

Middle and Late Jurassic tube-dwelling polychaetes from the Polish Basin: diversity, palaeoecology and comparisons with other assemblages
JAKUB SŁOWIŃSKI, OLEV VINN, MANFRED JÄGER, and MICHAŁ ZATOŃ
Słowiński, J., Vinn, O., Jäger, M., and Zatoń, M. 2022. Middle and Late Jurassic tube-dwelling polychaetes from the Polish Basin: diversity, palaeoecology and comparisons with other assemblages. Acta Palaeontologica Polonica 67 (4): 827–864.
This study describes diverse assemblages of serpulid and sabellid polychaetes from various Middle and Upper Jurassic (upper Bajocian to lower Kimmeridgian) deposits of the Polish Basin. Twenty four taxa are reported in total, including two new species (Cementula radwanskae sp. nov. and Filogranula spongiophila sp. nov.). Abundance, distribution, and colonization patterns of serpulids and sabellids significantly depended on many palaeoenvironmental variables including the nature of the colonized substrate and its overall shape, food supply, and hydrodynamism. The most diverse assemblages have been noted on the Middle Jurassic shells inhabiting soft muddy substrates, on hardgrounds and oncoids, whereas the lowest biodiversity levels have been found on the Middle Jurassic hiatus concretions and Kimmeridgian oyster shell beds. Some species are clearly associated with certain substrate types, whereas stratigraphic interval is not that important. Middle Jurassic mobile rockgrounds (hiatus concretions and oncoids) and hardgrounds are characterized by the most similar species associations, while Middle Jurassic shelly substrates from soft-bottom environments and Upper Jurassic shell beds and sponge build-ups are most dissimilar with respect to the colonizing tube dwelling polychaete taxa. Among the diverse assemblages of the encrusting faunas, serpulid and sabellid tubeworms are the most abundant constituents in the majority of settings, what is explained by their opportunism and ability to effectively outcompete other contenders. In the majority of locations, the most abundant tube-dwelling polychaete is the ubiquitous sabellid Glomerula gordialis, followed by the serpulid species Propomatoceros lumbricalis. The dominance of these species is congruent with many other serpulid and sabellid communities inhabiting various Jurassic palaeoenvironments.
Key words: Sabellidae, Serpulidae, exrusters, taxonomy, tubeworms, Mesozoic, Poland.
Jakub Słowiński [jakub.slowinski@us.edu.pl] and Michał Zatoń [mzaton@wnoz.us.edu.pl], University of Silesia in Katowice, Institute of Earth Sciences, Będzińska 60, 41-200 Sosnowiec, Poland.
Olev Vinn [olev.vinn@ut.ee], Department of Geology, University of Tartu, Ravila 14A, 50411 Tartu, Estonia.
Manfred Jäger [langstein.jaeger@web.de], Lindenstrasse 53, 72348 Rosenfeld, Germany.
Received 16 May 2022, accepted 26 October 2022, available online 7 December 2022.
Copyright © 2022 J. Słowiński et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Among the all tube-dwelling polychaete families, only Serpulidae dwell exclusively in calcareous tubes, while in the Sabellidae and Cirratulidae, calcareous species are restricted to single genera in each family, Glomerula and Dodecaceria, respectively (Perkins 1991; Fischer et al. 1989, 2000; Vinn et al. 2008a). Moreover, another extinct cirratulid genus—Diplochaetetes—dwelled in partly calcareous tubes (Kočí et al. 2021). During the Jurassic, calcareous cirratulids were absent (Fischer et al. 2000), and thus the only representatives bearing hard, mineralized skeletons were sabellids and, obviously, the most abundant serpulids.
Tube-dwelling polychaetes are sessile, almost exclusively marine (but see Bosák et al. 2004; Kupriyanova et al. 2009) invertebrates predominantly attaching to hard substrates, such as rocks and shells. The oldest known serpulids are middle Permian in age (Sanfilippo et al. 2017, 2018). However, there are only very few reports of true tube-dwelling polychaetes of the late Permian (e.g., Ramsdale 2021) to Early Triassic age. In the Middle Triassic (Assmann 1937; Flügel et al. 1984; Stiller 2000; Senowbari-Daryan et al. 2007), the abundance and diversity of serpulids, as well as their palaeogeographic distribution, started to increase, which continued further during the Late Triassic (Ziegler and Michalík 1980; Berra and Jadoul 1996; Cirilli et al. 1999). Tube-dwelling polychaetes have been widespread since the Early Jurassic, and their major diversification took place during the Middle and Late Jurassic, and continued during the Cretaceous (for review see Ippolitov et al. 2014). Such an increase in both diversity and abundance of sessile polychaete fauna during the Middle and Late Jurassic coincided with an overall evolutionary radiation of various organisms colonizing hard substrates (e.g., Palmer and Fürsich 1974; Wilson and Palmer 1990; Feldman and Brett 1998; Taylor and Wilson 2003; Wilson et al. 2008; Zatoń and Taylor 2009b; Zatoń et al. 2011a, b; Breton et al. 2020). This sclerobiont (sensu Taylor and Wilson 2002) “bloom” during the Jurassic resulted from an increase of carbonate, lithified substrates and hard, calcareous skeletons of diverse sessile organisms (see e.g., Taylor and Wilson 2003 for a comprehensive review). The last factor might have also been a response to increasing levels of predation during the so-called Mesozoic Marine Revolution (Vermeij 1977).
In spite of their large number and wide distribution, tube-dwelling polychaetes are a group which seems to have been omitted quite often in palaeontological research, possibly due to their morphological simplicity and a common opinion that they are an unimportant group for biostratigraphy. Among the best studied serpulid and sabellid taxa are those from the Upper Cretaceous (e.g., Brünnich Nielsen 1931; Jäger 1983, 2005, 2011; Macellari 1984; Tapaswi 1988; Radwańska 1996; Kočí and Jäger 2015a, b; Kočí et al. 2017), whereas other stratigraphic intervals are characterized by scattered reports. Although in recent years much more effort has been made towards a better recognition of Jurassic serpulids and sabellids (e.g., Ippolitov 2007a, b; Jäger and Schubert 2008; Vinn and Wilson 2010; Kočí et al. 2019), the more complex treatments of Jurassic representatives are now clearly outdated (e.g., Parsch 1956). Despite extensive Middle and Upper Jurassic outcrops in Poland, with deposits bearing a vast abundance of tube-dwelling polychaetes, there is only one publication focusing only on the taxonomy and palaeoecology of the Oxfordian (now Kimmeridgian, see Loba and Radwańska 2022) species from the Kuyavia region in central Poland (Radwańska 2004). Some other, single reports either treated a single species (Filograna socialis from the Tithonian (Upper Jurassic) of Sławno, see Radwańska 2003) or only mentioned serpulids and sabellids on the palaeoecological background and were not the main scope of the research (e.g., Kaim 2011; Zatoń et al. 2011a, b, 2012).
The lack of a comprehensive study of Middle and Upper Jurassic tube-dwelling polychaetes from Poland hampers our understanding of evolution and ecology of encrusting faunas of the Jurassic Polish Basin and Jurassic serpulids and sabellids in general. This serious gap in knowledge is here filled by our systematic and palaeoecological study of serpulids and sabellids derived from numerous outcrops of Middle and Upper Jurassic deposits, representing various palaeoenvironments in the Polish Basin.
Nomenclatural acts.—This published work and the nomenclatural acts it contains have been registered in ZooBank: urn:lsid:zoobank.org:pub:1F0C99C5-769A-4C82-80C8-1A92584BF19D
Institutional abbreviations.—GIUS, Institute of Earth Sciences, University of Silesia in Katowice, Poland.
Other abbreviations.—CEBS, Central European Basin System; ITS, Internal Tube Structures; UPGMA, unweighted pair group method with arithmetic mean algorithm.
Geological setting
Palaeogeographical background.—During the Mesozoic, the epicontinental Polish Basin constituted the easternmost part of the larger system of epicontinental seas called the Central European Basin System (CEBS). During the Middle Jurassic, the Polish Basin was restricted from the north, east and south by Fennoscandian, Ukrainian, Meta-Carpathian and Bohemian landmasses, respectively (Fig. 1A), so its connection with other Central European basins was quite limited and only existed to the west via the Germanic Basin and to the south-east via the East Carpathian Gate which linked the Polish Basin with the Tethys Ocean (Dayczak-Calikowska and Moryc 1988; Dayczak-Calikowska et al. 1997). From the Aalenian, the basin progressively widened during gradual transgression, punctuated by some regressive events, and in the late Bathonian the entire area of the Polish Lowlands was submerged (Matyja and Wierzbowski 1998). During this time, sedimentation was dominated by clastics derived from the surrounding land masses (especially from the largest Fennoscandian land, Marynowski et al. 2007), the depositional systems of which followed transgressive-regressive cycles (Feldman-Olszewska 1997; Leonowicz 2015a). During the Callovian (Middle Jurassic), the Polish Basin witnessed further progressive deepening with a peak transgression noted in the late Callovian (Wierzbowski et al. 2009). The Late Jurassic was a time of reorganization of the Polish Basin and the onset of carbonate platform deposition. After this time, the Polish Basin became part of the northern shelf of the Tethys Ocean (Kutek 1994; Matyja and Wierzbowski 2006), where the development of the carbonate platform was controlled by sea-level and climate changes, as well as the architecture of the Paleozoic basement and synsedimentary tectonics (Kutek 1994; Gutowski et al. 2005; Krajewski et al. 2011, 2016, 2017; Matyszkiewicz et al. 2012, 2016). Characteristic bioherms formed by siliceous sponges and microbial consortia were extensively developed during the Oxfordian (Late Jurassic; e.g., Trammer 1982; Ostrowski 2005; Matyja 2006; Matyszkiewicz et al. 2012) and various carbonate facies commenced during the Kimmeridgian (Late Jurassic), including oolites and oncolites with hardgrounds, and shell-beds in a variety of marine environments (Kutek 1994; Machalski 1998; Matyja et al. 2006; Krajewski et al. 2017).
Investigated outcrops.—In total, 11 localities exposing Middle to Upper Jurassic deposits in the Polish Jura area and the Mesozoic margin of the Holy Cross Mountains have been sampled (Fig. 1B, C; Table 1). As the details of the sampled sites have already been presented by Słowiński et al. (2020), here we provide only those stratigraphic and palaeoenvironmental data which are most important for the present paper. In ascending stratigraphic order, these are as follows:
Ogrodzieniec-Świertowiec: The outcrop is located 1 km south of the town of Ogrodzieniec, Polish Jura. The serpulid and sabellid fauna has been found encrusting large oncoids encased within condensed, sandy and carbonate deposits underlain by the dark mudstone of the Częstochowa Ore-bearing Clay Formation. Dinoflagellate cysts and foraminifers found in the oncoid cortices (Słowiński 2019), as well as ammonites found within the host rocks, indicate that the oncoid-bearing deposits are confined to the upper Bajocian–lower Bathonian (up to the Morphoceras macrescens Subzone of the Zigzagiceras zigzag Zone, see Zatoń and Taylor 2009a; Zatoń et al. 2012). The palaeoenvironment was interpreted as a shallow-water marine habitat within the photic zone and slightly below fair-weather wave base (Zatoń et al. 2012).
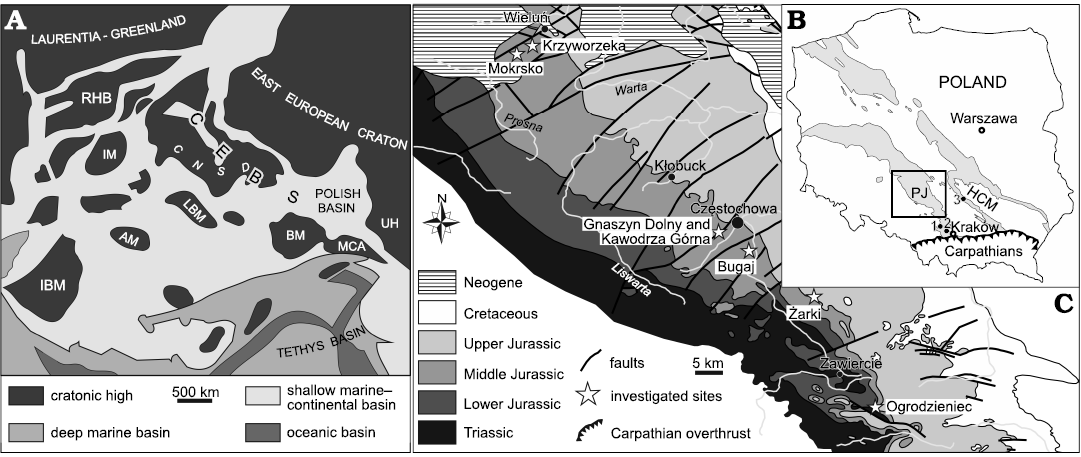
Fig. 1. Palaeogeography and geology of the investigated area. A. Paleogeographical sketch-map of Europe during the Middle Jurassic (from Leonowicz 2016, modified after Ziegler 1990). AM, Armorican Massif; BM, Bohemian Massif; CEBS, Central European Basin System; CNSD, Central North Sea Dome; IBM, Iberian Meseta,; IM, Irish Massif; LBM, London-Brabant Massif; MCA, Meta-Carpathian Arc; RHB, Rockall-Hatton Bank; UH, Ukrainian High. B. Geological sketch-map of Poland without the Cenozoic cover with three sampled localities indicated. HCM, Holy Cross Mountains; PJ, Polish Jura; 1, Bolęcin; 2, Zalas; 3, Małogoszcz. C. Geological map of the Polish Jura area without Quaternary cover, with sampled localities indicated (after Zatoń and Taylor 2009b).
Ogrodzieniec, Żarki, Bugaj, Kawodrza Górna, Gnaszyn Dolny, Mokrsko, and Krzyworzeka: These seven sites are located in the Polish Jura (Fig. 1B, C) area and contain Middle Jurassic (upper Bajocian–Bathonian) siliciclastic deposits in the form of dark mudstone and siltstone beds with siderite nodules and calcite hiatus concretions belonging to the Częstochowa Ore-bearing Clay Formation (e.g., Majewski 2000; Matyja and Wierzbowski 2000; Zatoń et al. 2011a; Leonowicz 2015b). The tube-dwelling polychaetes have been found encrusting bivalve shells and belemnite rostra collected in Kawodrza Górna (lower Bathonian) and Gnaszyn Dolny (middle Bathonian), as well as the hiatus concretions from Mokrsko (upper Bajocian), Bugaj (middle Bathonian), Ogrodzieniec, Żarki, and Krzyworzeka (upper Bathonian). Mudstone beds of the Częstochowa Ore-bearing Clays were deposited in various bathymetric regimes, ranging from lower offshore to offshore transition, e.g., below, and above storm wave-base (Gedl et al. 2012; Leonowicz 2015a). The horizons with tubeworm-bearing hiatus concretions mark distinct decreases (or even pauses) in sedimentation rate and erosion of the seafloor (Zatoń et al. 2011a; Leonowicz 2015b).
Bolęcin: This site is about 6 km to the east of the town of Chrzanów, between Katowice and Kraków, Polish Jura (Fig. 1B). The trench dug in a nearby wood exposed highly fossiliferous, condensed sandy limestone with abundant quartz pebbles and ooids, which most probably correspond to the so-called “Balin Oolite” of upper Bathonian–lower Callovian (Middle Jurassic), with possible base of middle Callovian present (Tarkowski et al. 1994; Mangold et al. 1996; Taylor 2008). The presence of a diverse macrofauna, including ammonites (Mangold et al. 1996), may indicate an open marine palaeoenvironment. Tube-dwelling polychaetes were found encrusting various shells and skeletal remains of different mollusks.
Zalas: The active quarry is located in the Zalas village near Krzeszowice, southern part of the Polish Jura (Fig. 1B). The serpulid and sabellid fauna has been derived from two different lithologies and stratigraphic units. Stratigraphically older specimens have been found encrusting various macrofossils (especially the large bivalve Ctenostreon proboscideum (Sowerby and Sowerby, 1820) encased within a hardground of middle Callovian–earliest late Callovian age (Middle Jurassic; Giżejewska and Wieczorek 1977; Dembicz and Praszkier 2007), and originating in an open-sea, offshore environment (Dembicz and Praszkier 2007; Zatoń et al. 2011b). Stratigraphically younger specimens were found on lower Oxfordian (Upper Jurassic) sponge-forming bioherms (Matyja 2006; Matyszkiewicz et al. 2012).
Małogoszcz: Serpulid and sabellid fauna has been collected in an active quarry situated in the southwestern part of the Mesozoic margin of the Holy Cross Mountains, ca. 1 km north of Małogoszcz town centre (Fig. 1B). The specimens encrusted bivalve shells, especially specimens of Actinostreon gregareum (Sowerby, 1815), derived from the lower Kimmeridgian (Upper Jurassic) shell-beds referred to as the Skorków Lumachelle (Kutek 1994; Machalski 1998; Matyja et al. 2006; Zatoń and Machalski 2013), which resulted from storm-induced deposition in a relatively shallow marine environment (Machalski 1998).
Material and methods
The investigated material encompasses in total 2314 specimens of fossil serpulids and sabellids (Table 1), most of which are relatively well-preserved, allowing for proper taxonomic assignment. All strongly abraded tubes, whose determination was ambiguous, were eliminated from further examination.
