythermal as they live in the colder deep waters of the Atlantic. All these eurybathic species are present in the eastern Atlantic, whereas only five species are also found in the western Atlantic. Nine of the eleven still living eurybathic genera lived also in the Mediterranean Pliocene–Pleistocene, from where they are much more frequently reported from shallow-water settings.
Conclusions
Our study on the biodiversity of the Mediterranean Messinian to present day echinoids indicates a general progressive decrease of their diversity, particularly regarding the Irregularia.
Though very few genera survived the Mediterranean transformation caused by the Messinian Salinity Crisis, 92% of the pre-evaporitic genera repopulated the basin at the beginning of the Pliocene, with the re-establishment of the normal marine conditions. Only three genera disappeared definitely from the Mediterranean in the late Messinian. Therefore, similarly to what has been shown by Néraudeau et al. (2001) for the Irregularia, the whole echinoid diversity drop after the evaporitic event was not as drastic as for other marine groups, at least at the genus level. Differently, at the species level the Mediterranean assemblage radically changed at the beginning of the Pliocene. Changes were due to the species impoverishment that affected many of the well diversified genera in the Late Miocene, and, from a taxonomical point of view, to the significant species replacement that involved several genera.
Two new taxa entered from the Atlantic in the Pliocene: Arbacia, a genus of tropical affinity, and Gracilechinus, represented by Gracilechinus acutus, today a subtropical-temperate species.
The Pliocene–Pleistocene climate change determined the main modifications of the Mediterranean echinoid assemblages. Fourteen taxa (13 genera and one species) of tropical affinity, about 40% of the warm Pliocene genus-level assemblage, disappeared during the Piacenzian (Late Pliocene), likely for the onset of the Northern Hemisphere glaciation and especially for the significant cooling event occurred at about 3 Ma ago. This mid-Piacenzian echinoid crisis correlates with the dramatic drop of the Mediterranean bivalve diversity due to the disappearance of warm-water species recorded at the end of the MPMU1 zone of Monegatti and Raffi (2001). This suggests that the late Pliocene climatic deterioration was critical for the Mediterranean tropical shallow-water fauna in general, likely much more than the lower Early Pleistocene settlement of a high-seasonality climate regime. Among the tropical echinoid taxa only Arbacia temporarily disappeared during the Piacenzian crisis and reappeared in the Mediterranean in the warm Late Pleistocene.
The Early Pleistocene progressive climate cooling allowed the entrance of the Atlantic temperate taxa Placentinechinus, Paracentrotus, and Sphaerechinus, and the spread of Gracilechinus, another temperate Atlantic host that had entered the Mediterranean during the Zanclean (Early Pliocene). Three thermophilous shallow-water echinoids, which had survived the Piacenzian climate deterioration, disappeared during the Early Pleistocene: Clypeaster, during the Gelasian (early Lower Pleistocene); Schizechinus and Echinolampas in the Calabrian (late Lower Pleistocene).
A small group of Mediterranean cold and deep-water echinoids, including Histocidaris, Stirechinus, and Cidaris margaritifera, whose modern relatives are restricted to tropical areas, are not found in deposits younger than the Calabrian. The presence of this group in the Pliocene–Early Pleistocene Mediterranean was due to the oceanographic conditions, which allowed the persistence of the psychrosphere, rather than to the climate settings.
Records of Middle–Late Pleistocene echinoids are lacking or so scarce that it is impossible to delineate the biodiversity trend at that time interval. We know, however, that the warm climate of the last interglacial, particularly of the marine isotope stage 5e, promoted the (re)appearance of the thermophilous taxon Arbacia, which likely disappeared at the beginning of the following glacial period. Neolampas likely entered the Mediterranean only after the last glaciation. The Lessepsian Diadema represents the latest Mediterranean acquisition of a tropical genus.
The extant Mediterranean echinoid fauna, consisting of 21 genera, partly derives from the Late Miocene fauna, with which it shares 15 genera (5 regulars and 10 irregulars). Compared to the Late Miocene–Early Pliocene faunas, the Recent echinoids, similarly to what we observed for the Late Pliocene–Pleistocene examples, shows a consistent drop in biodiversity (about 43%) at the genus level.
During the latest Cenozoic of the Mediterranean domain most of the strictly thermophilic echinoids, currently living in tropical to equatorial waters, occurred most importantly within the warmest Early Pliocene, at the climatic optimum, and secondarily during the Late Pliocene, in the mid-Piacenzian. At these times a tropical climate produced the highest Mediterranean echinoid diversity ever recorded in the very late Cenozoic, with a taxonomic setting remarkably different from that today known from the same area. According to the latest projection, a tropical condition similar to that occurred in the warm Pliocene is expected to recur in the Mediterranean by the end of the 21st century (Dowsett et al. 2013). Therefore our review can be of help to better understand what we could expect in term of changes in the echinoid diversity on a regional or global scale because of global warming.
Acknowledgements
We warmly thank Andreas Kroh (Naturhistorisches Museum Wien, Austria) and Pedro Pereira (Universidade Aberta, Lisbon, Portugal) for the accurate revision of the submitted manuscript. Editorial revision was also appreciated. With great sadness we acknowledge the contributon of the late Dimitri Bertolaso (Società Reggiana di Scienze Naturali, Reggio Emilia, Italy), who passed away prematurely in 2020, for providing very useful data on the Cenozoic echinoids from the northern Italy. We are grateful to Ildefonso Bajo Campos (Museo de Alcalá de Guadaíra, Sevilla, Spain), Gianna Innocenti (Museo di Storia Naturale, University of Florence, Italy), Marco Taviani (Institute of Marine Sciences, ISMAR-CNR, Bologna, Italy), Diego Garcia Ramos (Asociación Cultural Paleontológica Murciana, Murcia, Spain), Philippe Nicolleau (Société d’Histoire Naturelle du Boulonnais, France), Paolo Stara (Centro Studi di Storia Naturale del Mediterraneo, Museo di Storia Naturale “Aquilegia”, Masullas, Italy) and Leonardo Hernandez (Alicante, Spain), for providing information on the stratigraphy and biogeography of some echinoids. Pieter Provoost (OBIS Data Manager) provided useful information on the sea surface temperatures collected by OBIS.ORG.
References
Agassiz, A. 1863. List of the Echinoderms sent to different Institutions in Exchange for other Specimens, with Annotations. Bulletin of the Museum of Comparative Zoology at Harvard College 1 (2): 17–28.
Agassiz, A. 1869. Preliminary Report on the Echini and Starfishes dredged in deep water between Cuba and the Florida Reef, by L.F. de Pourtalès, Assist. U.S. Coast Survey. Bulletin of the Museum of Comparative Zoology at Harvard College 1 (9): 253–308.
Agassiz, A. 1880. Reports on the results of dredging, under the supervision of Alexander Agassiz, in the Caribbean Sea in 1878–79, and along the Atlantic Coast of the United States during summer of 1880, by the U.S. Coast Survey Steamer “Blake”. IX. Preliminary report on the Echini. Bulletin of the Museum of Comparative Zoology at Harvard College 8 (2): 69–84.
Agassiz, A. 1883. Report on the results of dredging under the supervision of Alexander Agassiz, in the Gulf of Mexico (1877–1878), in the Caribbean Sea (1878–79), and along the Atlantic coast of the United States (1880), by the U.S. Coast Survey Steamer “Blake”, Lieut.-Com. C.D. Sigsbee, U.S.N., and Commander J.R. Bartlett, U.S.N., commanding. XXIV, Part 1. Report on the Echini. Memoirs of the Museum of Comparative Zoology at Harvard College 10 (1): i–v, 1–94.
Agassiz, L. 1836. Prodrome d’une monographie des Radiaires ou Échinodermes. Mémoires de la Société des Sciences Naturelles de Neuchâtel 1: 168–199.
Agassiz, L. 1840. Catalogus systematicus Ectyporum Echinodermatum fossilium Musei Neocomiensis, secundum ordinem zoologicum dispositus; adjectis synonymis recentioribus, nec non stratis et locis in quibus reperiuntur. Sequuntur characteres diagnostici generum novorum vel minus cognitorum. 20 pp. Petitpierre, Neuchâtel. Crossref
Agassiz, L. 1841. Monographies d’Échinodermes vivants et fossiles. Échinites. Famille des Clypéasteroides. Seconde Monographie. Des Scutelles. 149 pp. Petitpierre, Neuchâtel.