Table 1. Data on provenance and number of all sabellid and serpulid specimens.
|
Taxa |
Mokrsko |
Kawodrza |
Gnaszyn |
Bugaj |
Ogrodzieniec |
Krzyworzeka |
Żarki |
Ogrodzieniec- |
Bolęcin |
Zalas |
Zalas |
Małogoszcz |
Total number of individuals (per taxon) |
|
Glomerula gordialis |
66 |
13 |
80 |
52 |
60 |
62 |
8 |
174 |
102 |
181 |
36 |
45 |
879 |
|
Metavermilia cf. striatissima |
|
|
|
|
2 |
33 |
1 |
18 |
24 |
56 |
|
|
134 |
|
Metavermilia? sp. |
|
|
11 |
|
|
|
|
|
|
|
|
|
11 |
|
Filogranula runcinata |
3 |
3 |
13 |
5 |
|
|
|
5 |
15 |
11 |
|
|
55 |
|
Filogranula spongiophila sp. nov. |
|
|
|
|
|
|
|
|
|
|
16 |
|
16 |
|
Cementula spirolinites |
|
|
|
|
|
|
|
|
|
|
122 |
|
122 |
|
Cementula radwanskae sp. nov. |
|
|
|
|
|
|
|
|
4 |
29 |
|
|
33 |
|
Cementula cf. circinnalis |
|
|
|
|
|
|
|
38 |
|
|
|
|
38 |
|
“Serpula cingulata” |
|
|
|
|
|
|
|
|
|
|
3 |
|
3 |
|
Propomatoceros lumbricalis |
21 |
31 |
228 |
81 |
33 |
23 |
9 |
156 |
81 |
149 |
|
28 |
840 |
|
Propomatoceros sp. 1 |
1 |
|
4 |
|
|
|
|
|
|
|
|
|
5 |
|
Propomatoceros sp. 2 |
|
|
|
|
|
|
|
|
|
1 |
|
|
1 |
|
Propomatoceros sp. 3 |
|
|
|
|
|
|
|
|
|
|
|
1 |
1 |
|
Nogrobs aff. quadrilatera |
|
|
36 |
|
|
|
|
|
|
|
|
|
36 |
|
Nogrobs? aff. tricristata |
|
|
8 |
|
|
|
|
|
|
|
|
|
8 |
|
Nogrobs aff. tetragona |
|
|
29 |
|
|
|
|
|
|
|
|
|
29 |
|
Mucroserpula tricarinata |
|
|
|
|
|
|
|
|
|
2 |
|
|
2 |
|
Mucroserpula? sp. |
|
|
2 |
|
|
|
|
|
|
|
|
|
2 |
|
Placostegus planorbiformis |
|
|
|
|
|
|
|
|
|
|
7 |
|
7 |
|
Pseudovermilia sp. |
|
|
|
|
|
|
|
|
|
4 |
|
|
4 |
|
Serpulidae sp. 1 |
|
|
|
|
|
|
|
23 |
5 |
43 |
|
|
71 |
|
Serpulidae sp. 2 |
|
|
1 |
|
|
|
|
|
|
|
|
|
1 |
|
Serpulidae sp. 3 |
|
|
10 |
|
|
|
|
|
|
|
|
|
10 |
|
Serpulidae sp. 4 |
|
|
|
|
|
|
|
|
|
|
|
6 |
6 |
|
Total number of individuals |
91 |
47 |
422 |
138 |
95 |
118 |
18 |
414 |
231 |
476 |
184 |
80 |
2314 |
Sufficiently well-preserved specimens have been cleaned and carefully studied under a binocular microscope paying special attention to all the diagnostic characters of the tubes, which allowed for a reliable identification to the lowest possible taxonomic level. Following their taxonomic identification, the total number of specimens, their relative abundance and distribution in relation to and within the specific substrate have been assessed, employing biodiversity indices such as Dominance (D), Simpson’s (1-D), Shannon’s (H) and evenness (eH/S) using the PAST software (Hammer et al. 2001).
In order to analyse the relationship of particular tube-dwelling polychaete assemblages inhabiting different substrates and palaeoenvironments, cluster analysis using the PAST software (Hammer et al. 2001) was performed. In order to quantitatively examine the similarities of the communities between particular substrates/palaeoenvironments, Q-mode cluster analysis was used. The analysis was performed using the Raup-Crick similarity index and the unweighted pair group method with arithmetic mean (UPGMA) algorithm. The Raup-Crick index was applied as it uses Monte Carlo randomization, which compares the observed number of taxa occurring within two associations with the distribution of their co-occurrences on the basis of 200 random interactions.
The specimens were coated with ammonium chloride prior to photography. All the material investigated is housed at the Institute of Earth Sciences in Sosnowiec, under collection numbers GIUS 8-3589 (Callovian of Zalas), GIUS 8-3730 (Gnaszyn Dolny and Kawodrza Górna), GIUS 8-3745 (Bolęcin), GIUS 8-3746 (Oxfordian of Zalas), GIUS 8-3747 (Małogoszcz), GIUS 8-3750 (Ogrodzieniec-Świertowiec), GIUS 8-3751 (Mokrsko, Bugaj, Ogrodzieniec, Krzyworzeka, and Żarki).
Systematic palaeontology
Phylum Annelida Lamarck, 1802
Class Polychaeta Grube, 1850
Subclass Sedentaria Lamarck, 1818
Infraclass Canalipalpata Rouse and Fauchald, 1997
Order Sabellida Levinsen, 1883
Family Sabellidae Latreille, 1825
Subfamily Sabellinae Chamberlin, 1919
Genus Glomerula Brünnich Nielsen, 1931
Type species: Serpulites gordialis (Schlotheim, 1820); Schlotheim (1820) lists seven specimens from the Alps and Jurassic limestone, from different localities; Switzerland; no precise location mentioned; Heidenheim, South Germany, Upper Jurassic; Ermreuth, South Germany, Jurassic (possibly Middle or Upper Jurassic); more precise stratigraphy unknown.
Glomerula gordialis (Schlotheim, 1820)
Figs. 2A–H, 3A, B, E, F, 5D, 10A, 12A.
1820 Serpulites gordialis sp. nov.; Schlotheim 1820: 96.
1831 Serpula gordialis Schloth.; Goldfuss 1831: 234, pl. 69: 8a–c.
1956 Serpula (Cycloserpula) gordialis Schlotheim, 1820; Parsch 1956: 214, pl. 20: 15, 16.
pars 1983 Glomerula gordialis (Schlotheim, 1820); Jäger 1983: 26, pl. 2: 1, non pl. 2: 2–18 (with a large synonymy including Cretaceous and Palaeogene specimens which Jäger [2005: 127–131, pl. 1: 1–10] later considered as belonging to two separate species).
2004 Glomerula gordialis (Schlotheim, 1820); Radwańska 2004: 38, pl. 1: 1–10.
2010 Glomerula gordialis (Schlotheim, 1820); Vinn and Wilson 2010: 36: 6.7, 6.8.
Material.—879 variably preserved specimens encrusting hiatus concretions (66 from Mokrsko, 52 from Bugaj, 60 from Ogrodzieniec, 62 from Krzyworzeka, and 8 from Żarki), oncoids (174 from Ogrodzieniec-Świertowiec), bivalve, cephalopod, and gastropod shells (and moulds to a lesser extent) (13 from Kawodrza Górna, 80 from Gnaszyn Dolny, 102 from Bolęcin, and 181 from the Callovian of Zalas), and sponges (36 from the Oxfordian of Zalas from the Middle–Upper Jurassic of the Polish Jura and 45 from Upper Jurassic of Małogoszcz) (see Table 1); GIUS 8-3589, GIUS 8-3730, GIUS 8-3745, GIUS 8-3746, GIUS 8-3747, GIUS 8-3750, GIUS 8-3751.
Description.—Tubes small to large, up to 100–150 mm long, forming numerous, usually irregularly curved loops and dense, coiled aggregations; some specimens are also represented by straight or slightly meandering, only occasionally complex and coiled tubes. Tube diameter nearly constant along the whole length, and cross-section always circular to subcircular. Relative to the diameter of the tube (up to 2 mm, most often 1 mm or less), the wall is relatively thick. Tubes do not possess any flanges or attachment structures, and the base is not distinctly flattened. Surface entirely smooth, lacking ornamentation and peristomes. Interior smooth without any internal tube structures.
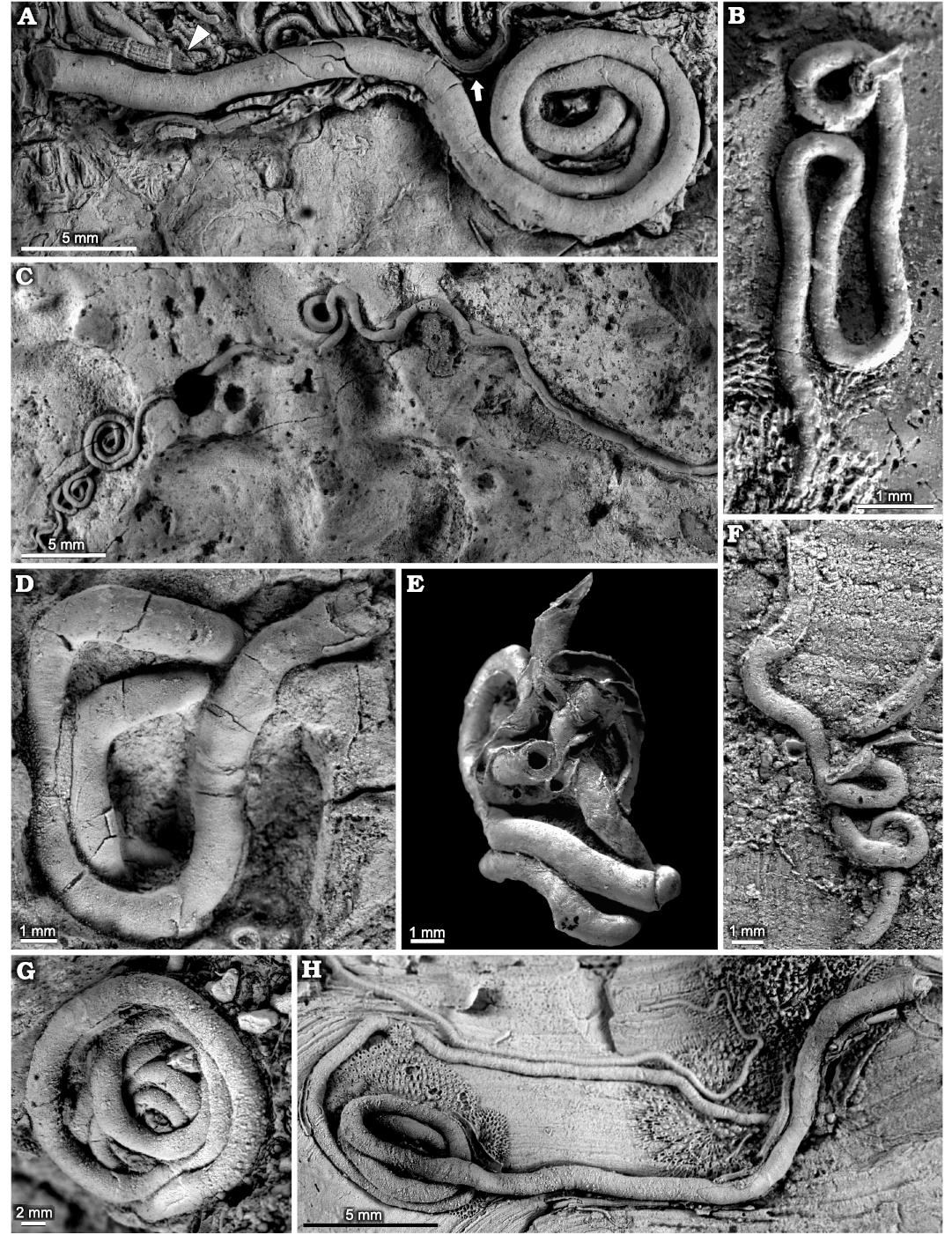
Fig. 2. Sabellid and serpulid polychaetes from the Jurassic of Poland. A. Sabellid polychaete Glomerula gordialis (Schlotheim, 1820), tiny serpulid polychaete Metavermilia cf. striatissima (Fürsich, Palmer, and Goodyear, 1994) (arrowhead), and tiny, juvenile serpulid polychaete Propomatoceros lumbricalis (Schlotheim, 1820) (arrow) encrusting oncoid from the upper Bajocian–lower Bathonian of Ogrodzieniec-Świertowiec (GIUS 8-3750/1). B–H. Glomerula gordialis, specimen encrusting: oncoid from the upper Bajocian–lower Bathonian of Ogrodzieniec-Świertowiec (B, GIUS 8-3750/2); hiatus concretions from the upper Bathonian of Ogrodzieniec (C, GIUS 8-3751/1; D, GIUS 8-3751/2); shell fragments from the upper Bathonian–lower Callovian of Bolęcin (F, GIUS 8-3745/1) and Callovian of Zalas (G, GIUS 8-3589/1; H, GIUS 8-3589/2); specimen partially detached from a belemnite rostrum from the middle Bathonian of Gnaszyn Dolny (E, GIUS 8-3730/8).
Remarks.—The specimens studied are assigned to Glomerula gordialis because of their irregularly curved loops and dense aggregations, which are characteristic of the species. Specimens also lack any internal tube structures, which—if present—cause a trilobate shape of the lumen. Some specimens with coiled tube parts alternating with straight to curved parts somewhat resemble Glomerula flaccida (Goldfuss, 1831); however, presumably all such specimens (especially those lacking characteristic knee-like bends) belong in fact to G. gordialis, which is abundant on substrates with unlimited surface. Some specimens of G. gordialis (both in this collection and elsewhere) are superficially similar to Glomerula serpentina (Goldfuss, 1831) in having their tubes coiled in a characteristic, meandering fashion. However, the primary character distinguishing G. gordialis from G. serpentina is the potential ability of G. serpentina to form trilobate constrictions of the tube’s lumen (see e.g., Jäger 1983: 191, pl. 2: 14B, 17). Such constrictions have been relatively common only since the Late Cretaceous, during the Early Cretaceous they occur rarely, but are totally absent in the Jurassic (Jäger 2005). Thus, similarly to Glomerula gordialis tubes resembling G. flaccida, those resembling G. serpentina are presumably also a result of ecophenotypic variation resulting in a specific shape of G. gordialis. Therefore, a specific shape of the Glomerula tube alone cannot be considered as a good taxonomic indicator.
Stratigraphic and geographic range.—In the material studied the species occurs in the Bajocian–lower Oxfordian (Middle–Upper Jurassic) of the Polish Jura and in the lower Kimmeridgian (Upper Jurassic) of the Mesozoic margin of the Holy Cross Mountains (Małogoszcz). Previously, tubes identified as G. gordialis were reported from Jurassic (Pugaczewska 1970; Radwańska 2004; Zatoń et al. 2011a), Cretaceous (Radwańska 1996), and Paleocene localities (Pugaczewska 1967) in Poland. The species is widespread in the Jurassic of England (Sowerby 1844), Germany (Parsch 1956), Czech Republic (Kočí et al. 2019), and France (Breton et al. 2020). It has also been described from the Jurassic of Israel (Wilson et al. 2008; Vinn and Wilson 2010). Glomerula tubes cited as G. gordialis are also widespread in the Cretaceous of Europe (e.g., in Germany, Jäger 1983) and India (Chiplonkar and Tapaswi 1973).
Family Serpulidae Rafinesque, 1815
Genus Metavermilia Bush, 1905
Type species: Vermilia multicristata (Philippi, 1844); Recent, Mediterranean Sea.
Metavermilia cf. striatissima (Fürsich, Palmer, and Goodyear, 1994)
Figs. 2A, 3C–F, 5D, 7B.
Material.—134 well-preserved specimens encrusting hiatus concretions (2 from Ogrodzieniec, 33 from Krzyworzeka, and 1 from Żarki), bivalve shells (and moulds to a lesser extent) (24 from Bolęcin and 56 from the Callovian of Zalas), and oncoids (18 from Ogrodzieniec-Świertowiec) from the Bajocian–Callovian (Middle Jurassic) of the Polish Jura (see Table 1); GIUS 8-3589, GIUS 8-3745, GIUS 8-3759, GIUS 8-3751.
Description.—Tubes irregularly and strongly curved, but not coiled. Solitary tubes relatively small, however, some specimens may be up to 30–40 mm long. Tube diameter nearly constant throughout the entire length, rarely exceeding 1 mm. Cross-section subcircular or more frequently angular due to a multi-keeled tube. In some specimens, the cross-section may be more subtriangular due to a widened tube base. Tubes usually lack specific attachment structures and are attached to the substrate by a flattened and occasionally widened base, without a free apertural tube part. Tubes usually covered by six longitudinal keels, evenly spaced from the top of the tube to its flanges. Ampullacea-type peristomes (proles ampullacea) usually thick, prominent and irregularly distributed. Apart from longitudinal ornamentation and peristomes, the tube’s external surface is variable, being smooth or ornamented by corrugations and wrinkles. Some tubes bear a characteristic combination of transverse and longitudinal ornamentation, resulting in a strongly developed but very irregular reticulate “honeycomb” structure.
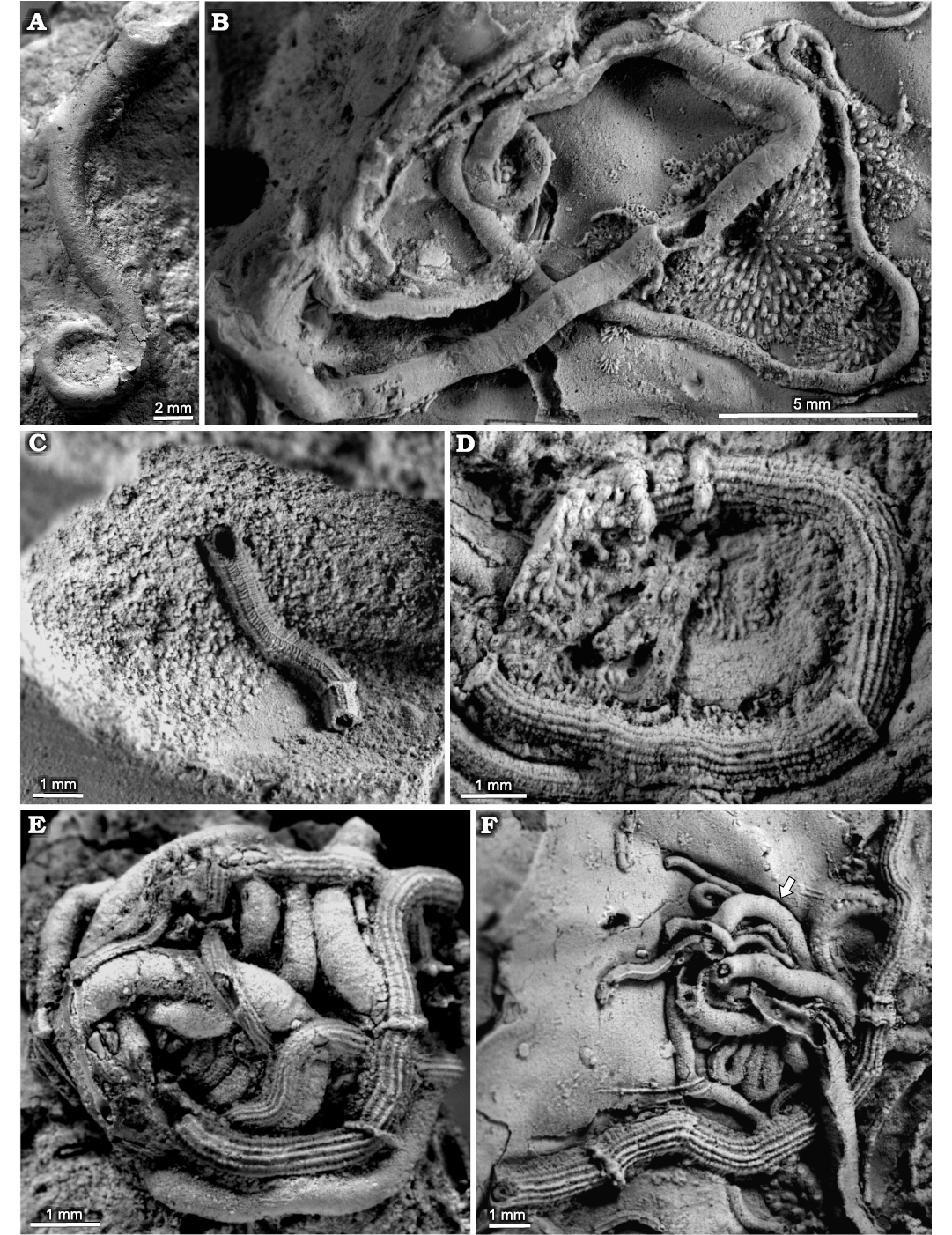
Fig. 3. Sabellid and serpulid polychaetes from the Jurassic of Poland. A. Sabellid polychaete Glomerula gordialis (Schlotheim, 1820) encrusting a sponge fragment from the Oxfordian of Zalas (GIUS 8-3746/1). B. Glomerula gordialis and cyclostome bryozoan colony encrusting an oyster fragment from the lower Kimmeridgian of Małogoszcz (GIUS 8-3747/1). C. Serpulid polychaete Metavermilia cf. striatissima (Fürsich, Palmer, and Goodyear, 1994) encrusting the interior of the boring Gastrochaenolites from the upper Bathonian of Krzyworzeka (GIUS 8-3751/3); note the additional, delicate transverse elements. D–F. Metavermilia cf. striatissima encrusting shell fragments from the Callovian of Zalas (D, GIUS 8-3589/3; E, GIUS 8-3589/4; F, GIUS 8-3589/5), note specimen in E is intertwined with Glomerula gordialis tube, specimen in F is encrusted by a juvenile Propomatoceros lumbricalis; Glomerula gordialis indicated by an arrow.
Remarks.—The type specimens of Metavermilia striatissima from the Tithonian (Upper Jurassic) of southern England (Fürsich et al. 1994) are somewhat smaller than our specimens. Except for the specimens from the type area, five or six-keeled specimens like the one described here have been relatively rarely reported from the Jurassic of western and central Europe. Metavermilia cf. striatissima may locally be a common species in the Jurassic of Poland. This species encompasses two kinds of populations slightly differing in details. The general tube aspect of both groups is very similar, characterized by such features as: presence of six (rarely five) distinct keels consistent in outline; subcircular to subangular cross-section; presence of irregularly scattered ampullacea-type peristomes, and the mode of curvature. The slight differences are in the ornamentation. The populations from Zalas and Bolęcin (hardgrounds) have smooth outer surfaces (between keels and peristomes) and bear better developed flanges (Fig. 3D–F), whereas the other group (primarily from Krzyworzeka) bears some additional transverse elements resulting in a delicate “honeycomb” structure (Fig. 3C; see also Kupriyanova 1993: 155, for comparison), and less developed attachment structures, although basal margins are widened in some specimens as well. These slight differences presumably resulted from ecophenotypic variations.
Metavermilia? sp.
Fig. 4A.
Material.—Eleven specimens attached to belemnite rostra from the middle Bathonian (Middle Jurassic) of Gnaszyn Dolny, Polish Jura (see Table 1); GIUS 8-3730.
Description.—Tubes medium-sized (ca. 15 mm long), gently curved to straight without peristomes, very slowly increasing in diameter (up to 1 mm in total diameter). Tubes attached to the substrate along their entire length and have well-developed, widened flanges. The tubes have three prominent keels which are continuous and rather unmodified along the whole length of the tube and are separated by well-developed furrows. Keels are directed outwards forming a centrifugal pattern. Tubes covered with usually weak perpendicular growth lines. Cross-section subcircular, slightly angular.
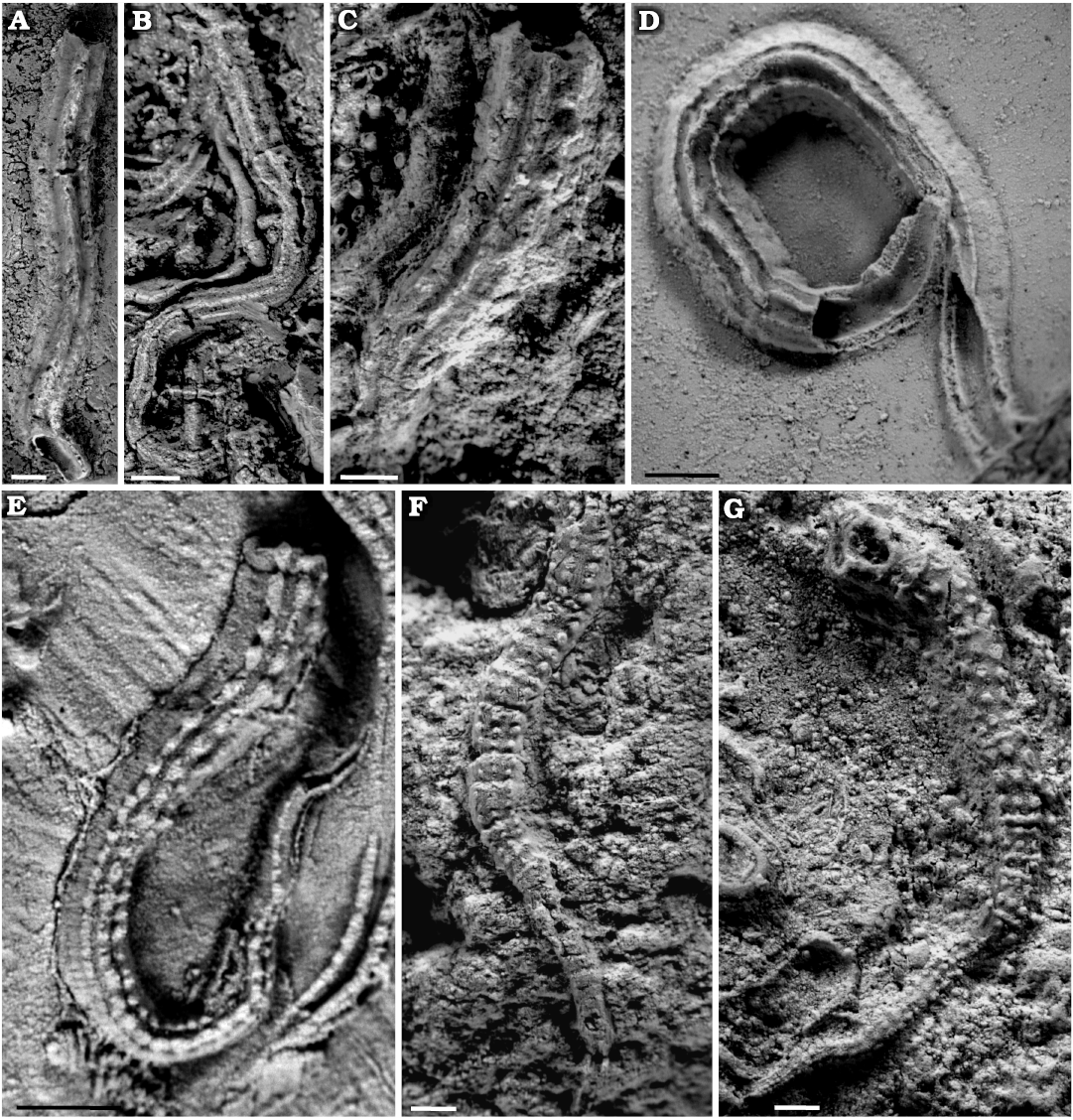
Fig. 4. Serpulid polychaetes from the Jurassic of Poland. A. Metavermilia? sp. encrusting a belemnite rostrum from the middle Bathonian of Gnaszyn Dolny (GIUS 8-3730/9). B–E. Filogranula runcinata (Sowerby, 1829), specimens encrusting: an oncoid from the upper Bajocian–lower Bathonian of Ogrodzieniec-Świertowiec (B, GIUS 8-3750/3); a hiatus concretion from the upper Bajocian of Mokrsko (C, GIUS 8-3751/4); shell fragments from the middle Bathonian of Gnaszyn Dolny (D, GIUS 8-3730/10), the Callovian of Zalas (E, GIUS 8-3589/6). F, G. Filogranula spongiophila sp. nov. encrusting sponge fragments from the Oxfordian of Zalas (F, holotype, GIUS 8-3746/2; G, paratype, GIUS 8-3746/3). Scale bars 1 mm.
Remarks.—The specimens studied are tentatively assigned to an unidentified species of Metavermilia because of similarity in ornamentation to other species of this genus which usually bear 3 to 7 keels. Alternatively, the fossils might belong to Mucroserpula. In spite of the fact that tubes assigned to the latter genus commonly tend to form curved loops and may considerably increase in diameter, the studied specimens have been found only on straight belemnite rostra, where they might have not been able to form curves and loops. Moreover, Ippolitov (2007b) suggested that during the Bathonian–Callovian (Middle Jurassic), some straight, three-keeled Mucroserpula representatives might have coexisted with those of Propomatoceros, which indicates both genera diverged earlier. The arrangement of the three keels of Metavermilia? sp. described here remains somewhat similar to “Tetraserpula quinquangularis (Goldfuss, 1831)” sensu Parsch 1956: 224, pl. 21: 25), however, “Tetraserpula quinquangularis” sensu Parsch (1956) presumably should be synonymized with Mucroserpula tricarinata (Sowerby, 1829). The specific name “quinquangularis” is doubtful; in any case, the overall appearance of the tubes does not fully resemble species of Mucroserpula sensu stricto, and more likely is a member of the genus Metavermilia. It differs from Mucroserpula tricarinata in the much wider arrangement of the lateral keels, the keels being less prominent and only slightly undulating.
Genus Filogranula Langerhans, 1884
Type species: Filogranula gracilis (Langerhans, 1884); Recent, Atlantic.
Filogranula runcinata (Sowerby, 1829)
Figs. 4B–E, 12B.
1829 Serpula runcinata sp. nov.; Sowerby 1829: 227, pl. 608: 6.
1955 Serpula tricarinata Sowerby, 1829; Gerasimov 1955: 29, pl. 9: 1–14.
2007 Filogranula runcinata (Sowerby, 1829); Ippolitov 2007a: 263, pl. 7: 7–12.
2020 “Filogranula” runcinata (J. de C. Sow.); Kosenko and Ippolitov 2020: 117, pl. 1: 2, pl. 2: 2a.
Material.—55 mostly well-preserved specimens encrusting bivalves, belemnites, nautiloids, ammonites (3 from Kawodrza Górna, 13 from Gnaszyn Dolny, 15 from Bolęcin, and 11 from The Callovian of Zalas), hiatus concretions (3 from Mokrsko and 5 from Bugaj), and oncoids (5 from Ogrodzieniec-Świertowiec) from the Middle Jurassic of the Polish Jura (see Table 1); GIUS 8-3589, GIUS 8-3730, GIUS 8-3745, GIUS 8-3750, GIUS 8-3751.
Description.—Tubes small, most often slightly undulating, but curved specimens also occur, though these are not loop-forming. The tubes are attached to the substrate along their entire length and grow to rather small diameter (usually up to 1 mm). Except for the lower lateral tube parts, which remain almost smooth, the tubes are distinctly ornamented by three parallel and longitudinal keels (of which the middle one is usually more elevated) separated by grooves. Each keel is either topped or replaced by a row of distinctive denticles, usually distributed evenly throughout the entire length of the keels; however, the appearance of the keels is sometimes inconsistent, varying in shape and size, depending on locality. Denticles are directed forward, sometimes forming a centrifugal pattern. Peristomes absent. Cross-section subtriangular, slightly flattened, relatively narrow at the top, widens downward, and sometimes widens even more at the base.
Remarks.—The specimens are assigned to Filogranula runcinata due to their distinctive three keels, which are most often topped or replaced by pronounced denticles. The species most similar to F. runcinata is Metavermilia goldfussi (Ippolitov, 2007a), which co-occurs at some European localities (Ippolitov 2007a). These two species bear similarly well-developed external ornamentation. However, M. goldfussi differs from F. runcinata mainly by the lack of such specific features as stronger developed/more denticulate keels or lack of free anterior tube portion. Although M. goldfussi usually bears better developed basal margins and attachment structures, which are present in a part of the specimens studied here, this feature seems to be insufficient for an unequivocal designation. Within Filogranula runcinata, a few populations consisting of very similar tubes (sometimes represented by only several specimens in each locality) can be distinguished in different localities. The tubes, however, only slightly differ in details, presumably depending on (i) different palaeoenvironments recorded in particular localities, and/or (ii) evolutionary change. Nevertheless, despite minor differences, these few forms are included into single species due to the presence of distinct features such as: (i) three strongly denticulate, rather consistent keels present throughout entire ontogeny; (ii) most often slightly widened tube base; (iii) lateral walls being smooth in their lowermost part and strongly ornamented closer to the upper part; (iv) tube attached to the substrate along its entire length; (v) universal lack of peristomes. It must be also noticed that due to the differences in wall microstructures between F. runcinata and other species of Filogranula, the former was also referred to as “Filogranula” runcinata by Ippolitov et al. (2014), suggesting its uncertain affiliation to this genus, and even to different clade than geologically younger species of Filogranula (see also Kočí and Jäger 2015a).
Stratigraphic and geographic range.—The specimens of Filogranula runcinata studied herein come from the Bajocian–Callovian (Middle Jurassic) of the Polish Jura. This species was reported also from the Middle and Upper Jurassic of Russia (Gerasimov 1955; Ippolitov 2007a; Kosenko and Ippolitov 2020) and UK (Sowerby 1829).
Filogranula spongiophila sp. nov.
Fig. 4F, G.
Zoobank LSID: urn:lsid:zoobank.org:act:D9C5AD2A-271B-4D41-B6 C9-0D02C21E584D
Etymology: After the substrate (sponges), the sole substrate this species encrusts.
Type material: Holotype: GIUS 8-3746/2 (almost completely preserved tube attached to a sponge substrate). Paratype: GIUS 8-3746/3 (almost completely preserved tube attached to a sponge substrate) from the type locality and horizon.
Type locality: Zalas near Krzeszowice, southern Poland.
Type horizon: Lower Oxfordian, Upper Jurassic.