Agassiz, L. and Desor, P.J. 1846. Catalogue raisonné des familles, des genres, et des espèces de la classe des échinodermes. Annales des Sciences Naturelles, Troisième Série, Zoologie 6: 305–374. Crossref
Agassiz, L. and Desor, P.J. 1847a. Catalogue raisonné des espèces, des genres, et des familles d’échinides. Annales des Sciences Naturelles, Troisième Série, Zoologie 7: 129–168. Crossref
Agassiz, L. and Desor, P.J. 1847b. Catalogue raisonné des espèces, des genres, et des familles d’échinides. Annales des Sciences Naturelles, Troisième Série, Zoologie 8: 5–35, 355–380. Crossref
Agiadi, K., Hohmann, N., Carnevale, G., Gliozzi, E., Faranda, C., Lozar, F., Harzhauser, M., Iliopoulos, G., Caruso, A., Kontakiotis, G., Taviani, M., Mancini, A.M., Borghi, E., Bajo Campos, I., Moissette, P., Thivaiou, D., Zarkogiannis, S., Besiou, E., Garcia-Castellanos, D., and Camerlenghi, A. 2020. The impact of the Messinian Salinity Crisis on marine biota. EGU General Assembly 2020, Online, 4–8 May 2020 [published online, https://doi.org/10.5194/egusphere-egu2020-5799]. Crossref
Airaghi, C. 1901. Echinidi terziari del Piemonte e della Liguria. Palaeontographia Italica 7: 22–126.
Ali, M.S.M. 2017. Middle Eocene echinoids from Gebel Qarara, Maghagha, Eastern Desert, Egypt. Journal of African Earth Sciences 133: 46–73. Crossref
Aymé, M. and Roman, J. 1954. Découverte d’une nouvelle espèce d’Amphiope dans le Pliocène des environs d’Alger. Publication du service de la Carte Géologique de l’Algérie (Nouvelle Série). Travaux des Collaborateurs 1 (for 1953): 165–172.
Backman, J., Raffi, I., Rio, D., Fornaciari, E., and Pälike, H. 2012. Biozonation and biochronology of Miocene through Pleistocene calcareous nannofossils from low and middle latitudes. Newsletters on Stratigraphy 45: 221–244. Crossref
Bajo, I. and Borghi, E. 2009. Tripneustes gahardensis (Echinoidea) en el Mioceno de la cuenca del Guadalquivir. Batalleria 14: 11–20.
Bajo, I., Rico García, A., Cárdenas Carretero, J., Maestre, V., and Borghi, E. 2008. Asociación de equinoideos en las calcarenitas messinienses de Guadaira (Sevilla, SO España). Geogaceta 45: 55–58.
Bak, R.P.M. 1990. Patterns of echinoid bioerosion in two Pacific coral reef lagoons. Marine Ecology Progress, Series 66: 267–272. Crossref
Barras, C.G. 2007. Phylogeny of the Jurassic to Early Cretaceous “disasteroid” echinoids (Echinoidea; Echinodermata) and the origins of Spatangoids and Holasteroids. Journal of Systematic Palaeontology 5: 134–161. Crossref
Bartoletti, E., Bossio, A., Esteban, M., Mazzanti, R., Mazzei, R., Salvatorini, G., Senesi, G., and Squarci, P. 1986. Studio geologico del territorio comunale di Rosignano Marittimo in relazione alla carta geologica alla scala l:25.000. In: R. Mazzanti (ed.), La Scienza della Terra nuovo strumento per lettura e pianificazione del territorio di Rosignano marittimo. Quaderno del Museo di Storia Naturale, Livorno 6 (Supplement l): 33–12.
Belaústegui, Z., Muñiz, F., James H. Nebelsick, J.H., Domènech, R., and Martinell, J. 2017. Echinoderm ichnology: bioturbation, bioerosion and related processes. Journal of Paleontology 91: 643–661. Crossref
Blainville, H.M. 1825. Dictionnaire des Sciences Naturelles, Séries 37: 59–102.
Borghi, E. 1993a. Echinocyamus pusillus (Müller, 1776) nel Pliocene e Pleistocene dell’Emilia. Notiziario della Società Reggiana di Scienze Naturali 13 (4): 1–28.
Borghi, E. 1993b. Echinus acutus Lamarck, 1816 nel Pliocene e Pleistocene dell’Emilia. Notiziario della Società Reggiana di Scienze Naturali 13 (3): 1–12.
Borghi, E. 1993c. Un echinide attuale rinvenuto allo stato fossile: Plagiobrissus (Rhabdobrissus) costai (Gasco, 1876). Notiziario della Società Reggiana di Scienze Naturali 13 (1): 1–6.
Borghi, E. 1994a. Cidaris cidaris (Philippi, 1845) e Stylocidaris affinis (Philippi, 1845) nel Pliocene e Pleistocene dell’Emilia. Notiziario della Società Reggiana di Scienze Naturali 14 (2): 1–18.
Borghi, E. 1994b. Echinolampas (Macrolampas) hoffmanni Desor, 1847 nel Pliocene dell’Emilia. Notiziario della Società Reggiana di Scienze Naturali 14 (1): 1–15.
Borghi, E. 1995a. Paracentrotus lividus (Lamarck, 1816) nel Pleistocene dell’Emilia. Notiziario della Società Reggiana di Scienze Naturali 15 (1): 1–23.
Borghi, E. 1995b. Segnalazione di una nuova forma affine al Genocidaris maculata A. Agassiz, 1869. Bibliotheca 1995: 1–14.
Borghi, E. 1997. Il genere Brissopsis nel Plio-Pleistocene dell’Emilia. Notiziario della Società Reggiana di Scienze Naturali 17 (1): 1–20.
Borghi, E. 1999. Echinodermi fossili dell’Emilia: i Cidaridi del Plio-Pleistocene. Parva Naturalia 1: 105–120.
Borghi, E. 2020. Gli echinoidi della Formazione di Pantano (Miocene medio-inferiore) dell’Emilia. Notiziario della Società Reggiana di Scienze Naturali 2019: 30–48.
Borghi, E. 2021a. Il genere Psammechinus (Echinoidea) nel Plio-Pleistocene dell’Emilia. Notiziario della Società Reggiana di Scienze Naturali 2020: 2–13.
Borghi, E. 2021b. Gli schizasteridi (Echinoidea) del Plio-Pleistocene dell’Emilia. Notiziario della Società Reggiana di Scienze Naturali 2020: 20–38.
Borghi, E. and Ciappelli, F. 2014. Prima segnalazione del genere Hypsoclypus (Echinoidea) nel Pliocene italiano. Notiziario della Società Reggiana di Scienze Naturali 2013–2014: 9–18.
Borghi, E. and Garilli, V. 2017. A new subtropical-temperate brooding echinoid with no marsupium: the first Mediterranean and the last European Temnopleuridae from the early Pleistocene of Italy. Journal of Systematic Palaeontology 15: 313–337 [published online 2016]. Crossref
Borghi, E., Bajo, I., and Rico Garcia, A. 2006. Arbacina romana (Merian, 1858) from the lower Pleistocene of Favignana Island (Sicily). Parva Naturalia 7: 47–71.
Borghi, E., Bajo Campos, I., and Gatt, M. 2018. Il genere Schizechinus Pomel, 1869 (Echinoidea) nel Pleistocene dell’Emilia. Notiziario della Società Reggiana di Scienze Naturali 13: 1–22.
Borghi, E., Garilli, V., and Bonomo, S. 2014. Plio-Pleistocene Mediterranean bathyal echinoids: evidence of adaptation to psychrospheric conditions and affinities with Atlantic assemblages. Palaeontologia Electronica 17.3.44A: 1–26. Crossref
Borghi, E., Rondelli, R., and Magnani, S. 2022. First record of the Mazettia genus (Echinoidea) in the Pliocene. Atti della Società dei Naturalisti e Matematici di Modena 153: 89–98.
Botto Micca, L. 1896. Contribuzione allo studio degli Echinidi Terziarii del Piemonte (famiglia Spatangidi). Bollettino della Società Geologica Italiana 15: 341–375.