Material.—16 specimens encrusting sponges from the type locality and horizon (see Table 1); GIUS 8-3746.
Diagnosis.—A species of the genus Filogranula which lacks continuous keels; instead, it has three longitudinal rows of large granules. Moreover, occasionally a free apertural part and peristomes occurring like thickenings on the tube may be present.
Description.—Tubes small to medium-sized (5–20 mm long; tube diameter 0.5–1.5 mm), usually straight or only slightly undulating, and attached to the substrate by most of their length; however, in some specimens the anterior part is detached by angular folding and erected upwards. Lateral tube walls are almost parallel, only slightly rounded. Tube base is not widened and lacks any flanges or attachment structures. Longitudinal ornamentation is strongly developed and becomes most distinctive in the anterior part of the tube. Keels are lacking; instead, each tube bears three longitudinal rows of large granules which are present along the entire tube length. They may project over the aperture. The median line of granules may be slightly weaker in some specimens. Transverse elements are occasionally represented by irregularly distributed, thick, somewhat nodular (due to the granules), very slightly flaring peristomes occurring rather like thickenings on the tube. However, peristomes are not always present. Tube cross-section rounded pentagonal or petaloid.
Remarks.—Filogranula spongiophila sp. nov. differs from F. runcinata by having rows of granules instead of continuous keels. Moreover, it differs from Filogranula tricristata (Goldfuss, 1831) by its thicker peristomes and the presence of occasionally a free-apertural part. Filogranula spongiophila sp. nov. differs from other species investigated in the present study in the presence of petaloid aperture sometimes raised above the substrate, and keels substituted by rows of granules. However, these regularly ornamented, denticulated forms occur solely within the lower Oxfordian sponge facies of Zalas and are clearly different from Filogranula runcinata from the upper Bajocian–Callovian of other localities mentioned above. To the best of our knowledge, these and similar fossils have not been found in any other locality and currently are known only encrusting sponges from Zalas. Thus, their assignment to a new species is justified.
Stratigraphic and geographic range.—Lower Oxfordian (Upper Jurassic), Zalas, Poland.
Genus Cementula Regenhardt, 1961
Type species: Cementula sphaerica (Brünnich Nielsen, 1931); Maastrichtian (Upper Cretaceous), Nørre Uttrup, Denmark.
Cementula spirolinites (Münster in Goldfuss, 1831)
Fig. 5A–C.
1831 Serpula spirolinites sp. nov.; Münster in Goldfuss 1931: 229, pl. 68: 5a–c.
1956 Serpula (Dorsoserpula) spirolinites Münster, 1831; Parsch 1956: 221, pl. 21: 29.
2004 Cementula spirolinites (Münster in Goldfuss, 1831); Radwańska 2004: 39, pl. 2: 6–8.
2019 Cementula spirolinites (Münster in Goldfuss, 1831); Kočí et al. 2019: 317: 4D.
Material.—122 specimens attached to sponges from the lower Oxfordian (Upper Jurassic) of Zalas, Polish Jura (see Table 1); GIUS 8-3746.
Description.—Tubes up to 100 mm long. Predominantly straight or slightly undulating tube portions alternate with planispiral coils. Tube diameter small (not exceeding 2 mm), almost constant throughout the length of the tube and apparently small also if compared to the diameter of the spiral. Within most spirals, the tube not overgrowing its previous whorls, so that all whorls most often remain visible. About five whorls usually tightly contiguous; however, in some loops the whorls without tight contact and more or less irregularly coiled, and most often with a small open umbilicus in the center of the spiral. Usually, the tubes attached to the substrate by indistinctive basal flanges. A single well-developed median keel present along the entire length of the tube. Transverse elements represented by rare, irregularly occurring alae-type peristomes, strongly developed on top of the tube and less strongly and less often developed in the upper-lateral position where the peristome may resemble a pair of “ears”. However, they stay rather faint outside these positions and close to the basal margins. Apart from the median keel and peristomes, the tube’s external surface smooth and without any additional ornamentation. The cross-section usually rounded-triangular.

Fig. 5. Serpulid polychaetes from the Jurassic of Poland. A–C. Cementula spirolinites (Münster in Goldfuss, 1831), specimen encrusting: sponge fragment from the Oxfordian of Zalas (A, GIUS 8-3746/4; B, GIUS 8-3746/5; C, GIUS 8-3746/6). D. Cementula radwanskae sp. nov., holotype (GIUS 8-3589/7, arrow) encrusting a shell fragment from the Callovian of Zalas; partially encrusting another C. radwanskae, sabellid Glomerula gordialis (Schlotheim, 1820) (white arrowhead), and serpulid Metavermilia cf. striatissima (Fürsich, Palmer, and Goodyear, 1994) (black arrowhead). E–G. Cementula radwanskae sp. nov. encrusting shell fragments from the Callovian of Zalas (E, paratype, GIUS 8-3589/8; F, paratype, GIUS 8-3589/9; G, GIUS 8-3589/10).
Remarks.—Our specimens described above resemble tubes of Spiraserpula oligospiralis Ippolitov, 2007b, in several features: consistent median keel throughout entire length of the tube, alternation of straight portions with contiguous coiled spirals leaving an open umbilicus, and absence of ITS (Internal Tube Structures). However, they differ by the spirals growing larger in diameter and amounting to a considerably higher percentage of the total tube length, as well as by the keel being conspicuous already in the posterior tube portion. The genera Spiraserpula Regenhardt, 1961, and Cementula are hardly distinguishable due to similarities in external appearance, in spite of the fact that Spiraserpula tends to grow to a larger size forming a more complex tube system consisting of alternating straight tube portions and several spirals. A reliable distinction between the genera Spiraserpula and Cementula, as previously mentioned by Pillai (1993) and Pillai and Hove (1994), is based only on the presence of ITS, which are present in Spiraserpula, while in Cementula they are absent. Thus, the principal character allowing distinction between these two genera is the potential ability to form ITS in Spiraserpula. To prevent ambiguous determinations where ITS would be an exclusive feature determining this taxon, Ippolitov (2007b) proposed to consider Cementula as a subgenus of Spiraserpula. Although an ability to form ITS cannot be taken as the only taxonomic feature simply due to taphonomic reasons, possibly except for well-recognizable species within certain stratigraphic intervals (e.g., Cementula spirolinites), in principle this problem seems to refer to younger species than those described here. To the best of our knowledge, until now not a single ITS in tubes of any Spiraserpula species older than Campanian has been found (see Pillai 1993; Pillai and Hove 1994; Jäger 2005).
Stratigraphic and geographic range.—The material studied herein come from lower Oxfordian (Upper Jurassic) of Zalas, Polish Jura. This species was also reported from the Oxfordian (Upper Jurassic) of central Poland (Wapienno Quarry) by Radwańska (2004), Germany (Goldfuss 1831; Parsch 1956), and Czech Republic (Kočí et al. 2019).
Cementula radwanskae sp. nov.
Fig. 5D–G.
Zoobank LSID: urn:lsid:zoobank.org:act:67B0722D-4F88-4DDD-A 813-C090E89B4E54
Etymology: In honor of Urszula Radwańska in recognition of her studies on tube-dwelling polychaetes.
Type material: Holotype: GIUS 8-3589/7 (almost complete coiled tube attached to a bivalve shell fragment, partially encrusting another Cementula radwanskae sp. nov.). Paratypes: GIUS 8-3589/8, GIUS 8-3589/9 (two slightly eroded coiled tubes attached to a bivalve shell fragment). All from type locality and type horizon, see below.
Type locality: Zalas near Krzeszowice, southern Poland.
Type horizon: Middle–upper Callovian (Middle Jurassic).
Material.—33 well-preserved specimens encrusting mainly bivalves from the Middle Jurassic of the Polish Jura (4 from Bolęcin and 29 from the Callovian of Zalas) (see Table 1); GIUS 8-3589, GIUS 8-3745.
Diagnosis.—The tubes forming rather small spirals which are coiled in a compact tight mode. Keels and peristomes lacking. The entire surface covered by delicate wrinkles and corrugations and lines of tiny granules.
Description.—Tubes very small, planispirally coiled. Spirals reaching only up to 3 mm in diameter, usually consisting of up to five whorls. Predominately sinistrally coiled, but both directions of coiling may occur. Rarely, small anterior tube portions uncoiled. All the whorls are tightly adherent to each other. The umbilicus in the center of the spiral is tiny or absent. Tubes are attached to the substrate by their entire length and do not overgrow each other. Tube diameter (less than 0.5 mm) increasing only very slowly or constant in the anterior part. The tubes lacking any strongly developed ornamentation such as keels or peristomes, but usually the entire surface densely covered by somewhat irregular delicate corrugations, wrinkles and lines of tiny granules protruding slightly at the tube’s median line. Cross-section circular or subcircular.
Remarks.—The specimens studied are similar to Cementula cf. circinnalis (Münster in Goldfuss, 1831) occurring in Bajocian–Bathonian (Middle Jurassic) deposits of Ogrodzieniec-Świertowiec, but differ from this species in granulate ornamentation present on a vast majority of Cementula radwanskae sp. nov. specimens and more tightly coiled whorls. Cementula radwanskae sp. nov. bears features somewhat similar to the species Cementula sp. 2 from the Bajocian of Normandy, France, described by Breton et al. (2020), whose spirals, however, may reach more than twice the diameter of the spirals of C. radwanskae sp. nov. Cementula radwanskae sp. nov. differs from C. spirolinites by its much smaller size, compact coiling mode, and universal lack of keels and peristomes. In contrast to C. circinnalis, C. radwanskae sp. nov. possesses delicate wrinkles and corrugations on the entire surface and has more tightly coiled whorls. In contrast to Cementula complanata (Goldfuss, 1831) (see Jäger and Schubert 2008), C. radwanskae sp. nov. possesses occasionally straightened anteriormost tube portion and has delicate ornamentation.
Stratigraphic and geographic range.—The material studied herein comes from upper Bathonian–lower Callovian (Middle Jurassic) of Bolęcin, and Callovian (Middle Jurassic) of Zalas, Polish Jura. Possibly, the species may also occur in the Bajocian of Normandy, France (Breton et al. 2020).
Cementula cf. circinnalis (Münster in Goldfuss, 1831)
Fig. 6A, C.
Material.—38 well-preserved specimens exclusively encrusting oncoids from the upper Bajocian–lower Bathonian (Middle Jurassic) of Ogrodzieniec-Świertowiec, Polish Jura (see Table 1); GIUS 8-3750.
Description.—Tubes very small, planispirally coiled, usually consisting of three to five whorls. Sinistral and dextral spirals occur. The maximum diameter of entire spiral reaching only 3 mm, while the tube diameter does not exceed 0.5 mm. Most of the whorls very tightly coiled; however, in some specimens they are not completely adpressed, and show small chinks left between subsequent whorls. A minute umbilicus occasionally present in the center of the spiral. In some cases, anteriormost tube portions straight and not adhering to the previous whorl. The attachment area sometimes widened at the basal margins. The tube diameter increasing moderately fast in the early ontogenetic stages, but rather constant in the adult tube portions. The external surface of the tubes completely smooth. Cross-section circular to subcircular.
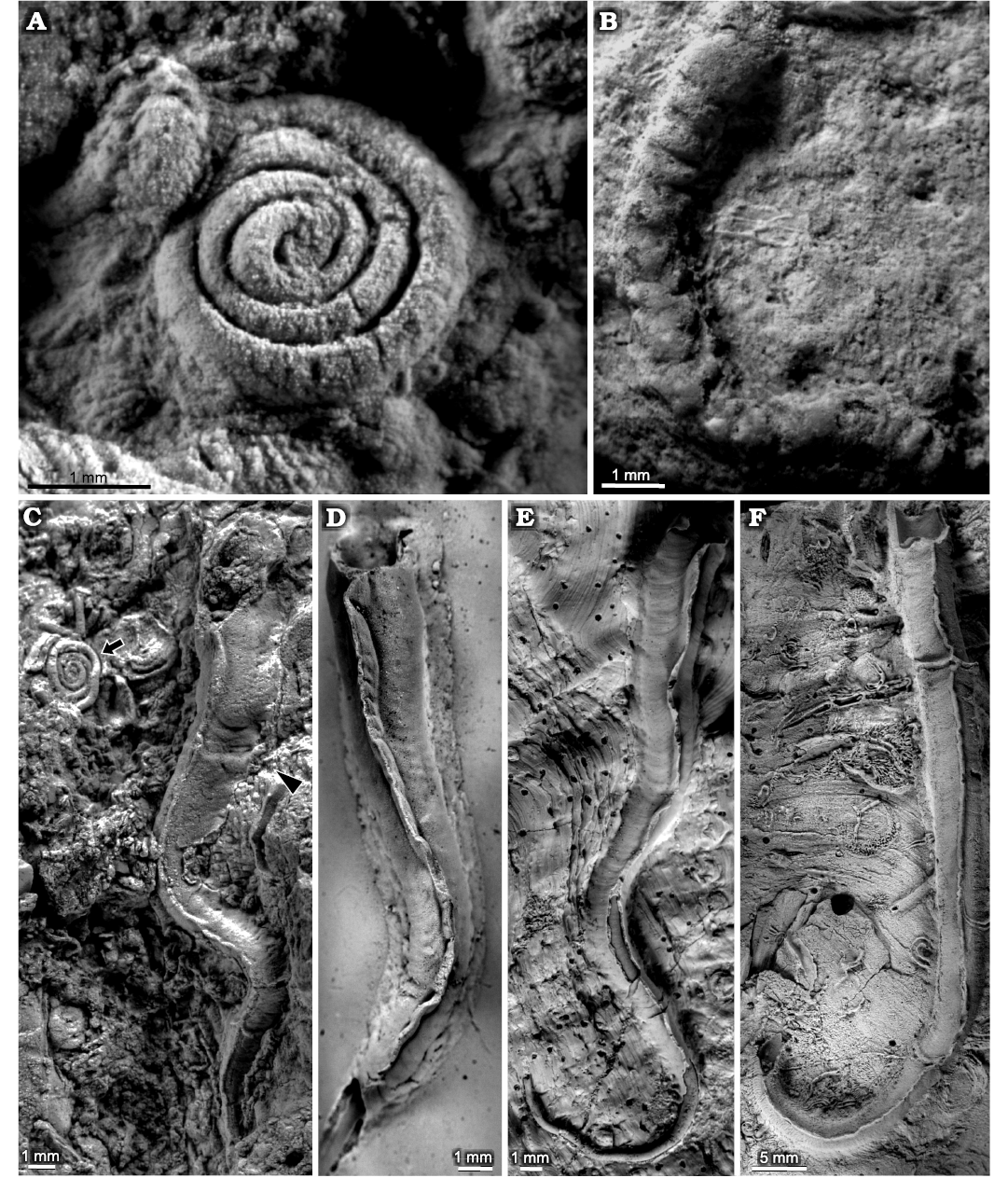
Fig. 6. Serpulid polychaetes from the Jurassic of Poland. A. Cementula cf. circinnalis (Münster in Goldfuss, 1831) encrusting an oncoid from the upper Bajocian–lower Bathonian of Ogrodzieniec-Świertowiec (GIUS 8-3750/4). B. “Serpula cingulata Münster in Goldfuss, 1831” encrusting a sponge fragment from the the Oxfordian of Zalas (GIUS 8-3746/7). C. Propomatoceros lumbricalis (Schlotheim, 1820) (arrowhead) and Cementula cf. circinnalis (arrow) encrusting an oncoid from the upper Bajocian–lower Bathonian of Ogrodzieniec-Świertowiec (GIUS 8-3750/5). D–F. Propomatoceros lumbricalis, specimen encrusting: a piece of a belemnite rostrum from the middle Bathonian of Gnaszyn Dolny (D, GIUS 8-3730/11); an oyster shell from the middle Bathonian of Gnaszyn Dolny (E, GIUS 8-3730/12); a shell fragment from the Callovian of Zalas (F, GIUS 8-3589/11).
Remarks.—In overall shape, these tubes are similar to those assigned to Cementula radwanskae sp. nov.; however, in all cases they differ in having an entirely smooth surface lacking any ornamentation, whereas C. radwanskae sp. nov. possesses granulate ornamentation. Coiling mode is also slightly different in the vast majority of spirals, the tubes are less tightly coiled than in C. radwanskae sp. nov. and straight tube portions in the anterior occur more frequently. Therefore, we consider them separate species. The type of Cementula circinnalis comes from the lower Aalenian (Middle Jurassic; Goldfuss 1831). Another possible affiliation is Cementula filaria (Goldfuss, 1831), but due to the unification of species from two presumably different genera under this name by Goldfuss (1831), the species name “filaria” seems to be not a good choice for these Middle Jurassic forms. Specimens presumably of the same species as those discussed here were described from the Bajocian of Normandy under the name Cementula sp. 1 by Breton et al. (2020).
Genus Serpula Linnaeus, 1758
Type species: Serpula vermicularis (Linnaeus, 1767); Recent, western European seas.
“Serpula cingulata Münster in Goldfuss, 1831”
Fig. 6B.
Material.—Three specimens attached to sponges from the lower Oxfordian (Upper Jurassic) of Zalas, Polish Jura (see Table 1); GIUS 8-3746.
Description.—Tubes relatively short (ca. 10 mm long), attached to the substrate along their entire length. Tube diameter increases slowly, up to 1 mm. Tubes slightly undulate forming delicate curves. Attachment structures absent, although basal parts of the tubes are slightly widened. Ornamentation consisting of thick, densely and regularly spaced, ring-like peristomes present along the whole length of the tube. Longitudinal elements are absent. Cross-section not well-visible; likely subcircular due to the overall shape of the tube.
Remarks.—The name “Serpula cingulata” is derived from Münster in Goldfuss (1831), although the true generic affiliation of the present species to the genus Serpula is unclear.
Stratigraphic and geographic range.—The material studied herein comes from the lower Oxfordian (Upper Jurassic) of Zalas, Polish Jura; present also in the Upper Jurassic sponge facies of Germany (Parsch 1956: 215).
Genus Propomatoceros Ware, 1975
Type species: Propomatoceros sulcicarinata (Ware, 1975); Aptian (Lower Cretaceous), Faringdon, UK.
Propomatoceros lumbricalis (Schlotheim, 1820)
Figs. 2A, 3F, 6C–F, 7A–D.
1820 Serpulites lumbricalis sp. nov.; Schlotheim 1820: 96.
1952 Serpula cf. lumbricalis Schlotheim; Makowski 1952: 4, pl. 2: 2, 3.
1956 Serpula (Dorsoserpula) lumbricalis (Schlotheim) 1820; Parsch 1956: 219, pl. 20: 18, 20.
2007 Propomatoceros lumbricalis (Schlotheim, 1820); Ippolitov 2007b: 432, pl. 12: 1c, 3, 6–8, 9c, 9d.
Material.—840 variably preserved specimens, mainly encrusting oysters and belemnites (31 from Kawodrza Górna, 228 from Gnaszyn Dolny, 81 from Bolęcin, and 149 from the Callovian of Zalas), but also hiatus concretions (21 from Mokrsko, 81 from Bugaj, 33 from Ogrodzieniec, 23 from Krzyworzeka, and 9 from Żarki) and oncoids (156 from Ogrodzieniec-Świertowiec) from the Middle Jurassic of the Polish Jura and Upper Jurassic of Małogoszcz (28) (see Table 1); GIUS 8-3589, GIUS 8-3730, GIUS 8-3745, GIUS 8-3747, GIUS 8-3750, GIUS 8-3751.
Description.—Tubes of different, sometimes large sizes (up to 80 mm long), straight to strongly curved, very rarely forming loops; large and robust tubes dominate in the majority of localities. Tubes grow in diameter (up to 6 mm) at a moderately fast rate. Attachment structures well developed, forming characteristic tubulae, sometimes resulting in a widened tube base; however, in some specimens flanges are less developed. Tubulae are most often divided into chambers which are visible in the abraded tube parts where the irregularly distributed transverse elements occur; hollow tubulae are rare. A prominent median keel on the top of the tube running along its entire length. In the middle and anterior parts, the keel sometimes tends to undulate. Curved alae-type peristomes, most often well developed, occur occasionally at irregular intervals. Growth lines most prominently near the median keel, seldom visible along the whole length of the tube. The tube surface usually smooth, only occasionally covered irregularly by tiny granules. Cross-section depending on the ontogenetic stage, most often being triangular, and subtriangular at early ontogenetic stages, and becoming more subcircular at later stages, with the lateral walls becoming more convex, and the longitudinal keel on top only delicately marked.
Remarks.—Among fossil serpulids, Propomatoceros is one of the most common, geographically widespread and geologically long-ranging genera. Its occurrence in Jurassic and Cretaceous deposits combined with conservative morphology, which varies intraspecifically and depending on palaeoenvironment and ontogeny, makes reliable species determinations within this genus remarkably difficult. The species name Serpulites lumbricalis is historically the oldest available species name which can be included into Propomatoceros. It was introduced by Schlotheim (1820) for Middle and Late Jurassic species, but unfortunately without providing any figure or type specimen. Nevertheless, as this is the oldest available name, we decided to use it, including also some informal names such as e.g., forma “limax”, which Goldfuss (1831) had introduced as a species name for Bajocian (Middle Jurassic) serpulids from Southern Germany. In contrast to Schlotheim (1820), Goldfuss (1831) provided figures and more detailed information on several species which now can be included into Propomatoceros. Parsch (1956) and Ippolitov (2007b) validated the species status of Promopatoceros lumbricalis, and they considered forma “limax” of Goldfuss (1831) as its subjective synonym.
Considering the difficulties in proposing a morphologically well-defined species concept for this genus with a well-defined stratigraphic range, here, we consider all specimens having a similar suite of features as belonging to a single species Propomatoceros lumbricalis. Although the specimens are slightly variable within certain populations, they display rather congruent morphology as all the easily-distinguishable features of species of Propomatoceros, such as e.g., distinctive keel which may vary inter- and intraspecifically, and ontogenically. Thus, we regard such small-scale morphological differences which might have led to the introduction of separate species as a result of intraspecific, palaeoenvironmentally controlled changes within a given population. However, for the sake of coherence, we use such taxonomic names as forma “limax” and forma “conformis” (both of Goldfuss 1831) only as names of particular morphotypes. In fact, a part of the studied specimens of Propomatoceros lumbricalis might have been represented by the genuine representatives of Propomatoceros limax (Goldfuss, 1831) and Propomatoceros conformis (Goldfuss, 1831).
Propomatoceros lumbricalis sensu stricto differs from the forma “limax” by its more prominent keel, better developed but less common peristomes, and smooth surface, as well as less convex lateral walls due to a usually faster growth. It differs from forma “conformis” in the more prominent and rather more undulating median keel and in the more rounded cross-section. Our specimens also exhibit a striking morphological resemblance to this species as described and figured by Ippolitov (2007b; see also Słowiński et al. 2020).
Further detailed studies are needed concerning the affinity and the true systematic position of the genus Propomatoceros within the “Spirobranchus group”, as well as a reliable intrageneric division of the genus. Detailed morphological studies engaging statistical methods within and between certain groups of Propomatoceros serpulids inhabiting different palaeoenvironments, both different-aged and coeval, together with microstructural data of different morphotypes will possibly help to solve this problem. Perhaps, it will allow to draw a proper universal concept of Jurassic species of Propomatoceros. All in all, at the moment it is not possible to provide unambiguous determination of species, proper differentiation between them, and exact stratigraphic ranges of all Propomatoceros fossils studied herein.
Stratigraphic and geographic range.—The material studied herein comes from the middle Bathonian–Callovian (Middle Jurassic) of the Polish Jura, and lower Kimmeridgian (Upper Jurassic) of the Mesozoic margin of the Holy Cross Mountains (Małogoszcz). This species was also reported from the Middle and Upper Jurassic of Germany (Schlotheim 1820; Parsch 1956) and the Middle Jurassic of Central Russia (Ippolitov 2007b).
Propomatoceros sp. 1
Figs. 7E, 8A, B.
Material.—Five variously preserved specimens, four of which encrust oyster shells and one encrusting a hiatus concretion from the upper Bajocian (Middle Jurassic) of Mokrsko and the middle Bathonian (Middle Jurassic) of Gnaszyn Dolny, Polish Jura (see Table 1); GIUS 8-3730, GIUS 8-3751.
Description.—Tubes large (up to 60 mm long) and robust, strongly curved or serpentine, rarely forming loops, but also with straight portions. Tube diameter growing moderately slowly and reaching up to 4 mm. Uneroded tube parts with an indistinct median keel presumably present along the entire tube length and forming only a slightly marked denticle above the aperture. Tubes with densely and regularly spaced, chevron-shaped transverse growth lines; interspaces between the growth lines only slightly wider than the width of growth lines. Common but indistinct alae-type peristomes occur at irregular intervals. The shape of growth lines and peristomes is identical, but peristomes are about twice as prominent as growth lines. Cross-section subtriangular to subcircular due to strongly convex walls in later ontogenetic stages. The tube wall is thick and often breaks along the boundary between its two layers.
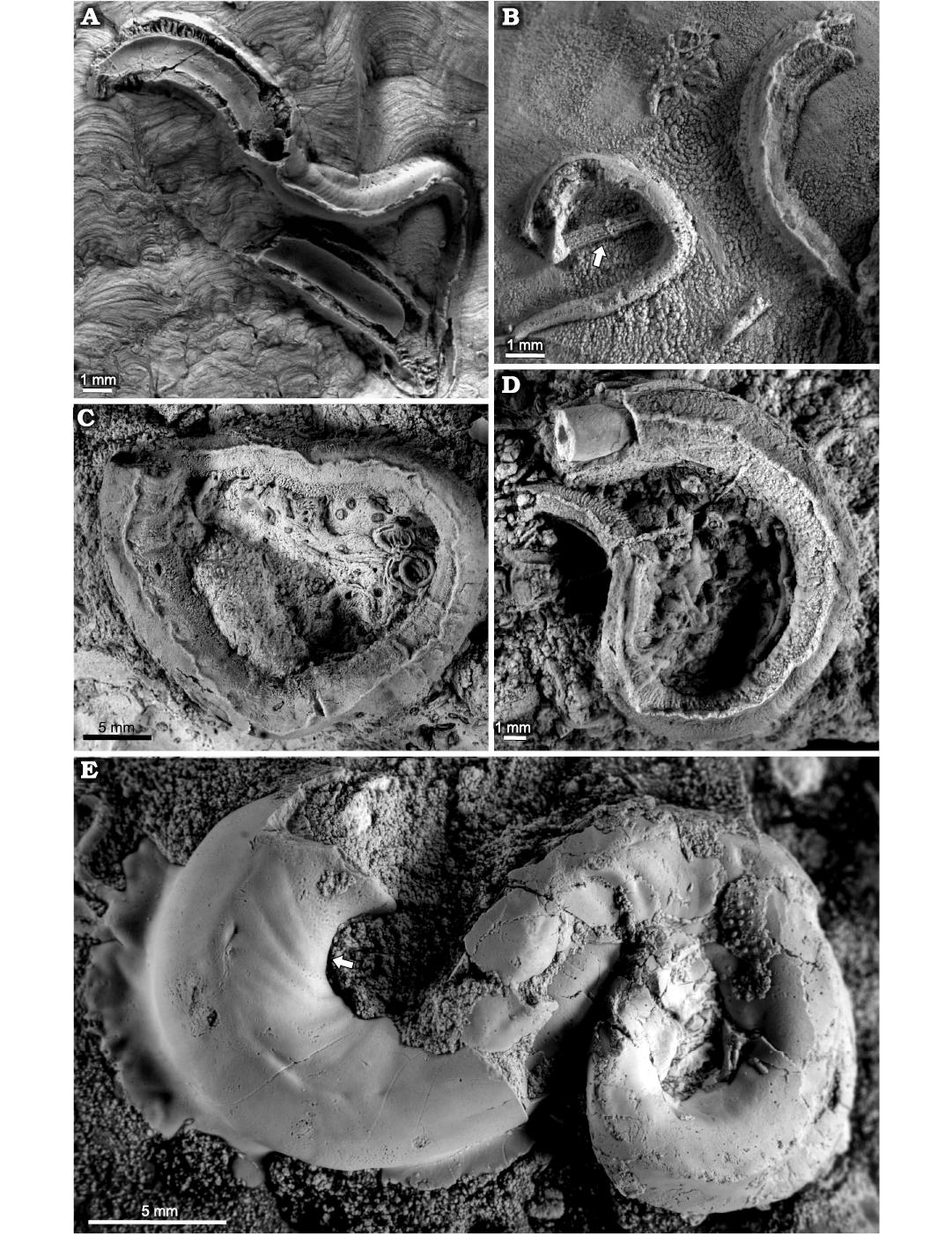
Fig. 7. Representatives of the serpulid polychaete Propomatoceros spp. from the Jurassic of Poland. A. Partially eroded Propomatoceros lumbricalis (Schlotheim, 1820) encrusting an oyster shell from the middle Bathonian of Gnaszyn Dolny (GIUS 8-3730/13); eroded tube fragments show well-developed tubules, which are divided into chambers by densely spaced septa. B. Two specimens of Propomatoceros lumbricalis forma “conformis” and partially preserved, straight, tiny serpulid Metavermilia cf. striatissima (Fürsich, Palmer, and Goodyear, 1994) (arrow) inside an unclosed loop of P. lumbricalis encrusting a shell fragment from the upper Bathonian–lower Callovian of Bolęcin (GIUS 8-3745/2). C, D. Propomatoceros lumbricalis forma “limax” encrusting shell fragments from the Callovian of Zalas (C, GIUS 8-3589/12; D, GIUS 8-3589/13). E. Robust Propomatoceros sp. 1 encrusting a hiatus concretion from the upper Bajocian of Mokrsko (GIUS 8-3751/5); the arrow points to the delicate growth lines.

Fig. 8. Representatives of the serpulid polychaete Propomatoceros spp. from the Jurassic of Poland. A, B. Propomatoceros sp. 1 detached from fragmented shell from the middle Bathonian of Gnaszyn Dolny (A, GIUS 8-3730/14, B, GIUS 8-3730/15). C. Propomatoceros sp. 2 encrusting a shell fragment of Ctenostreon proboscideum (Sowerby and Sowerby, 1820) from the Callovian of Zalas (GIUS 8-3745/3); top (C1) and lateral (C2) views. Note the anterior tube part overgrowing older portion of the tube and rising above the substrate (C2). D. Propomatoceros sp. 3 encrusting a small piece of a shell from the Kimmeridgian of Małogoszcz (GIUS 8-3747/2).
Remarks.—The specimens studied superficially resemble Propomatoceros semicostatus (Regenhardt, 1961) sensu Luci et al. (2013) in their large size, strong, characteristic ornamentation, and typical coiling. However, the type specimen figured by Regenhardt (1961) is smaller than Propomatoceros sp. 1 studied herein and more straight. In addition, the type specimen of Propomatoceros semicostatus as well as the tubes described by Luci et al. (2013) were found in Lower Cretaceous deposits; therefore, it is rather unlikely that our specimens belong to P. semicostatus. Our tubes may represent a new species; however, due to the highly limited number of specimens, we refrain from designation of the new species.
Propomatoceros sp. 2
Fig. 8C.
Material.—One specimen encrusting a small shell fragment of Ctenostreon proboscideum (Sowerby and Sowerby, 1820) from the Callovian (Middle Jurassic) of Zalas, Polish Jura (see Table 1); GIUS 8-3589.
Description.—The tube diameter reaching 2.5 mm, but the diameter of the entire tightly coiled specimen not exceeding 10 mm. Posterior tube parts planispirally coiled and attached to the substrate along their entire length, the anterior part of the tube overgrows older portions, forming a loop with an open but narrow umbilicus, and rising above the substrate. The tube robust and possessing a not very high but consistent, only slightly undulating median keel. Otherwise the surface smooth. The base not distinctly widened; however, attachment structures visible due to expansion of the lowermost tube parts. Lateral walls distinctly convex and the tube is delicately flattened, resulting in a subtriangular or even almost circular cross-section and slightly lowered lateral sides below the median keel.
Remarks.—The single tube is assigned to the Propomatoceros due to its low but distinctive keel and relatively large size. The specimen is characterized by a kind of tight coiling, which, although not common, is not so rare in Propomatoceros.
Propomatoceros sp. 3
Fig. 8D.
Material.—One specimen attached to a bivalve shell from the lower Kimmeridgian (Upper Jurassic) of Małogoszcz, the Mesozoic margin of the Holy Cross Mountains, Poland (see Table 1); GIUS 8-3747.
Description.—The tube large, robust, and significantly increasing in diameter (up to 4 mm). The entire specimen coiled and forms a loop. A very prominent, slightly undulating keel on the top of the tube, which results in its subtriangular cross-section. Otherwise, the surface completely smooth, lacking any ornamentation. Flanges are very well-developed.
Remarks.—The specimen is assigned to the genus Propomatoceros because of the large size, the very distinctive keel and very well-developed flanges. Nevertheless, it seems to differ from other species of Propomatoceros by its completely smooth surface lacking any growth lines or ornamentation except for the keel.
Genus Nogrobs Montfort, 1808
Type species: Nogrobs vermicularis Montfort, 1808, Middle Jurassic (presumably Stephanoceras humphriesanum Zone of the Bajocian), Muttenz, Switzerland.
Nogrobs aff. quadrilatera (Goldfuss, 1831)
Fig. 9A–C.
Material.—36 specimens, the majority of which are well-preserved, encrusting belemnite rostra from the middle Bathonian (Middle Jurassic) of Gnaszyn Dolny, Polish Jura (see Table 1); GIUS 8-3730.
Description.—Tubes medium-sized (up to 25 mm long), straight to slightly curved, adjusting to the available solid substrates to which they are attached: small to medium-sized, but relatively long belemnite rostra. Tubes growing relatively fast in diameter in the early ontogenetic stages where some of the specimens forming either a loose or a tight spiral, whereas in the adult anterior tube portions increase in diameter (up to 1.5 mm) slowly or very slowly. The tube base sometimes delicately widened and rarely possesses hollow flanges, which are visible in a few cases where the tubes are partly worn out. Tubes distinctly flattened on top and have three median longitudinal crests, of which the two marginal ones more conspicuous than the central one, which is faint or barely present. The vast majority of specimens attached to the substrate along their entire length; only in a few tubes the anterior portions raised above a substrate. Transverse ornamentation represented by well-visible, regular growth lines which are especially well-developed between the keels. Weakly developed nodular peristomes occasionally occur. Lateral walls nearly parallel, resulting in a subquadrangular to subcircular cross-section, in some specimens slightly convex in profile. The tube wall composed of two layers.