Bronn, H.G. 1831. Ubersicht der Fossilen Uberreste in den tertiären subappeninischen Gebirgen. Italiens Tertiär-Gebilde und deren organische Einschlüsse. xii + 176 pp. Karl Groos, Heidelberg. Crossref
Bronstein, O., Georgopoulou, E., and Kroh, A. 2017. On the distribution of the invasive long-spined echinoid Diadema setosum and its expansion in the Mediterranean Sea. Marine Ecology Progress Series 583: 163–178. Crossref
Capozzi, R. and Picotti, V. 2003. Pliocene sequence stratigraphy, climatic trends and sapropel formation in the Northern Apennines (Italy). Palaeogeography, Palaeoclimatology, Palaeoecology 190: 349–371. Crossref
Cappelletti, E. 2008. Gli Echinoidi miocenici dell’Arcipelago Maltese: studio sistematico ed affinità biogeografia. 225 pp. Facoltà di Scienze Geologiche, sistema ETD (Electronic Theses and dissertations N. 213848), Università di Pisa, Pisa.
Carboni, M.G. and Di Bella, L. 1997. The Plio-Pleistocene of Anzio coast (Rome). Bollettino della Società Paleontologica Italiana 36 (12): 135–159.
Carter, B.D. 2003. Diversity Patterns in Eocene to Oligocene Echinoids. In: D.R. Prothero, L.C. Ivany, and E.A. Nesbitt (eds.), From Greenhouse to Icehouse: the Marine Eocene–Oligocene Transition, 354–365. Columbia University Press, New York.
Cau, S., Franchi, F., Roveri, M., and Taviani, M. 2015. The Pliocene-age Stirone River hydrocarbon chemoherm complex (Northern Apennines, Italy). Marine and Petroleum Geology 66: 582–595. Crossref
Ceregato, A., Raffi, S., and Scarponi, D. 2007. The circalittoral/bathyal paleocommunities in the Middle Pliocene of Northern Italy: The case of the Korobkovia oblonga–Jupiteria concava paleocommunity type. Geobios 40: 555–572. Crossref
Challis, G.R. 1980. Palaeoecology and Taxonomy of Mid Tertiary Maltese echinoids. 401 pp. Ph.D. Thesis (unpublished), Department of Geology, Bedford College, University of London, London.
Checchia Rispoli, G. 1907. Gli echinidi viventi e fossili della Sicilia. Parte 2: gli Echinidi del piano Siciliano dei dintorni di Palermo. Palaeontographia Italica 13: 199–231.
Checchia Rispoli, G. 1916. Gli echinidi fossili e viventi della Sicilia. Parte 4: Echinidi pliocenici. Palaeontographia Italica 22: 229–242.
Checchia Rispoli, G. 1917. Gli Echinidi viventi e fossili della Sicilia. Parte quinta: Echinidi miocenici. Palaeontographia Italica 23: 55–78.
Checchia Rispoli, G. 1919. Su alcuni Rhabdocidaris ed in particolar modo sul Rhabdocidaris remiger (Ponzi) del Monte Vaticano (Roma). Bollettino della Società Geologica Italiana 37 (1): 71–81.
Checchia Rispoli, G. 1923. Gli echinidi del pliocene di Anzio. Memorie per servire alla descrizione della Carta Geologica d’Italia 9: 1–29.
Clark, H.L. 1907. The Cidaridae. Bulletin of the Museum of Comparative Zoology at Harvard College 51: 165–230.
Clark, H.L. 1912. Hawaiian and other Pacific Echini. The Pedinidae, Phymosomatidae, Stomopneustidae, Echinidae, Temnopleuridae, Strongylocentrotidae and Echinometridae. Memoirs of the Museum of Comparative Zoology at Harvard College 34: 213–385.
Clark, A.M. and Rowe, F.W.E. 1971. Monograph of Shallow-Water Indo-West Pacific Echinoderms. 238 pp. Trustees of the British Museum (Natural History), London.
Clauzon, C., Suc, J.-P., Gautier, F., Berger, A., and Loutre, M.-F. 1996. Alternate interpretation of the Messinian salinity crisis: Controversy resolved? Geology 24: 363–366. Crossref
Cohen, K.M., Finney, S.C., Gibbard, P.L., and Fan, J.-X. 2013, updated. The ICS International Chronostratigraphic Chart. Episodes 36: 199–204. Crossref
Colantoni, B. and Borsetti, A.M. 1973. Geologia e stratigrafia dell’isola di Pianosa (Arcipelago Toscano, Mar Tirreno). Giornale di Geologia 39: 287–302.
Coll, M., Piroddi, C., Steenbeek, J., Kaschner, K., Ben Rais Lasram F., Aguzzi, J., Ballesteros, E., Bianchi, C.N., Corbera, J., Dailianis, T., Danovaro, R., Estrada, M., Froglia, C., Galil, B. S., Gaso, J.M., Ruthy Gertwagen, R., Gil, J., Guilhaumon, F., Kesner-Reyes, K., Kitsos, M.-S., Athanasios Koukouras, A., Nikolaos Lampadariou, N., Elijah Laxamana, E., López-Fé de la Cuadra, C.M., Lotze, H.K., Martin, D., Mouillot, D., Oro, D., Raicevich, S., Rius-Barile, J., Saiz-Salinas, J.I., San Vicente, C., Somot, S., Templado, J., Turon, X., Vafidis, D., Villanueva, R., and Voultsiadou, E. 2010. The biodiversity of the Mediterranean Sea: estimates, patterns, and threats. PLoS ONE 5 (8): e11842. Crossref
Cotteau, G. 1895. Description des Échinides recueillis par M. Lovisato dans le Miocène de la Sardaigne. Mémoires de la Société Géologique de France, Paléontologie 13: 5–56.
Cotteau, G., Peron, A., and Gauthier, V. 1891. Échinides fossiles de l’Algérie, 10e fasc.; Étages Miocène et Pliocène. 273 pp. G. Masson, Paris.
Crippa, G., Azzarone, M., Bottinia, C., Crespi, S., Felletti, F., Marinia, M., Petrizzo, M.R., Scarponi, D., Raffi, S., and Raineri, G. 2019. Bio- and lithostratigraphy of lower Pleistocene marine successions in western Emilia (Italy) and their implications for the first occurrence of Arctica islandica in the Mediterranean Sea. Quaternary Research 92: 1–21. Crossref
Cronin, T.M., De Martino, D.M., Dwyer, G.S., and Rodriguez-Lazaro, J. 1999. Deep-sea ostracode species diversity: response to late Quaternary climate change. Marine Micropaleontolology 37: 231–249. Crossref
Cronin, T.M., Whatley, R., Wood, A., Tsukagoshi, A., Ikeya, N., Brouwers, E.M., and Briggs, W.M. Jr. 1993. Microfaunal evidence for elevated Pliocene temperatures in the Arctic Ocean. Paleoceanography 8: 161–173. Crossref
Da Silva, C.M. 2002. Novos dados sobre los Moluscos Pliocénicos Marinhos de Portugal: Implicações Paleoceanográficas e Paleobiogeográficas. Pliocénica 2: 117–125.
Da Silva, C.M., Landau, B., Domenec, R., and Martinell, J. 2010. Pliocene Atlantic molluscan assemblages from the Mondego Basin (Portugal): age and palaeoceanographic implications. Palaeogeography, Palaeoclimatology, Palaeoecology 285: 248–254. Crossref
De Shepper, S., Gibbard, P.L., Salzmann, U., and Ehlers, J. 2014. A global synthesis of the marine and terrestrial evidence for glaciation during the Pliocene Epoch. Earth-Science Review 135: 83–102. Crossref
Des Moulins, C. 1837. Troisième Mémoire sur les Échinides. Synonymie générale. Actes de la Société Linnéenne de Bordeaux 9 (6): 45–364.
Des Moulins, C. 1870. Spécification et noms légitimes de six Échinolampes. Actes de la Société Linnéenne de Bordeaux 27: 309–322.
Desor, P.J. 1855–1858. Synopsis des échinides fossiles. ixviii, 490 pp. Reinwald, Paris. Crossref
Di Bella, L., Carboni, M.G., and Pignatti, J., 2005. Paleoclimatic significance of the Pliocene Amphistegina levels from the Tyrrhenian margin of Central Italy. Bollettino della Società Paleontologica Italiana 44 (3): 219–229.
Dominici, S., Benvenuti, M., Garilli, V., Uchmann, A., Pollina, F., and David, A. 2020. Pliocene–Pleistocene stratigraphic paleobiology at Altavilla Milicia (Palermo, Sicily): tectonic, climatic and eustatic forcing. Bollettino della Società Paleontologica Italiana 59 (1): 57–83. Crossref
Donovan, S.K. 2018. The internal morphology of primary spines of extant regular echinoids in the tropical Western Atlantic: a SEM atlas. Swiss Journal of Palaeontology 137: 363–377. Crossref
Donovan, S.K., Lewis, D.N., and Davis, P. 2005. Fossil echinoids from Belize. Caribbean Journal of Science 41: 323–328.
Dowsett, H.J. and Caballero-Gill, R.P. 2010. Pliocene climate. Stratigraphy 7: 106–110.
Dowsett, H.J., Foley, K.M., Stoll, D.K., Chandler, M.A., Sohl, L.E., Bentsen, M., Otto-Bliesner, B.L., Bragg, F.J., Chan, W.-L., Contoux, C., Dolan, A.L., Haywood, A.M., Jonas, J.A., Jost, A., Kamae, Y., Lohmann, G., Lunt, D.J., Nisancioglu, K.H., Abe-Ouchi, A., Ramstein, G., Riesselman, C.R., Robinson, M.M., Rosenbloom, N.A., Salzmann, U., Stepanek, C., Strother, S.L., Ueda, H., Yan, Q., and Zhang, Z. 2013. Sea surface temperature of the mid-Piacenzian ocean: a data-model comparison. Scientific Reports 3: 1–8. Crossref
Dudicourt, J.C., Néraudeau, D., Nicolleau, P., Ceulemans, L., and Boutin, F. 2005. Une faune remarquable d’échinides marsupiaux dans le Pliocène de Vendee (Ouest de la France). Bulletin de la Société Géologique de France 176: 545–557. Crossref
Duncan, P.M. 1889. A revision of the genera and great groups of the Echinoidea. Journal of the Linnaean Society, Zoology 23: 1–311. Crossref
Emig, C.C. and Geistdoerfer, P. 2004. The Mediterranean deep-sea fauna: historical evolution, bathymetric variations and geographical changes. Carnets de Géologie/Notebooks on Geology, Maintenon 2004/01: CG2004-A01-CCE-PG. Crossref
Esteban, M. 1979–1980. Significance of the Upper Miocene coral reefs of the Western Mediterranean. Palaeogeography, Palaeoclimatology, Palaeoecology 29: 169–188. Crossref
Esu, D. and Kotsakis, T. 1981. Osservazioni su un Clypeaster (Echinodermata) pliocenico figurato da M. Mercati. Atti della Accademia Nazionale dei Lincei. Classe di Scienze Fisiche, Matematiche e Naturali. Rendiconti Lincei 71: 183–190.
Fedorov, A.V., Brierley, C.M., Lawrence, K.T., Liu, Z., Dekens, P.S., and Ravelo, A.C. 2013. Patterns and mechanisms of early Pliocene warmth. Nature 496: 43–49. Cossref
Fell, H.B. 1954. Tertiary and Recent Echinoidea of New Zealand: Cidaridae. Paleontological Bulletin of the New Zealand Geological Survey 23: 1–62.
Fell, H.B. and Pawson, D.L. 1966. Echinacea. In: R.C. Moore (ed.), Treatise on Invertebrate Paleontology. U. Echinodermata 3 (2): U367–U440. Geological Society of America, Boulder and University Kansas Press, Lawrence.
Flecker, R. and Ellam, R.M. 2006. Identifying Late Miocene episodes of connection and isolation in the Mediterranean–Paratethyan realm using Sr isotopes. Sedimentary Geology 188–189: 189–203. Crossref
Flecker, R., De Villiers, S., and Ellam, R.M. 2002. Modelling the effect of evaporation on the salinity-87Sr/86Sr relationship in modern and ancient marginal-marine systems: The Mediterranean Messinian salinity crisis. Earth and Planetary Science Letters 203: 221–233. Crossref
Forbes, E. 1841. A History of British Starfishes and Other Animals of the Class Echinodermata. 267 pp. J. Van Voorst, London. Crossref
Foresi, L.M., Aldinucci, M., Sandrelli, F., and Cornamusini, G. 2007. Guida per l’escursione GeoSed all’isola di Pianosa. 44 pp. GeoSed, Siena.
Garcia-Castellanos, D. and Villaseñor, A. 2011. Messinian salinity crisis regulated by competing tectonics and erosion at the Gibraltar arc. Nature 480: 359–363. Crossref
Garilli, V. 2011. Mediterranean Quaternary interglacial molluscan assemblages: Palaeobiogeographical and palaeoceanographical responses to climate change. Palaeogeography, Palaeoclimatology, Palaeoecology 312: 98–114. Crossref
Gasco, F. 1876. Descrizione di alcuni echinodermi nuovi o per la prima volta trovati nel Mediterraneo. Rendiconti della Reale Accademia delle Scienze Fisiche e Matematiche di Napoli 15 (2): 1–13.
Gillespie, J.M. and McClintock, J.B. 2007. Brooding in echinoderms: how can modern experimental techniques add to our historical perspective? Journal of Experimental Marine Biology and Ecology 342: 191–201. Crossref
Gray, J.E. 1825. An attempt to divide the Echinida, or sea eggs, into natural families. Annals of Philosophy, New Series 10: 423–431.
Gray, J.E. 1835. On the genera distinguishable in Echinus Lam. Proceedings of the Zoological Society of London 3: 57–59. Crossref
Gray, J.E. 1851. Descriptions of some new genera and species of Spatangidae in the British Museum. The Annals and Magazine of Natural History, 2nd Series 7: 130–134.
Hammond, L.S. 1981. An analysis of grain size modification in biogenic carbonate sediments by deposit-feeding holothurians and echinoids (Echinodermata). Limnology and Oceanography 26: 898–906. Crossref
Harzhauser, M., Kroh, A., Mandic, O., Piller, W.E., Göhlich, U., Reuter M., and Berning, B. 2007. Biogeographic responses to geodynamics: A key study all around the Oligo-Miocene Tethyan Seaway. Zoologischer Anzeiger Journal of Comparative Zoology 246: 241–256. Crossref
Harzhauser, M., Piller, W.E., and Steininger, F.F. 2002. Circum-Mediterranean Oligo/Miocene biogeographic evolution—the gastropods’ point of view. Palaeogeography, Palaeoclimatology, Palaeoecology 183: 103–133. Crossref
Haywood, A.M. and Valdes, P.J. 2004. Modelling Pliocene warmth: contribution of atmosphere, oceans and cryosphere. Earth and Planetary Science Letters 218: 363–377. Crossref
Haywood, A.M., Sellwood, B.W., and Valdes, P.J. 2000. Regional warming: Pliocene (3 Ma). Paleoclimate of Europe and the Mediterranean. Geology 28 (12): 1063–1066. Crossref
Head, M.J. 1998. Pollen and dinoflagellates from the Red Crag at Walton-on-the-Naze, Essex: evidence for a mild climatic phase during the early Late Pliocene of eastern England. Geological Magazine 135: 803–817. Crossref
Head, M.J. and Gibbard, P.L. 2005. Early–Middle Pleistocene transitions: an overview and recommendation for the defining boundary. In: M.J. Head and P.L. Gibbard (eds.), Early–Middle Pleistocene Transitions: The Land–Ocean Evidence. Geological Society, London, Special Publications 247: 1–18. Crossref
Heimann, K.O. and Marcopoulou-Diacantoni, A. 1977. Sur une faune d’échinides dans la série messinienne de Céphalonie (Grèce) [in Greek]. Annales Géologiques des Pays Helléniques 29: 542–550.
Hopkins, M.J. and Smith, A.B. 2015. Dynamic evolutionary change in post-Paleozoic echinoids and the importance of scale when interpreting changes in rates of evolution. PNAS 112: 3758–3763. Crossref
Hsu, K.J., Ryan, W.B.F., and Cita, M.B. 1973. Late Miocene desiccation of the Mediterranean. Nature 242: 240–244. Crossref
Isaji, Y., Yoshimura, T., Kuroda, J., Tamenori, Y, Jiménez-Espejo, F.J., Lugli, S., Manzi, V., Roveri, M., Kawahata, H., and Ohkouchi, N. 2019. Biomarker records and mineral compositions of the Messinian halite and K-Mg salts from Sicily. Progress in Earth and Planetary Science 6 (60): 1–15. Crossref
Jeffery, C.H. and Emlet, R.B. 2003. Macroevolutionary consequences of developmental mode in temnopleurid echinoids from the Tertiary of southern Australia. Evolution 57: 1031–1048. Crossref
Jiménez-Moreno, G., Pérez-Asensio, J.N., Larrasoaña, J.C., Sierro, F.J., Garcia-Castellanos, D., Salazar, Á., Josep Maria Salvany, J.M., Ledesma, S., Mata, M.P., and Mediavilla, C. 2019. Early Pliocene climatic optimum, cooling and early glaciation deduced by terrestrial and marine environmental changes in SW Spain. Global and Planetary Change 180: 89–99. Crossref
Kastens, K.A. 1992. Did glacio-eustatic sea level drop trigger the Messinian salinity crisis? New evidence from Ocean Drilling Program Site 654 in the Tyrrhenian Sea. Paleoceanography 7: 333–356. Crossref
Kier, P.M. 1965. Evolutionary trends in Paleozoic echinoids. Journal of Paleontology 39: 436–465.