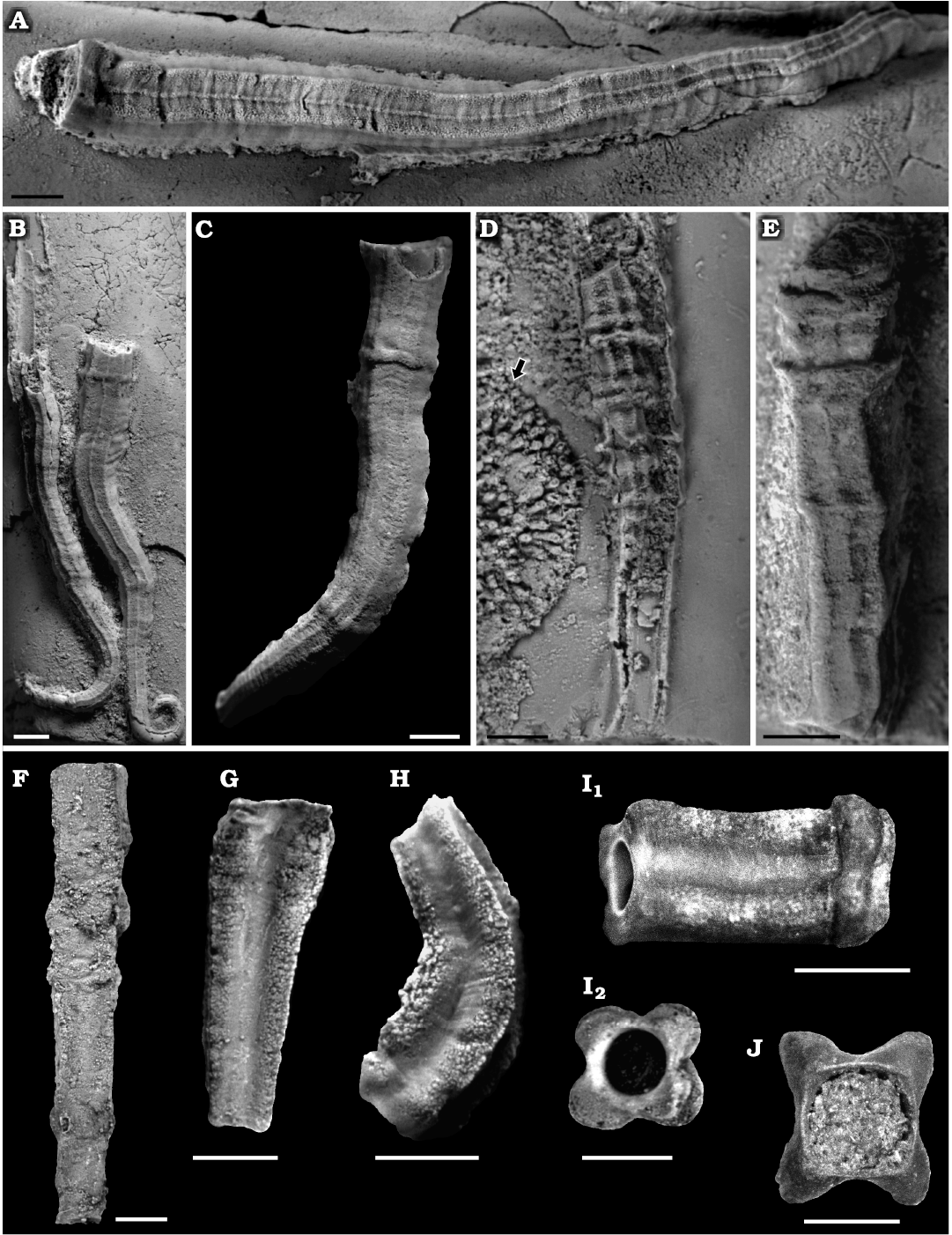
Fig. 9. Representatives of the serpulid polychaete Nogrobs spp. from the middle Bathonian (Middle Jurassic) of Gnaszyn Dolny, Poland. A–C. Nogrobs aff. quadrilatera (Goldfuss, 1831) encrusting belemnite rostra (A, GIUS 8-3730/16; B, GIUS 8-3730/17; C, GIUS 8-3730/18); the specimen in C has been partially detached from its substrate. D, E. Nogrobs? aff. tricristata (Goldfuss, 1831) encrusting belemnite rostra (D, GIUS 8-3730/19; E, GIUS 8-3730/20); ctenostomate bryozoan colony indicated by arrow in D. F–J. Free-lying tubes of Nogrobs aff. tetragona (Sowerby, 1829) (GIUS 8-3730/21–25, respectively); lateral (I1) and cross-section (I2) view; I2 shows characteristic quadrangular cross-section with distinctly concave walls between the edges. Notice characteristic quadrangular cross-section with distinctly concave walls between the edges visible in J. Scale bars 1 mm.
Remarks.—The tubes are assigned to Nogrobs aff. quadrilatera (Goldfuss, 1831) because their cross-section, shape and ornamentation are characteristic for this species. Similarly to our specimens, N. quadrilatera sensu stricto has a flattened upper side, three small median keels and a subquadrangular cross-section. However, unlike in species of Nogrobs from many other localities (e.g., Germany, England), the broken-off, free tube portions which originally rose above the substrate are relatively rare in our materials. Our tubes somewhat resemble Nogrobs tricarinata (Goldfuss, 1831) (see Parsch 1956: 224, pl. 21: 21). Moreover, Serpula tricarinata (Goldfuss, 1831), is a junior homonym of Serpula tricarinata Sowerby, 1829, the latter correctly affiliated to Mucroserpula by Ippolitov (2007b). To replace Goldfuss’ (1831) invalid junior homonym, Ippolitov (2007a) proposed a new combination Metavermilia goldfussi. Nogrobs aff. quadricarinata reported by Vinn et al. (2014: fig. 4G, H) bears similar growth lines to those present in our specimens; however, these are less visible.
Nogrobs? aff. tricristata (Goldfuss, 1831)
Fig. 9D, E.
Material.—Eight, mostly well-preserved specimens encrusting belemnite rostra from the middle Bathonian (Middle Jurassic) of Gnaszyn Dolny, Polish Jura (see Table 1); GIUS 8-3730.
Description.—Tubes medium-sized (up to 20 mm long), straight or only slightly undulating; however, in two cases they form a loose loop in the early ontogenetic stages. The upper surface flattened and bears three consistent longitudinal keels. The central keel rather weakly developed, the lateral two are more distinct. All three keels well-visible and present in all specimens along the entire tube length. The lateral walls nearly parallel. The tubes with many irregularly distributed, flaring peristomes which in some specimens slightly protrude at the keels to form short and blunt spines. In some specimens, after forming a sharp, angular folding, the anterior tube part projecting upwards and rising above the substrate. In the anteriormost tube part, walls slightly concave. Prominent and regular growth lines covering the flattened upper surface between the lateral keels. Perpendicular growth lines well-visible also on the lateral tube sides. The tubes gently curved, quadrangular in cross-section, and slowly growing in diameter up to 1 mm. The tube wall composed of two layers.
Remarks.—The specimens studied are tentatively assigned to Nogrobs because of their overall shape, quadrangular cross-section and perpendicular growth lines. However, these specimens may also be referred to Filogranula due to a characteristic rising above substrate in the anterior part, often slightly flaring and weakly spiny peristomes, and three consistent keels running along the entire tube, although the keels are not denticulate. Specimens discussed here are also similar in general shape to Nogrobs aff. quadrilatera described above and might also be a variation of that species from the same locality; however, differences comprise flaring and more frequent peristomes, three better developed keels, and parallel or delicately concave lateral walls (see Parsch 1956: 225, pl. 19: 17). Filogranula tricristata (Goldfuss, 1831) described from Toarcian–Aalenian (Lower–Middle Jurassic) deposits of Germany has occasionally widened base of the tubes resulting in the trapezoidal cross-section (MJ own observations), which clearly differs from the tubes described here. Moreover, Filogranula tricristata is attached to the substrate along its entire length, while the specimens discussed herein have their anterior portions occasionally raised up. This supports the distinction between the studied specimens from Poland and the typical Filogranula tricristata from the Toarcian and Aalenian from Germany.
Nogrobs aff. tetragona (Sowerby, 1829)
Fig. 9F–J.
Material.—29 specimens from the middle Bathonian (Middle Jurassic) of Gnaszyn Dolny, Polish Jura (see Table 1); GIUS 8-3730; three of the specimens encrust two belemnite rostra and the rest are free-lying/detached.
Description.—Tubes small (up to 10 mm long), almost straight or only slightly curved. Nearly all free-lying; only three partially attached to the substrate. Only the attached posterior tube portion bearing a small median keel on the tube’s upper side, whereas the free anterior portion with no median keel. No growth lines visible except those on the lateral walls. Peristomes occasionally occurring; they consist of four thick nodes situated at the edges of the square. The tube diameter expanding very slowly, except for the anterior end where it (up to 1 mm) increases more abruptly. The most distinctive character of the tube, especially of its free anterior portion, is the quadrangular cross-section, with all the walls markedly concave between the edges.
Remarks.—Nogrobs tetragona was described from claystone of late Oxfordian–early Kimmeridgian age in England, and is characterized by masses of densely entangled clusters of tubes (Sowerby 1829), which are not attached to the substrate, lack any attachment scars and never tend to form a compact spiral in the posterior tube part (MJ own observations). However, N. tetragona has been used in the literature in a wider sense (e.g., Sowerby 1829; Gerasimov 1955; Ippolitov 2007a) for predominantly free tube portions, which lack or possess only inconspicuous, rare peristomes with a quadrangular cross-section, found in many localities from the Middle and Upper Jurassic of England (Sowerby 1829) and Germany (Parsch 1956). The tubes described here match N. tetragona well, if it is understood in that wider sense. The specimens described show a very close resemblance to “Serpula (Tetraserpula) tetragona” (see Parsch 1956: 223, pl. 21: 14), “Tetraserpula tetragona” (see Ippolitov 2007a), and to a lesser extent to “Serpula (Tetraserpula) quadrisulcata” (see Parsch 1956: 227, pl. 21: 15), which bears more prominent and sharp margins.
Genus Mucroserpula Regenhardt, 1961
Type species: Mucroserpula mucroserpula (Regenhardt, 1961); Hauterivian (Lower Cretaceous), Schandelah, north Germany.
Mucroserpula tricarinata (Sowerby, 1829)
Fig. 10A.
1829 Serpula tricarinata sp. nov.; Sowerby 1829: 226, pl. 608: 3, 4.
1956 Serpula (Tetraserpula) quinquangularis Goldfuss 1831; Parsch 1956: 224, pl. 19: 9, pl. 20: 13, pl. 21: 25.
2007 Mucroserpula tricarinata (J. de C. Sowerby, 1829); Ippolitov 2007b: 429, pl. 12: 1a, 1b, 2.
Material.—Two partially preserved specimens attached to bivalve shells from the Callovian (Middle Jurassic) of Zalas, Polish Jura (see Table 1); GIUS 8-3589.
Description.—Tubes medium-sized (less than 20 mm long), curved in a loose loop, moderately increasing in diameter (ca. 1 mm). The tubes possessing a consistent, slightly undulating median keel and two weaker lateral keels. Delicate, perpendicular growth lines visible along most of the tubes’ length. Specimens are attached to the substrate along their entire length, and the tube base is widened, resulting in a triangular to subtriangular cross-section; however, the anteriormost parts are pentagonal due to the three keels and the edges of the base.
Remarks.—The specimens described are assigned to the genus Mucroserpula due to their characteristic three keels and the mode of coiling. In spite of the fact that the tubes are not very well-preserved, the features indicative of Mucroserpula are sufficiently visible. Our specimens are small compared to other specimens of Mucroserpula tricarinata, presumably representing juveniles. Mucroserpula jaegeri Radwańska, 2004, from the lower Kimmeridgian (Upper Jurassic; see Wierzbowski et al. 2016) of central Poland differs from M. tricarinata by its very regular spiral coiling: the posterior tube portion forms a tightly coiled spiral, whereas the anterior, which is still attached all along its length, forms a wide open spiral curve. Moreover, M. jaegeri has weaker developed lateral keels and a more distinctly flattened tube.
Stratigraphic and geographic range.—The material studied here come from Callovian (Middle Jurassic) of Zalas, Polish Jura. Mucroserpula tricarinata was also reported from the Middle and Upper Jurassic of England (Sowerby 1829), Germany (Parsch 1956), and Russia (Ippolitov 2007b).
Mucroserpula? sp.
Fig. 10B.
Material.—Two specimens attached to an oyster shell and a belemnite from the middle Bathonian (Middle Jurassic) of Gnaszyn Dolny, Polish Jura (see Table 1); GIUS 8-3730.
Description.—Tubes relatively small, up to 15 mm long, increase slowly in diameter (up to ca. 1 mm), straight and attached to the substrate along their entire length. Tubes with three slightly developed keels, of which the median one may be slightly undulating and with very delicate alae-type peristomes forming a short denticle above the aperture. The tube surface slightly rough and its base only gently widened. Cross-section pentagonal.
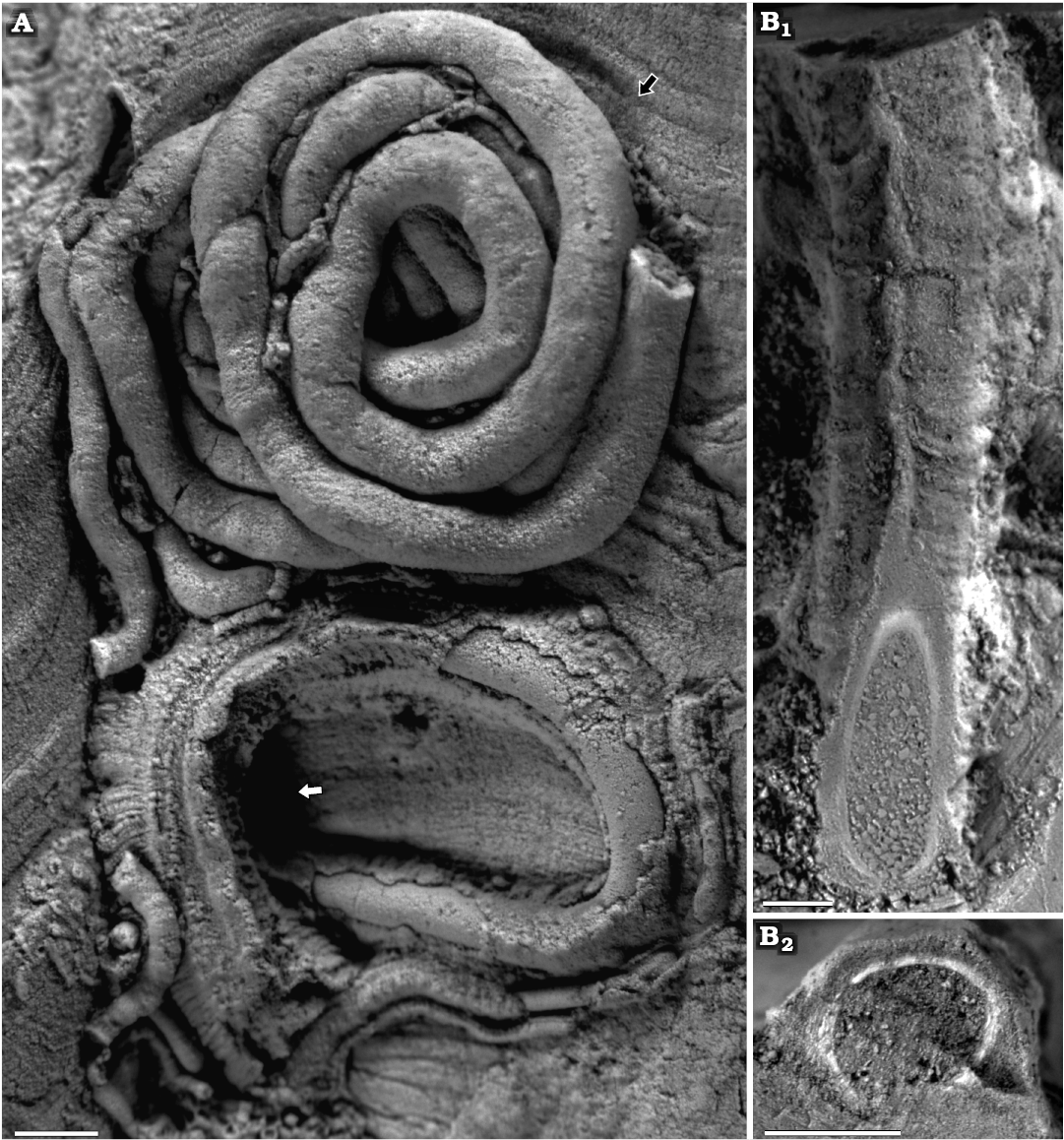
Fig. 10. Serpulid and sabellid polychaetes from the Jurassic of Poland. A. Mucroserpula tricarinata (Sowerby, 1829) (white arrow) and sabellid Glomerula gordialis (black arrow) encrusting a fragment of a shell from the Callovian of Zalas (GIUS 8-3589/14). B. Mucroserpula? sp. encrusting a fragment of a shell from the middle Bathonian of Gnaszyn Dolny (GIUS 8-3730/26); top (B1) and cross-section view (B2). Notice the characteristic pentagonal cross-section (B2) due to the presence of three keels. Scale bars 1 mm.