Kier, P.M. 1977. The poor fossil record of the regular echinoid. Paleobiology 3: 168–174. Crossref
Klotz, S., Fauquette, S., Combourieu-Nebout, N., Uhl, D., Suc, J.-P., and Mosbrugger, V. 2006. Seasonality intensification and long-term winter cooling as a part of the Late Pliocene climate development. Earth and Planetary Science Letters 241: 174–187. Crossref
Koehler, R. 1927. Echinodermes des Mers d’Europe. Tome 2. 320 pp. Doin, Paris.
Köppen, W. 1931. Die Klimate der Erde, Grundriss der Klimakunde, 2ª Ed. 368 pp. Walter de Gruyter & co, Berlin. Crossref
Kroh, A. 2005. Catalogus fossilium Austriae, Echinoidea neogenica. Verlag der Österreichischen Akademie der Wissenschaften 56: 1–210.
Kroh, A. 2007. Climate changes in the Early to Middle Miocene of the Central Paratethys and the origin of its echinoderm fauna. Palaeogeography, Palaeoclimatology, Palaeoecology 253: 169–207. Crossref
Kroh, A. 2011. Echinoids from the Triassic of St. Cassian—a review. Geo. Alp 8: 136–140.
Kroh, A. and Mooi, R. 2021. World Echinoidea Database. World Register of Marine Species https://www.marinespecies.org/aphia.php?p=taxdetails&id [accessed 2021.11.15].
Kroh, A. and Smith, A.B. 2010. The phylogeny and classification of post-Palaeozoic echinoids. Journal of Systematic Palaeontology 8: 141–212. Crossref
Kroh, A., Madeira, P., and Haring, E. 2011. Species distributions: virtual or real—the case of Arbaciella elegans (Echinoidea: Arbaciidae). Journal of Zoological Systematics and Evolutionary Research 50: 99–105. Crossref
Lachkhem, H. and Roman, J. 1995. Les échinoides irréguliers (Neognathostomes et Spatangoides) du Messinien de Melilla (Maroc septentrional). Annales de Paléontologie 81: 247–278.
Lacour, D. and Néraudeau, D. 2000. Évolution de la diversité des Brissopsis (Echinoida, Spatangoida) en Méditerranée depuis la ‘crise messinienne’: application paléoécologique aux B. lyrifera intragypses de Sorbas (SE Espagne). Geodiversitas 22: 509–523.
Lamarck, J.B. de 1801. Systême des animaux sans vertèbres, ou tableau général des classes, des ordres et des genres de ces animaux [...]. viii, 432 pp. Deterville, Paris. Crossref
Lamarck, J.B. de 1816. Histoire Naturelle des Animaux sans Vertèbres, présentant les caractères généraux et particuliers de ces animaux, leur distribution, leur classes, leurs familles, leurs génères, et le citation des principales espèces qui s’y rapportent; précédée d’une Introduction offrant la Détermination des caractères essentiels de l’animal, sa distinction du végétal et des autres corps naturels, enfin, l’Exposition des Principes fondamentaux de la Zoologie. Tome Troisième. 586 pp. Verdière, Paris. Crossref
Lambeck, K., Roubya, H., Purcella, A., Sunc, Y., and Sambridgea, M. 2014. Sea level and global ice volumes from the Last Glacial Maximum to the Holocene. PNAS 111: 15296–15303. Crossref
Lambeck, K., Yokoyama, Y., and Purcell, T. 2002. Into and out of the Last Glacial Maximum: sea-level change during Oxygen Isotope Stages 3 and 2. Quaternary Science Review 21: 343–360. Crossref
Lambert, J. 1907. Description des échinides fossiles des terrains miocéniques de la Sardaigne. Mémoires de la Société Paléontologique Suisse 34: 1–72.
Lambert, J. 1909. Description des échinides fossiles des terrains miocéniques de la Sardaigne. Mémoires de la Société Paléontologique Suisse 35 (for 1908): 73–142.
Lambert, J. 1915. Description des échinides des terrains néogènes du bassin Rhône. Fasc. 4. Mémoires de la Société Paléontologique Suisse 41: 155–240.
Lambert, J. and Thiéry, P. 1924–1925. Essai de Nomenclature Raisonnée des Échinides. Fasc. 6–7: 385–512 (Dec. 1924); fasc. 7–8: 513–607 (Feb. 1925). Ferrière, Chaumont.
Landau, B., Capelo, J.C., and da Silva, C.M. 2007. Patterns of extinctions and local disappearance of tropical marine gastropods; contrasting examples from across the North Atlantic. Açoreana 2007 (Supplement 5): 50–58.
Landau, B., Marquet, R., and Grigis, M. 2003. The early Pliocene Gastropoda of Estepona, Southern Spain, Part 1: Vetigastropoda. Palaeontos 3: 1–87.
Landini, W. and Sorbini. L. 2005. Evolutionary dynamics in the fish faunas of the Mediterranean basin during the Plio-Pleistocene. Quaternary International 140–141: 64–89. Crossref
Lauriat-Rage, A., Brébion, P., Cahuzac, B., Chaix, C., Ducasse, O., Ginsburg, L., Janin, M.-C., Lozouet, P., Margerel, J.-P., Nascimento, A., Pais, J., Poignant, A., Pouyets, S., and Roman, J. 1992. Palaeontological data about the climatic trends from Chattian to present along the Northeastern Atlantic frontage. Ciências da Terra (UNL) 12: 167–179.
Lefebvre, B., Sumrall, C.D., Shroat-Lewis, R.A., Reich, M., Webster, G.D., Hunter, A.W., Nardin, E., Rozhnov, S.V., Guensburg, T.E., Touzeau, A., Noailles, F., and Sprinkle, J. 2013. Palaeobiogeography of Ordovician echinoderms. In: D.A.T. Harper and T. Servais (eds.), Early Palaeozoic Biogeography and Palaeogeography. Geological Society London, Memoirs 38: 173–198. Crossref
Leske, N.G. 1778. Jacobi Theodori Klein naturalis disposition echinodermatum.., edita et descriptionibus novisque inventis et synonomis auctorem aucta. Addimenta ad J.T. Klein naturalem dispositionem Echinodermatum. xxii, 278 pp. G.E. Beer, Leipzig.
Lickorish, W.H. and Butler, R.W.H. 1996. Fold amplification and parasequences stacking patterns in syntectonic shoreface carbonates. Geological Society of America Bulletin 108: 966–967.
Linnaeus, C. 1758. Systema Naturæ per Regna tria Naturæ, secundum Classes, Ordines, Genera, Species, cum characteribus, differentiis, synonymis, locis. Edito 10, Reformata, Tomus I. 824 pp. Laurentii Salvii, Holmiæ. Crossref
Linnaeus, C. 1767. Systema Naturae per Regna tria Naturae: secundum Classes, Ordines, Genera, Species, cum characteribus, differentiis, synonymis, locis. Edito 12. Tomus 1, Regnum Animale. 2. 533–1327 pp. Laurentii Salvii, Holmiæ. Crossref
Lirer, F., Foresi, L.M., Iaccarino, S.M., Salvatorini, G., Turco, E., Cosentino, C., Sierro, F.J., and Caruso, A. 2019. Mediterranean Neogene planktonic foraminifer biozonation and biochronology. Earth-Science Review 196: 102869. Crossref
Loubere, P. and Moss, K. 1986. Late Pliocene climatic change and the onset of northern hemisphere glaciation as recorded in the northeast Atlantic Ocean. Geological Society of America Bulletin 97: 818–828. Crossref
Lovén, S. 1874. Études sur les échinoïdées. Kongelige Svenska Vetenskaps-Akademiens Handlingar 11: 1–91.
Madeira, P., Kroh, A., Cordeiro, R., De Frias, A.M., and Ávila, S.P. 2019. The Echinoderm fauna of the Azores (NE Atlantic Ocean). Zootaxa 4639: 1–231. Crossref
Madeira, P., Kroh, A., Cordeiro, R., Meireles, R., and Ávila, S.P. 2011. The fossil echinoids of Santa Maria Island, Azores (Northern Atlantic Ocean). Acta Geologica Polonica 61: 243–264.
Malatesta, A. and Zarlenga, F. 1986. Northern Guests in the Pleistocene Mediterranean Sea. Geologica Romana 25: 91–154.
Mancosu, A. and Nebelsick, J.H. 2016. Echinoid assemblages from the early Miocene of Funtanazza (Sardinia): A tool for reconstructing depositional environments along a shelf gradient. Palaeogeography, Palaeoclimatology, Palaeoecology 454: 139–160. Crossref
Manzi, V., Gennari, R., Hilgen, F., Krijgsman, W., Lugli, S., Roveri, M., and Sierro, F.J. 2013. Age refinement of the Messinian salinity crisis in the Mediterranean. Terra Nova 25: 315–322. Crossref
Marcopoulou-Diacantoni, A. 1967. La faune des échinides néogènes des Pays helléniques. Annales Géologiques des Pays Helléniques 18: 331–406.
Marcopoulou-Diacantoni, A. 1972. Échinides (Clypeaster, Schizaster, Spatangus, Brissopsis) de l’Helvétien de l’île de Crète centrale et Orientale. Annales Géologiques des Pays Helléniques 24: 133–160.
Marcopoulou-Diacantoni, A. 1974. Contribution a la connaissance des Echinides néogènes de la région au NW de Sitia (Crête). Annales Géologiques des Pays Helléniques 26: 251–261.
Marcopoulou-Diacantoni, A. 1977. Les échinides plio-pleistocénes de la région de Neapolis (Peloponese). Annales Géologiques des Pays Helléniques 29: 436–449.
Mariani, E. and Parona, C. 1887. Fossili Tortoniani di Capo San Marco in Sardegna. Atti della Società Italiana di Scienze Naturali 30: 1–153.
Massari, F. and Chiocci, F. 2006. Biocalcarenite and mixed cool-water prograding bodies of the Mediterranean Pliocene and Pleistocene: architecture, depositional setting and forcing factors, 95–120. In: H.M. Pedley and G. Carannante (eds.), Cool-Water Carbonates: Depositional Systems and Palaeoenvironmental Controls. The Geological Society, London, Special Publication 255: 1–373. Crossref
Mazzanti, R. 2016. Note Illustrative della Carta Geologica d’Italia alla scala 1:50000, foglio 284, Rosignano Marittimo. 189 pp. Istituto Superiore per la Protezione e la Ricerca Ambientale, Roma.
Mazzetti, G. 1882. Echinodermi fossili di Montese. Annuario della Società dei Naturalisti in Modena, Serie 2 15: 108–126.
Meneghini, G. 1862. Studi sugli Echinodermi fossili neogenici di Toscana, 61–89. In: L. Lazzeri (ed.), Siena e il suo Territorio. 562 pp. Tipografia del Regio Istituto dei Sordo-Muti, Siena.
Michelin, H. 1863. Monographie des Clypéastres Fossiles. Mémoires de la Société Géologique de France 7 (2): 101–147.
Mironov, A.N., Minin, K.V., and Dilman, A.B. 2015. Abyssal echinoid and asteroid fauna of the North Pacific. Deep Sea Research Part II. Topical Studies in Oceanography 111: 357–375. Crossref
Mokady, O. Lazar, B., and Loya, Y. 1996. Echinoid bioerosion as a major structuring force of Red Sea coral reefs. The Biological Bulletin 193: 367–372. Crossref
Monegatti, P. 2008. Evoluzione climatica del Mediterraneo durante gli ultimi 8 milioni di anni, come testimoniato dalla fauna fossile a molluschi. Notiziario della Società Reggiana di Scienze Naturali 2008: 75–82.
Monegatti, P. and Raffi, S. 2001. Taxonomic diversity and stratigraphic distribution of Mediterranean Pliocene bivalves. Palaeogeography, Palaeoclimatology, Palaeoecology 165: 171–193. Crossref
Monegatti, P. and Raffi, S. 2010. The Messinian marine molluscs record and the dawn of the eastern Atlantic biogeography. Palaeogeography, Palaeoclimatology, Palaeoecology 297: 1–11. Crossref
Monegatti, P., Canali, G., Bertoldi, R., and Albianelli, A. 2002. The classical Late Piacenzian Monte Falcone–Rio Crevalese section (Northern Italy): palynological evidence and biomagnetostratigraphic constraints for climatic cyclicity and local mollusc extinctions. Geobios 35 (Mémoire spécial 24): 219–227. Crossref
Mongiardino Koch, N. and Thompson, J.R. 2021. A total-evidence dated phylogeny of Echinoidea combining phylogenomic and paleontological data. Systematic Biology 70: 421–439. Crossref
Mongiardino Koch N., Coppard S.E., Lessios H.A., Briggs D.E.G., Mooi R., and Rouse G.W. 2018. A phylogenomic resolution of the sea urchin tree of life. BMC Evolutionary Biology 18: 189. Crossref
Mongiardino Koch, N., Thompson, J.R., Hiley, A.S., McCowin, M.F., Armstrong, A.F., Coppard, S.E., Aguilera, F., Bronstein, O., Kroh, A., Mooi, R., and Rouse, G.W. 2022. Phylogenomic analyses of echinoid diversification prompt a re-evaluation of their fossil record. eLife 2022 (11): e72460. Crossref
Montenat, C. and Roman, J. 1970. Echinides Néogènes d’Espagne (Provinces d’Alicante et de Murcie). Annales de Paléontologie 56: 89–138.
Mortensen, T. 1903. The Danish Ingolf-Expedition. Vol. 4. Echinoidea (part 1). 193 pp. Bianco Luno, Copenhagen. Crossref
Mortensen, T. 1907. The Danish Ingolf-Expedition 1895–1896. Vol. 4. Echinoidea (part 2). 200 pp. Bianco Luno, Copenhagen.
Mortensen, T. 1909. Die Echinoiden der Deutschen Südpolar-Expedition 1901–1903. In: E. von Drygalski (ed.), Deutsche Südpolar-Expedition 1901–1903 im Auftrage des Reichsamtes des Innern. XI. Zoologie III. Heft I. 114 pp. Georg Reimer, Berlin.
Mortensen, T. 1910. Arbaciella elegans. Eine neue Echiniden-Gattung a. d. Familie Arbaciidae. Mitteilungen aus dem Naturhistorischen Museum in Hamburg 27: 327–334.
Mortensen, T. 1913. Die Echinodermen des Mittelmeeres. Mitteilungen aus der Zoologischen Station zu Neapel 21: 1–39.
Mortensen, T. 1927. Handbook of the Echinoderms of the British Isles. 471 pp. W. Backhuys, Rotterdam [reprint 1977]. Crossref
Mortensen, T. 1928. A Monograph of the Echinoidea. I. Cidaroidea. 551 pp. Reitzel, Copenhagen.
Mortensen, T. 1935. A Monograph of the Echinoidea. II. Bothriocidaroida, Melonechinoida, Lepidocentroida and Stirodonta. vi, 647 pp. Reitzel, Copenhagen.
Mortensen, T. 1940. A Monograph of the Echinoidea. III. 1, Aulodonta. 320 pp. Reitzel, Copenhagen,
Mortensen, T. 1943. A Monograph of the Echinoidea. III. 3, Camarodonta. 446 pp. Reitzel, Copenhagen.
Mortensen, T. 1948a. A Monograph of the Echinoidea. IV. 1, Holectypoida, Cassiduloida. 363 pp. Reitzel, Copenhagen.
Mortensen, T. 1948b. A Monograph of the Echinoidea. Clypeasteroida, Clypeastridae, Arachnoididae, Fibulariidae, Laganidae, and Scutellidae. 471 pp. Reitzel, Copenhagen.
Mortensen T. 1951. A Monograph of the Echinoidea. V. Spatangoida 2, Amphisternata II. 593 pp. Reitzel, Copenhagen.