Remarks.—The specimens investigated are tentatively assigned to Mucroserpula due to their median and supralateral keels and resulting pentagonal cross-section. However, due to their slightly undulating median keel and relatively small size, the tubes may also belong to the genus Filogranula. Apart from the distinctive pentagonal cross-section and a short denticle marked above the aperture, both typical for the species of Mucroserpula, these tubes also resemble those of species of Propomatoceros from the same locality. The somewhat artificial classification of strictly single-keeled Propomatoceros and three-keeled Mucroserpula is not completely satisfactory, and it is highly subjective where to put a boundary between these genera. Cross-section and appearance of the keels may vary during ontogeny, making clear designations difficult. Additionally, diagenetic compression may also change the curvature and general outline. Although species of Mucroserpula are usually described as forming loop-like tubes (e.g., Ippolitov 2007b), coiling mode cannot be a primary indicative feature as the coiling may be facultative and strongly dependent on the kind of substrate and restriction of space for winding and coiling. Finally, the number of specimens described here is limited, hampering proper and unambiguous determination.
Genus Placostegus Philippi, 1844
Type species: Serpula tridentata (Fabricius, 1779); Recent, Greenlandic part of the Arctic Ocean.
Placostegus planorbiformis (Münster in Goldfuss, 1831)
Fig. 11A.
1831 Serpula planorbiformis sp. nov.; Münster in Goldfuss 1831: 231, pl. 68: 12a, b.
1956 Serpula (Tetraserpula) planorbiformis Münster 1831; Parsch 1956: 225, pl. 19: 10, pl. 20: 1, 2.
Material.—Seven relatively well-preserved specimens encrusting sponges from the lower Oxfordian (Upper Jurassic) of Zalas, Polish Jura; GIUS 8-3746.
Description.—Tubes medium-sized, planispirally (sinistrally) and tightly coiled; diameter of the tube up to 2.5 mm, while the diameter of the entire spiral is ca. 10 mm. Spiral leaves only a narrow but deep central umbilicus open; however, coiling is evolute, leaving parts of the inner whorls well visible and countable. The tubes consist of four whorls increasing moderately rapidly in diameter. Tubes attached to the substrate along most of their length, except for the anterior part which may be attached predominantly to the previous whorl. The tube base widened. Cross-section subtriangular due to the presence of an explicit keel running uniformly along the entire tube length and forms a spine above the aperture. The anteriormost part projecting upwards; possibly it grew vertically (see Radwańska 2004); however, this is not evident in the material studied. The tube wall thick.
Remarks.—The studied specimens are very similar to those described by Radwańska (2004) as Placostegus conchophilus (Radwańska, 2004). However, P. conchophilus possesses a rather convex outer wall, whereas in P. planorbiformis the cross-section is roof-shaped, topped by a low, consistent keel.
Stratigraphic and geographic range.—The material studied herein comes from lower Oxfordian (Upper Jurassic) of Zalas, Polish Jura. P. planorbiformis is also common in the Oxfordian (Upper Jurassic) of South Germany (e.g., Parsch 1956; MJ own observation).
Genus Pseudovermilia Bush, 1907
Type species: Spirobranchus occidentalis (McIntosh, 1885); Recent, The Bermuda Archipelago.
Pseudovermilia sp.
Fig. 11B.
Material.—Four tubes attached to rock fragments from the Callovian (Middle Jurassic) of Zalas, Polish Jura (see Table 1); GIUS 8-3589.
Description.—Tubes long (up to 50 mm), rather flat, irregularly curved, having nearly constant diameter (ca. 1 mm). Ornamentation composed of closely and regularly spaced transverse elements subdivided in the middle by a slightly thicker longitudinal crest running through the entire tube length, and two similar longitudinal crests situated at the lateral margins of the flat upper side. The combination of transverse and longitudinal ornamentation results in a regular, reticular pattern which is disturbed only by larger, slightly nodular peristomes. Some of the transverse elements are slightly curved forward near the longitudinal crests, but this curvature is not always well-developed. Structures resembling tubulae usually present within basal flanges occur uniformly throughout the entire tube.
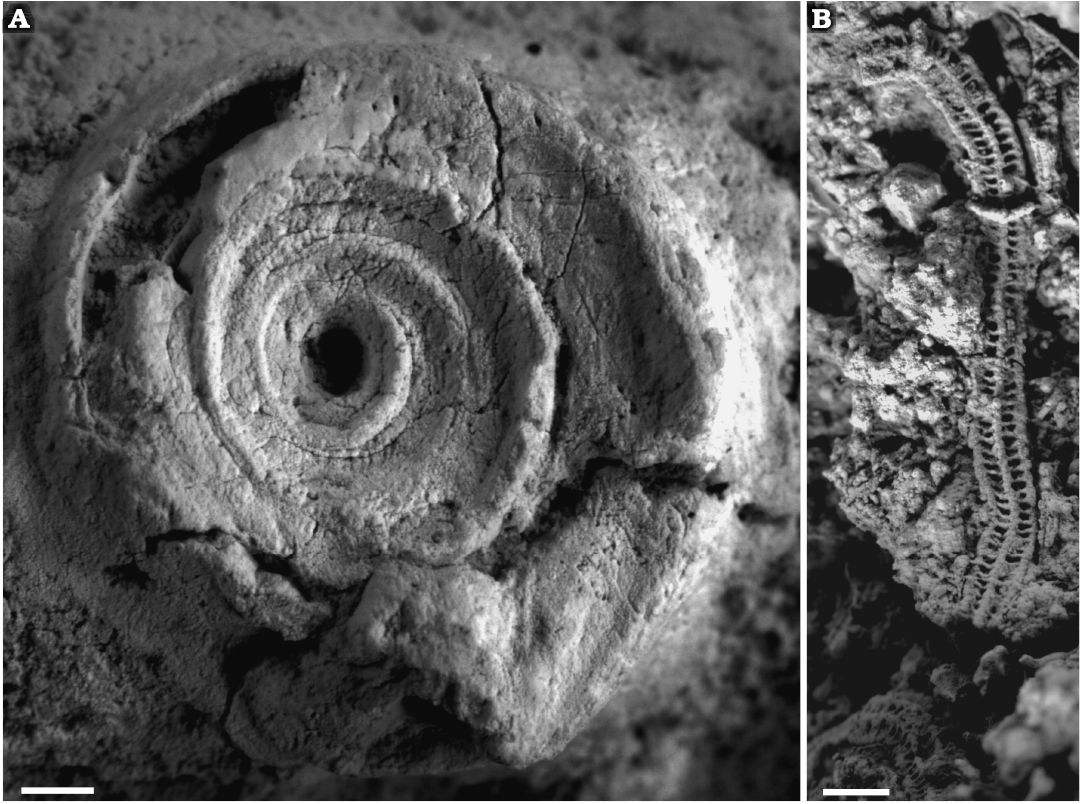
Fig. 11. Serpulid polychaetes from the Jurassic of Poland. A. Placostegus planorbiformis (Münster in Goldfuss, 1831) encrusting a sponge fragment from the Oxfordian of Zalas (GIUS 8-3746/8). B. Pseudovermilia sp. encrusting a rock fragment from the Callovian of Zalas (GIUS 8-3589/15). Scale bars 1 mm.
Remarks.—The four specimens studied are assigned to the genus Pseudovermilia due to their very distinctive, reticulate tube morphology (see Hove 1975; Jäger and Lang 2017). In the Mesozoic, Pseudovermilia occurs very rarely. The genus was previously reported by Jäger and Lang (2017) from the Kimmeridgian (Upper Jurassic), based on a single specimen only. Because of a very limited material, scarce reports, and scarce occurrences, we are unable to provide a reliable specific name. It is possible that our specimens represent a new species. To the best of our knowledge this is the first report of this genus from Poland.
Serpulidae sp. 1
Fig. 12A, B.
Material.—71 specimens attached to bivalve shells (and moulds to a lesser extent), and oncoids from the upper Bajocian–lower Bathonian (Middle Jurassic) of Ogrodzieniec-Świertowiec (23), upper Bathonian–lower Callovian (Middle Jurassic) of Bolęcin (5), and Callovian (Middle Jurassic) of Zalas (43), Polish Jura (see Table 1); GIUS 8-3589, GIUS 8-3745, GIUS 8-3750.
Description.—Tubes small to medium-sized (up to 100 mm long). Tubes are morphologically diverse, ranging from almost straight, gently curved to more strongly coiled. Tube diameter (slightly exceeding 1 mm) almost constant in the adult part. Depending on the specimen, tubes may be either smooth without any ornamentation, except for well-developed, irregularly scattered ampullacea-type to slightly nodular peristomes, or bear some corrugations and/or striae on the tube’s outer surface. On the upper side of some of the tubes a faint, crest-like keel present, as well as two longitudinal, lateral edges on the marginal parts of the tube. The tube attached to the substrate by its entire length with minor flanges. The tube base seems to be even narrowed below the lateral margins which are directed outwards. All the specimens are distinctly flattened which results in the rounded-rectangular, or at least angular, to subcircular cross-section.
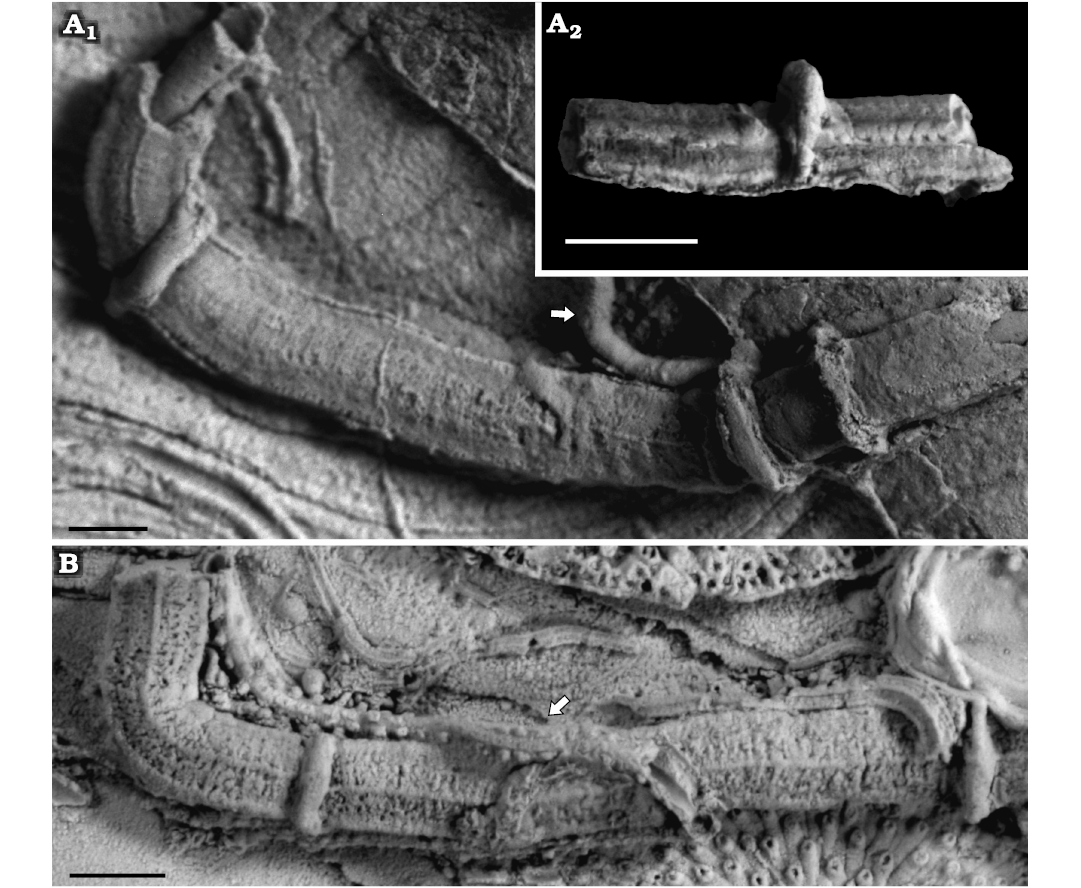
Fig. 12. Serpulidae sp. 1 from the Jurassic of Poland. A. Serpulidae sp. 1 and a tiny Glomerula gordialis (Schlotheim, 1820) (arrowed) encrusting a shell fragment from the upper Bathonian–lower Callovian of Bolęcin (GIUS 8-3745/4); top (A1) and lateral (A2) view; A2 shows a flattened shape of the tube. B. Serpulidae sp. 1 and a tiny, presumably juvenile Filogranula runcinata (Sowerby, 1829) (above, arrowed) encrusting a shell fragment from the Callovian of Zalas (GIUS 8-3589/16). Scale bars 1 mm.
Remarks.—The general shape of the tubes (irregularly curved, of nearly constant diameter and substantial length, bearing ampullacea-type to slightly nodular peristomes and longitudinal ornamentation) is similar to the Pseudovermilia sp. described above. The difference is the intensity of the reticulate ornamentation, which is very prominent in Pseudovermilia sp., whereas in the Serpulidae sp. 1 it is less pronounced, or even occasionally lacking in some parts of the tubes. The reticulate ornamentation of Recent tubes of Pseudovermilia may be very variable, but remains strong (Hove 1975), which rather excludes our specimens from this genus. Alternatively, at least a part of the tubes may represent a species of either Filogranula or Nogrobs with relatively well-developed transverse ornamentation.
Serpulidae sp. 2
Fig. 13A.
Material.—One unattached and partially eroded specimen from the middle Bathonian (Middle Jurassic) of Gnaszyn Dolny, Polish Jura (see Table 1); GIUS 8-3730.
Description.—The tube is thick and robust, 7 mm in diameter, less than 16 mm long, consisting of a small attached and a short free tube portion. bearing irregular but prominent ampullacea-type peristomes. The specimen bearing some well-visible, perpendicular growth lines. Cross-section circular, wall composed of two layers.
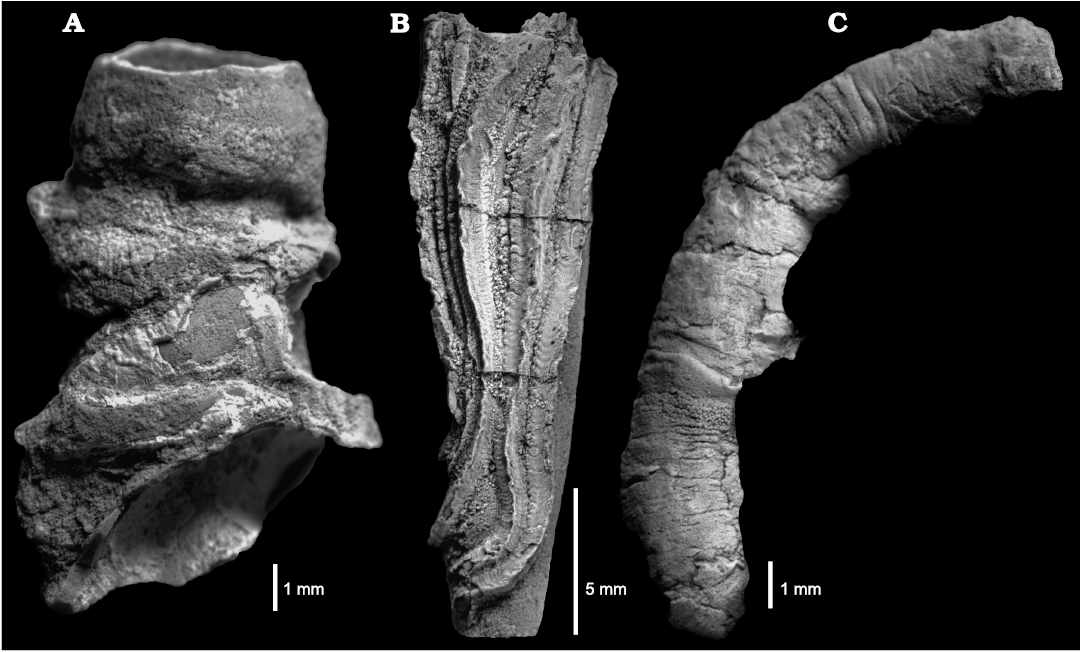
Fig. 13. Serpulid polychaetes from the Jurassic of Poland. A. Unattached Serpulidae sp. 2 from the middle Bathonian of Gnaszyn Dolny (GIUS 8-3730/27). B. Dense aggregation of closely spaced Serpulidae sp. 3 encrusting a fragment of a belemnite rostrum from the middle Bathonian of Gnaszyn Dolny (GIUS 8-3730/28). C. Serpulidae sp. 4 from the lower Kimmeridgian of Małogoszcz (GIUS 8-3747/3).
Remarks.—The specimen shows some superficial resemblance to Neovermilia ampullacea (Sowerby, 1829) (Jäger 1983: 41, pl. 5: 3, as “Proliserpula ampullacea”) due to distinctive, thick, bulge-like ampullacea-type peristomes and circular cross-section.