Mudelsee, M. and Raymo, M.E. 2005. Slow dynamics of the Northern Hemisphere glaciation. Paleoceanography 20: PA4022. Crossref
Müller, O.F. 1776. Zoologiæ Danicæ Prodromus, seu Animalium Daniæ et Norvegiæ indigenarum Characteres, Nomina, et Synonyma Imprimis Popularium. xxxii, 282 pp. Typis Hallageriis, Havniæ. Crossref
Néraudeau, D. and Masrour, M. 2008. Evolution de la biodiversité et de la distribution paléobiogéographique des échinides sur les côtes atlantiques du Maroc du Tortonien à l’Actuel. Geodiversitas 30: 211–232.
Néraudeau, D., Barbe, S., Mercier, D., and Roman, J. 2003. Signatures paléoclimatiques des échinides du Néogène final atlantique à faciès Redonien. Annales de Paléontologie 89: 153–170. Crossref
Néraudeau, D., Borghi, E., and Roman, J. 1998. Le genre d’échinide Spatangus dans les localités du Pliocène et du Pléistocène d’Émilie (Italie du Nord). Annales de Paléontologie 84: 243–264. Crossref
Néraudeau, D., Goubert, E., Lacour, D., and Rouchy, J.M. 2001. Changing biodiversity of Mediterranean irregular echinoids from the Messinian to the Present-day. Palaeogeography, Palaeoclimatology, Palaeoecology 175: 43–60. Crossref
Néraudeau, D., Roman, J., and Borghi, E. 1999. Impact of the Messinian crisis on the Mediterranean echinoid fauna. In: M.D. Candia Carnevali and F. Bonasoro (eds.), Proceedings of the 5th European Conference on Echinoderms, Milan, Italy, 7–12 September 1998, 355–360. Balkema, Rotterdam.
Noordenburg, H, 2008. Sea Urchins of the Philipphines—the Irregulars. 153 pp. Artificial Harmonics, Utrecht.
OBIS 2021. Ocean Biodiversity Information System. https://obis.org [accessed March 2021].
Ohneiser, C., Florindo, F., Stocchi, P., Roberts, A.P., De Conto, R.M., and Pollard, D. 2015. Antarctic glacio-eustatic contributions to late Miocene Mediterranean desiccation and reflooding. Nature Communication 6: 8765. Crossref
Pennant, T. 1777. British Zoology. Crustacea, Mollusca, Testacea. Vol. 4. 136 pp. B. White, London.
Péquignat, E. 1964. Description d’une espèce nouvelle de grande taille, repérée dans trios localités entre Marseille et Genès: Echinocardium fenauxi Péquignat. Bulletin Institut Océanographique de Monaco 62 (1291): 1–22.
Pereira, P. 2008. Echinoids from the Neogene of Portugal Mainland: Systematics, Palaeoecology Palaeogegraphy. 200 pp. Ph.D. Thesis (unpublished), Department of Geology, University of Lisbon, Lisbon.
Pérès, J.M. 1967. The Mediterranean benthos. In: H. Barnes (ed.), Oceanography and Marine Biology: An Annual Review 5: 449–533.
Pervesler, P., Uchman, A., Hohenegger, J., and Dominici, S. 2011. Ichnological record of environmental changes in Early Quaternary (Gelasian–Calabrian) marine deposits of the Stirone section, northern Italy. Palaios 26: 578–593. Crossref
Peters, W.K. 1855. Über die an der Koste von Mossambique beobachten Seeigel und insbesondere uber die Gruppe von Diademen. Abhandlungen der Koenig Akademie der Wissenschaften Berlin 1854: 1–109.
Phelan, T. 1970. A field guide to the Cidaroid Echinoids of north-western Atlantic Ocean, Gulf of Mexico and Caribbean Sea. Smithsonian Contributions to Zoology 40: 1–22. Crossref
Philippe, M. 1998. Les Échinides miocènes du Bassin du Rhône révision systématique. Nouvelles Archives du Muséum d’Histoire Naturelle de Lyon 36 (1–2): 3–241, 249–441. Crossref
Philippi, R.A. 1845. Beschreibung einiger neuer Echinodermen nebst kritischen Bemerckungen über einige weniger bekannte Arten. Archiv für Naturgeschichte 11: 344–359.
Picton, B.E. 1993. A Field Guide to the Shallow-Water Echinoderms of the British Isles. 96 pp. Immel Publishing, London.
Pomel, A. 1869. Revue des Échinodermes et de leur classification pour servir d’introduction à l’étude des fossiles. 67 pp. Deyrolle, Paris.
Pomel, A. 1883. Classification méthodique et Genera des Échinides vivante et fossiles. Thèses présentées a la Faculté des Sciences de Paris pour obtenir le Grade de Docteur ès Sciences Naturelles. 131 pp. Aldolphe Jourdan, Alger. Crossref
Pomel, A. 1887. Paléontologie ou descriptions des animaux fossiles de l’Algérie. Zoophytes 2e Fascicule: Echinodermes 2e Livraison. 344 pp. Fontana et Cie, Alger.
Pomel, A. 1888. Notes d’Échinologie synonymique. Bulletin de la Société Géologique de France, séries 3 16: 441–453
Ponzi, G. 1858. Note sur les diverses zones de la formation pliocène des environs de Rome. Bulletin de la Société Géologique de France, 2 séries 15: 374–375.
Popov, S.V., Rögl, F., Rozanov, A.Y., Steininger, F.F., Shcherba, I.G., and Kovae, M. 2004. Lithological-paleogeographic maps of Paratethys. 10 maps (Late Eocene to Pliocene). Courier Forschungsinstitut Senckenberg 250: 1–46.
Por, F.D. 2009. Tethys returns to the Mediterranean: Success and limits of tropical re-colonization. In: F. Krupp, J.L. Musselman, M.M.A. Kotb, and I. Weidig (eds.), Environment, Biodiversity and Conservation in the Middle East. Proceedings of the First Middle Eastern Biodiversity Congress, Aqaba, Jordan, 20–23 October 2008. BioRisk 3: 5–19. Crossref
Queirós, A.M., Birchenough, S.N.R., Bremner, J., Godbold, J.A., Parker, R.E., Romero-Ramirez, A., Reiss, H., Solan, M., Somerfield, P.J., Van Colen, C., Van Hoey, G., and Widdicombe, S. 2013. A bioturbation classification of European marine infaunal invertebrates. Ecology and Evolution 3: 3958–3985. Crossref
Raffi, S. 1986. The significance of marine boreal molluscs in the Early Pleistocene faunas of the Mediterranean area. Palaeogeography, Palaeoclimatology, Palaeoecology 52: 171–193. Crossref
Raymo, M.E. 1994. The initiation of Northern Hemisphere glaciation. Annual Review of Earth and Planetary Science 22: 353–383. Crossref
Raymo, M.E. and Nisancioglu, K. 2003. The 41 kyr world: Milankovitch’s other unsolved mystery. Paleoceanography 18: 1011. Crossref
Rögl, F. 1999. Mediterranean and Paratethys. Facts and hypotheses of an Oligocene to Miocene paleogeography (short overview). Geologica Carpathica 50: 339–349. Crossref
Roman, J. 1965. Morphologie et évolution des Echinolampas (Échinides, Cassiduloïdes). Mémoires du Muséum National d’Histoire Naturelle Nouvelle, Série, Science de la Terre, Série C 15: 1–341.
Roman, J. 1983. Echinides “marsupiaux” (famille des Temnopleuridae) dans le Neogene de l’Ouest européen. Annales de Paléontologie 69: 13–39.
Roman, J. 1989a. Echinodermes actuels et fossiles. Actes du VI° Séminaire international sur les échinodermes, 19–22 septembre 1988. Vie Marine, hors série 10 1989: 39–48.
Roman, J. 1989b. Les échinoides pliocènes de l’Ouest de la France. Vue d’ensemble. Géologie de la France 1–2: 227–234.
Roman, J. and Saint-Martin, J. 1987. Echinoneus lorioli, rare echinoide (holectypoïde) récifal dans le Messinien (Miocène) d’Oranie (Algérie). Actes 112e Congrès national des Sociétés Savantes, Lyon 2: 15–25.
Roman, J. and Soudet, H.J. 1990. Les échinides du Néogène récent bétique. Documents et Travaux de l’I.G.A.L. 12–13: 53–56.
Rouchy, J.M. and Caruso, A. 2006. The Messinian salinity crisis in the Mediterranean basin: a reassessment of the data and an integrated scenario. Sedimentary Geology 188–189: 35–67. Crossref
Roveri, M. and Taviani, M. 2003. Calcarenite and sapropel deposition in the Mediterranean Pliocene: shallow- and deep-water record of astronomically driven climatic events. Terra Nova 15: 279–286. Crossref
Roveri, M., Flecker, R., Krijgsman, W., Lofi, J., Lugli, S., Manzi, V., Sierro, F.J., Bertini, A., Camerlenghi, A., De Lange, G., Govers, R., Hilgen, F.J., Hübscher, C., Meijer, P.T., and Stoica, M. 2014. The Messinian salinity crisis: past and future of a great challenge for marine sciences. Marine Geology 352: 25–58. Crossref
Rose, P.F. and Wood, J.L. 1999. Clypeaster and Echinolampas (Echinoidea: Irregularia) from Neogene basins of south-east Spain, and the mediterranean Messinian “Salinity Crisis”. In: M.D.C. Carnevali and F. Bonasoro (eds.), Echinoderm Research 1998, 377–381. A.A. Balkema, Rotterdam.
Ruggieri, G. 1967. The Miocene and later evolution of the Mediterranean Sea. In: C.G. Adams and D.V. Ager (eds.), Aspects of Tethyan Biogeography, 283–290. Systematics Association Publications, Oxford.
Russell, M.P. 2013. Echinoderm Responses to variation in salinity. Advances in Marine Biology 66: 171–212. Crossref
Ryan, W.B. 2009. Decoding the Mediterranean salinity crisis. Sedimentology 56: 95–136. Crossref
Saint-Martin, J.P., Néraudeau, D., Lauriat-Rage, A., Secretan, S., Goubert, E., Babinot, J.F., Boukli-Hacene, S., Pouyet, S., Lacour, D., Pestrea, S., and Conesa, G. 2000. La faune interstratigraphique dans les gypses messiniens de Los Yesos (Bassin de Sorbas, SE Espagne): Implications paleoenvironnementales. Geobios 33: 637–649. Crossref
Saucède, T., Mooi, R. and David, B. 2007. Phylogeny and origin of Jurassic irregular echinoids (Echinodermata: Echinoidea). Geological Magazine 144: 333–359. Crossref
Seguenza, G. 1880. Le formazioni terziarie nella provincia di Reggio (Calabria). Memorie della Reale Accademia dei Lincei, Serie 3a, Memorie della Classe di scienze fisiche, matematiche e naturali 6 (for 1877): 1–446. Crossref
Serafy, D.K. 1979. Echinoids (Echinodermata: Echinoidea). Memoirs of the Hourglass Cruises 5 (3): 1–120.
Serrano, F. 2020. An approach to the paleoceanographic characteristics of the sea-surface at the Western Mediterranean (Balearic area) during the Pliocene and Gelasian. Geosciences 10: 302. Crossref
Seunes, M. 1896. Note sur quelques Echinides des faluns miocènes de la Bretagne. Bulletin de la Société des Sciences et Médecine de l’Ouest 5 (2): 82–89.
Simonelli, V. 1889. Terreni e fossili dell’isola di Pianosa nel mar Tirreno. Bollettino del Reale Comitato Geologico Italiano, serie 2 10: 193–237.
Sismonda, E. 1842. Appendice alla Monografia degli Echinidi Fossili del Piemonte. Memoria della Reale Accademia della Scienze di Torino, serie 2 4: 385–394.
Smith, A.B. and Gale, A.S. 2009. The pre-Messinian deep-sea Neogene echinoid fauna of the Mediterranean: Surface productivity controls and biogeographical relationships. Palaeogeography, Palaeoclimatology, Palaeoecology 281: 115–125. Crossref
Smith, A.B. and Kroh, A. (eds.) 2011. The Echinoid Directory. [available online, http://www.nhm.ac.uk/research-curation/projects/echinoid-directory, accessed April 2021].
Sorbini, L. 1988. Biogeography and climatology of Pliocene and Messinian fossil fish of eastern-central Italy. Bollettino del Museo Civico di Storia Naturale di Verona 14: 1–85.
Sorbini, C. and Tyler, J.C. 2004. Review of the fossil file fishes of the family Monacanthidae (Tetraodontiformes), Pliocene and Pleistocene of Europe, with a new genus, Frigocanthus, and two new species related to the Recent Aluterus. Bollettino del Museo Civico di Storia Naturale di Verona 28: 41–76.
Stara, P., Charbonnier, S., and Borghi, E. 2018. Redefinition of Prospatangus thieryi Lambert, 1909 (Echinoidea, Spatangoida), in Sardospatangus nov. gen. with two new species from Sardinia, Italy. Annales de Paléontologie 104: 309–327. Crossref
Stefanini, G. 1908. Echinidi del Miocene medio dell’Emilia. Parte prima. Palaeontographia Italica 14: 65–120.
Stefanini, G. 1909. Echinidi del Miocene medio dell’Emilia. Parte seconda. Palaeontographia Italica 15: 1–58.
Taviani, M. 2002. The Mediterranean benthos from late Miocene up to present: ten million years of dramatic climatic and geologic vicissitudes. Biologia Marina Mediterranea 9: 445–463.
Taviani, M., Bouchet, P., Metivier, B., Fontugne, M., and Delibrias, G. 1991. Intermediate steps of southwards faunal shifts testified by last glacial submerged thanatocoenoses in the Atlantic Ocean. Palaeogeography, Palaeoclimatology, Palaeoecology 86: 331–338. Crossref
Thiéry, P. 1909. Rectifications de nomenclature. Revue critique de paléozoologie 13: 136–137.
Tortonese, E. 1965. Fauna d’Italia. Echinodermata. 422 pp. Calderini, Bologna.
Tortonese, E. 1977. Recenti acquisizioni e rettifiche intorno ai crinoidi, oloturoidei, ofiuroidei ed echinoidi del Mediterraneo, con particolare riguardo alla fauna italiana. Atti della Società Italiana di Scienze Naturali 118: 333–352.
Valdinucci, A. 1974. “Aliaster” nuovo genere di echinide istituito per un piccolo gruppo di specie tratte dai generi Trachyaster ed Opissaster (Famiglia Hemiasteridae Clark). Bollettino del Servizio Geologico d’Italia 94: 454–479.
Van Phelsum, M. 1774. Brief aan den wel-eerwaardigen en zeer geleerden heere Cornelius Nozeman ... over de gewelv-slekken of zee-egelen: waar achter gevoegd zyn twee beschryvingen, de eene van zekere soort van zee-wier, de andere van maaden, in eene vuile verzweeringe gevonden. 145 pp. R. Arrenberg, Rotterdam. Crossref
Vertino, A., Stolarski, J., Bosellini, F.R., and Taviani, M. 2014. Mediterranean corals through time: from Miocene to Present. In: S. Goffredo and Z. Dubinsky (eds.), The Mediterranean Sea: Its History and Present Challenges, 257–274. Springer, Dordrecht. Crossref
Violanti, D. 2005. Pliocene foraminifera of Piedmont (north-western Italy): a synthesis of recent studies. Annali dell’Università degli Studi di Ferrara Museologia Scientifica e Naturalistica Volume Speciale: 75–88.
Wangensteen, O.S. 2013. Biology and phylogeography of the black sea urchin Arbacia lixula (Echinoidea: Arbacioida). 128 pp. Doctoral Thesis, Universitad de Barcelona, Barcelona.
Webb, P. 2017. Introduction to Oceanography. Online OER Textbook [published online, https:// rwu.pressbooks.pub/webboceanography/]
Wood, A.M. 2009. The phylogeny and palaeozoogeography of cold-water species of ostracod (Crustacea) from the Pre-Ludhamian Stage (late Pliocene–early Pleistocene), Red Crag Formation, East Anglia, England; with reference to the earliest arrival of Pacific species. Paleontological Research 13: 345–366. Crossref
Wright, T.W. 1855. On fossil echinoderms from the Island of Malta; with notes on the stratigraphical distribution of the fossil organisms in the Maltese beds. The Annals and Magazine of Natural History, 2nd Series 15: 101–127, 175–196, 262–277. Crossref
Wright, T. 1864. On the fossil Echinidae of Malta. Quarterly Journal of the Geological Society of London 20: 471–491. Crossref
Zachos, J., Pagani, M., Sloan, L., Thomas, E., and Billups, K. 2001. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 292: 686–693. Crossref
Acta Palaeontol. Pol. 67 (4): 781–805, 2022
https://doi.org/10.4202/app.00993.2022