Serpulidae sp. 3
Fig. 13B.
Material.—Ten specimens attached to belemnite rostra from the middle Bathonian (Middle Jurassic) of Gnaszyn Dolny, Polish Jura (see Table 1); GIUS 8-3730.
Description.—Tubes medium-sized (ca. 20 mm long), straight to slightly curved or slightly serpentine, nearly constant in diameter (up to 1 mm). The tubes with three very widely spaced keels; the two relatively weakly developed lateral keels located very close to the tube base. The prominent and thick median keel is occasionally slightly undulating. Small transverse ribs are most often well-visible, especially in the anterior tube part; they are densely and regularly spaced and curved forward, towards the three keels. Tubes most often attached to the substrate for their entire length with rare exceptions, where a part of the tube rises slightly above the substrate. The characteristic distribution of keels resulting in wide furrows in between them and thus triangular, subtriangular or otherwise angular cross-section.
Remarks.—Most of the tubes have been found on a single belemnite rostrum; they form a dense aggregation of closely spaced tubes. The specimens somewhat resemble species of several genera, but do not fully match any of those, so that we decided not to assign them to a definite genus. They resemble species of Propomatoceros and Placostegus in their triangular cross-section with a prominent median keel, and of Placostegus in the small and only slowly increasing tube diameter and small transverse ribs. However, they differ from these genera by possessing two lateral keels. Moreover, they slightly resemble Metavermilia? sp. described above; however, their longitudinal ornamentation is less pronounced; the median keel forms a simple upper edge to the tube (“Kante” sensu Jäger 1983: 14, fig. 4a), and the lateral crests are only very delicate, placed at the very bottom lateral parts of the tubes.
Serpulidae sp. 4
Fig. 13C.
Material.—Six specimens (four partially preserved, attached to oyster shells and two specimens detached) from the lower Kimmeridgian (Upper Jurassic) of Małogoszcz, Mesozoic margin of the Holy Cross Mountains, Poland (see Table 1); GIUS 8-3747.
Description.—Tubes large (up to 30 mm long, however, none of the specimens fully preserved), externally covered with prominent growth lines running perpendicularly to the tube long axis but showing no other ornamentation. Some thick, slightly curved tubes are attached to the substrate, but most specimens represent broken off anterior tube fragments. The cross-section is circular with the diameter up to 2 mm.
Remarks.—The specimens are superficially similar in general outline to Glomerula gordialis (Schlotheim, 1820) apart from the perpendicular growth lines and much thicker tubes.
Discussion
Serpulid and sabellid diversity and distribution across palaeoenvironments.—The use of cluster analysis allowed us to group the investigated fossils into several clusters (Fig. 14). The study shows that serpulids and sabellids are highly dependent on the habitat, especially the substrate they are cemented to, whereas the geological age or the stratigraphical distance between compared localities plays a minor role. This is in agreement with studies of other ancient serpulid and sabellid tubeworms. Although some morphotypes might have not been confined to certain substrates (e.g., Kočí et al. 2019), the nature and resultant physical properties of the substrate appear to have been among the most crucial factors inducing serpulid and sabellid colonization (see Ippolitov 2010). However, tube-dwelling polychaetes in general are also reliant upon factors other than substrate. Temperature, oxygen and salinity levels, as well as light availability might also influence larval settlement (Kupriyanova et al. 2019).
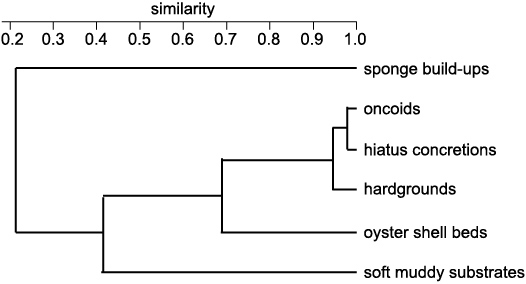
Fig. 14. Dendrogram showing clustering of substrates sharing similar taxa of tube-dwelling polychaetes from the Polish Basin.
The tube-dwelling polychaetes are preserved attached to a variety of available hard substrates (see Fig. 15). The highest similarity in serpulid and sabellid communities between certain localities is displayed between hiatus concretions and oncoids (Fig. 14), both of which served as mobile substrates prone to physical disturbances (e.g., Wilson 1987; Zatoń et al. 2011a, 2012), differing, however, in the nature of encrusted surface, which had a quantitative and (to a lesser extent) qualitative impact on the communities. Relatively similar to them are hardgrounds sensu stricto, where all taxa present on mobile rockgrounds occur as well. Hardgrounds, hiatus concretions and oncoids evidently witnessed, albeit to a different degree, time-averaging, so that factor could have potentially played a role in composition of tubeworm assemblages. The serpulid and sabellid faunas at the hardground localities are among the most diverse.

Fig. 15. Representative hard substrates colonized by the Middle and Upper Jurassic tube-dwelling polychaetes from the Polish Basin. A. Upper Bathonian hiatus concretion from Ogrodzieniec; sabellid Glomerula gordialis (Schlotheim, 1820) (GIUS 8-3751), indicated by arrows. B. Bajocian–Bathonian oncoid from Ogrodzieniec-Świertowiec; the entire oncoid is intensively encrusted by serpulids and sabellids (GIUS 8-3750). C. Callovian bivalve Ctenostreon proboscideum (Sowerby, 1820) from hardground of Zalas; black arrow indicates sabellid Glomerula gordialis, white arrow indicates juvenile serpulid Propomatoceros lumbricalis (GIUS 8-3589). D. Lower Kimmeridgian oyster from oyster shell beds of Małogoszcz; an arrow indicates sabellid Glomerula gordialis (Schlotheim, 1820) (GIUS 8-3747). E. Middle Bathonian oyster from soft muddy substrates of Gnaszyn Dolny; serpulid Propomatoceros lumbricalis (Schlotheim, 1820) is exemplified by arrows (black and white). White arrows point the specimens infested by the hydroid Protulophila gestroi (Rovereto, 1901) (GIUS 8-3730). F. Oxfordian sponge from Zalas; an arrow indicates serpulid Filogranula spongiophila sp. nov. (GIUS 8-3746). Scale bars 10 mm.
Tubeworm faunas encrusting oyster shell beds and those encrusting bivalve shells derived from soft muddy substrates exhibit moderate similarity levels (Fig. 14). Although the substrate available for tube dwelling polychaete colonization was similar, the prevailing conditions were quite different. Low taxonomic variability of serpulid and sabellid worm tubes encrusting parautochthonous oyster shells from the lower Kimmeridgian of Małogoszcz possibly was a result of impeded colonization in a relatively shallow marine environment affected by storm episodes (Machalski 1998). On the other hand, oyster shells and their aggregations, together with belemnite rostra, provided isolated benthic islands (e.g., Zuschin et al. 1999; Taylor and Wilson 2003) on otherwise muddy seafloor in relatively calm (below storm wave-base) palaeoenvironments (Gedl et al. 2012). On a soft-bottom, they offered a sufficiently stable substrate for colonization and further establishment of populations. Such conditions led to the highest taxonomic variability among the all palaeoenvironments.
The community from the lower Oxfordian (Upper Jurassic) sponge bioherms of Zalas shows the highest taxonomic distinctness (Fig. 14). Biotic substrate provided by sponges led to domination by usually compact (e.g., Placostegus planorbiformis) and fast-growing forms with minute diameters (e.g., Cementula spirolinites) and possibly hampered colonization of larger, slowly growing serpulids.
For the majority of localities and settings, moderate Simpson Index of Diversity (1-D) and Dominance (D) values indicate intermediate polychaete variability, with no species highly dominating (see Table 2). Such diversity and dominance values are confined to locations with intermediate levels of hydrodynamic and physical disturbances (e.g., Wilson 1987). Highest biodiversity has been noted on upper Bathonian–lower Callovian and Callovian hardgrounds (Bolęcin and Zalas, respectively) and upper Bajocian–lower Bathonian oncoids (Ogrodzieniec-Świertowiec) (see Table 2); it may attest to time-averaging of deposits with lowered sedimentation rates (hardgrounds) and particularly favourable conditions (oncoids). Slightly lower, but still among the highest diversity levels of Simpson and Dominance indices, are serpulid and sabellid communities derived from middle Bathonian skeletal remains from Gnaszyn Dolny, serving as benthic islands on the soft sediment (see Table 2). Such quiet water conditions may have fitted best with the feeding strategy of most tube-dwelling polychaete species. Based on the values of Shannon index (H), polychaetes from these substrates, together with hardgrounds, are also among the most diverse, followed by oncoids (see Table 2). Evenness values point to moderate (in the case of oncoids from Ogrodzieniec-Świertowiec and lower Kimmeridgian oyster shell beds from Małogoszcz, as well as a part of hiatus concretion localities, e.g., Ogrodzieniec, upper Bathonian), or even low biodiversity (in the case of a part of hiatus concretion localities, e.g., Krzyworzeka, upper Bathonian, and the Callovian hardground of Zalas) (see Table 2), which is an effect of similar proportions of different species’ representatives within these assemblages. The lowest evenness level in the case of the middle Bathonian (Middle Jurassic) of Gnaszyn Dolny (see Table 2) testifies to the highest species richness and abundance of both species and individuals at this locality, indicating highly advantageous palaeoenvironmental conditions (see Table 1).
Table 2. Biodiversity indices calculated for the investigated polychaete assemblages.
|
Index |
Mokrsko |
Kawodrza |
Gnaszyn |
Bugaj |
Ogrodzieniec |
Krzyworzeka |
Żarki |
Ogrodzieniec Świertowiec |
Bolęcin |
Zalas |
Zalas |
Małogoszcz |
|
Dominance (D) |
0.5805 |
0.481 |
0.335 |
0.4692 |
0.52 |
0.3795 |
0.4506 |
0.2991 |
0.3219 |
0.2642 |
0.4762 |
0.4403 |
|
Simpson (1-D) |
0.4195 |
0.519 |
0.665 |
0.5308 |
0.48 |
0.6205 |
0.5494 |
0.7009 |
0.6781 |
0.7358 |
0.5238 |
0.5597 |
|
Shannon (H) |
0.7334 |
0.8746 |
1.503 |
0.8491 |
0.7388 |
1.021 |
0.8676 |
1.4 |
1.334 |
1.546 |
1.005 |
0.9683 |
|
Evenness eH/S |
0.5205 |
0.7993 |
0.4087 |
0.7792 |
0.6978 |
0.9252 |
0.7937 |
0.6762 |
0.6325 |
0.5213 |
0.5463 |
0.6584 |
Mobile rockgrounds.—Both fossil (e.g., Wilson 1985; Lee et al. 1997; Zatoń et al. 2011a) and Recent (e.g., Osman 1977; Sousa 1979; Maughan and Barnes 2000; Kuklinski 2009) encrusting biotas inhabiting mobile lithic substrates are strongly affected and restrained by physical disruptions within marine environments, which strongly influences the ecology of substrate-dwellers. Generally, an increase in physical energy in the environment leading to frequent overturning of loose lithic substrates such as hiatus concretions and oncoids causes an increase in diversity of the fauna and hampers single species prevalence (Wilson 1987; Barnes and Kuklinski 2005). On the other hand, too high levels of physical disturbance, where the cobble and pebble overturning is continuous and frequent, may be destructive, leading to a lethal outcome even for the most opportunistic, robust, and scour-resilient serpulids, highly impeding any ecological succession.
Sediments from which the concretions were derived are interpreted to have been deposited in calm conditions, usually located below storm wave-base (e.g., Leonowicz 2013). Hiatus concretions (Majewski 2000; Zatoń et al. 2006, 2011a) and such sedimentological fabrics, as e.g., trace fossil associations, alternating laminated and bioturbated intervals, or biodeformational structures (Leonowicz 2015a) within the Middle Jurassic siliciclastic sediments of the Polish Jura indicate episodic storm events causing distinct sedimentation breaks and seafloor erosion. Rather rare episodic overturning of the mobile substrates resulted in relatively low diversity (in total five species, Table 1) leading to a predominance of robust, thick-walled species of Propomatoceros and ubiquitous Glomerula gordialis.
The shape and regularity of the concretions’ surfaces is another important factor strongly influencing the quantity and distribution of serpulids and sabellids (Wilson 1987). Larger and more rounded hiatus concretions (as well as oncoids; see below) were less resilient to hydrodynamic events and overturning on the sea bottom, compared to wider and more flattened ones. While resting on the seafloor, only the upper sides of these substrates were available for serpulid and sabellid colonization, while lower surfaces facing the sediment were inaccessible. It is possible that even such relatively stable concretions did not offer full stability against overturning. Minor differences in the encrustation pattern between certain localities seem to reflect slight disparities in the general concretions’ outlook which in turn presumably resulted from the frequency of episodic hydrodynamic activities, depth of concretions’ burial or biological activity of animals inhabiting firmgrounds (Zatoń et al. 2011a). The differences in concretions’ bioerosional patterns between the localities, likewise, reflect the position of the certain setting (proximal/distal) and thus the intensity and the frequency of palaeoenvironmental currents (Sadlok and Zatoń 2020). Organisms burrowing in the ambient sediment might have also loosened it enhancing exhumation of the concretions (Hesselbo and Palmer 1992).
The most important serpulid and sabellid adaptive strategies for persistence on mobile lithic grounds (Wilson 1987) were: (i) the morphological resistance to abrasion which was possible by hard and solid tubes; (ii) cavity dwelling; hazardous impact of repeated substrate overturning in highly energetic environments might have entailed attempts of withdrawal, whenever an opportunity had arisen. Tube-dwelling polychaetes often resolve to cryptic lifestyle (Kobluk 1988) hiding in places such as empty borings and concavities (e.g., Palmer and Fürsich 1974; Wilson and Palmer 1990; Palmer and Wilson 1990; Wilson 1998; Taylor and Wilson 2003; Mallela 2007; Schlögl et al. 2008). Such a solution facilitates also retreating from competition with dominating species. Anyhow, in spite of the presence of borings, some of the tubes encrust the surface of the concretions. A possible explanation is that such cavities have already been occupied by boring bivalves, which made the unavailable for nestling by tubeworms, thus of necessity colonizing the outer surface (see also Zatoń et al. 2011a).
Concretions from Mokrsko, Bugaj, Ogrodzieniec, Krzyworzeka, and Żarki experienced high taphonomic loss as the vast majority of encrusters inhabiting the surface of the concretions were prone to abrasion/corrasion, what is evidenced by many poorly preserved fossils, presumably representing various ecological successions.
Although serpulids and sabellids inhabiting large oncoids are only slightly more taxonomically diverse than that from hiatus concretions, attaining one species more (in total six species, Table 1), their abundance is much higher. Tube-dwelling polychaetes thrived inhabiting these coated grains in spite of the fact, that cryptic places were highly limited by the lack of borings, which were common on the hiatus concretions. Intense colonization and further flourishment of serpulids and sabellids must have been enhanced by the photic conditions, where cyanobacterial mats covering oncoids could develop (Zatoń et al. 2012; Słowiński 2019). A high availability of food supply, especially composed of mixed algae species, significantly induced larval development and subsequent growth (Leone 1970; Kupriyanova et al. 2001; Gosselin and Sewell 2013). Although algal mats covering substrates may hamper epibionts’ development (McKinney 1996; Kuklinski 2009; Zatoń et al. 2011b), they may also facilitate it (Wieczorek and Todd 1998; Kupriyanova et al. 2019). Additionally, in contrast to the tubeworms encrusting hiatus concretions, tubeworms colonizing oncoids were able to settle on the not yet lithified substrate during the formation of possibly still mucous biofilm forming on the oncoids (Taylor and Wilson 2003). Another advantage of oncoids over hiatus concretions (serving as a substrate for colonization) is that their stability might have been enhanced during growth. The formation of subsequent cortex layers increased the volume and thus reduced the susceptibility to overturning on the sea bottom.
Hardgrounds.—Serpulids and sabellids from hardgrounds are derived from two localities (Zalas and Bolęcin) of slightly different in palaeoecological conditions. Limited supply of sediment and resulting time-averaging (Giżejewska and Wieczorek 1977; Tarkowski et al. 1994; Mangold et al. 1996; Taylor 2008; Zatoń et al. 2011b) strongly influenced encrustation patterns of both communities. The species richness of tube-dwelling polychaetes in these localities could have resulted from favorable palaeoenvironmental conditions and long-term exposure of the hard substrates; however, some time-averaging responsible for the final assemblage preserved is not excluded (Taylor 2008; Zatoń et al. 2011b).
The Zalas deposits are characterized by a much more abundant and more taxonomically diverse sessile polychaete fauna (in total nine species, see Table 1) as compared to Bolęcin (in total six species, see Table 1). Such differences presumably resulted from more favourable conditions for the settlers, such as a relatively steady salinity level, a calm, sublittoral environment located within a dysphotic zone (Zatoń et al. 2011b), and a slow sedimentation rate at Zalas (Giżejewska and Wieczorek 1977). The total number of tube-dwelling polychaetes from Bolęcin, the deposits of which may be an equivalent of the uppermost Bathonian–lowermost Callovian Balin Oolite (e.g., Tarkowski et al. 1994; Mangold et al. 1996; Taylor 2008), is not among the lowest. However, the investigated number of substrate-serving fossils was ample. Therefore, the percentage of inhabiting sessile polychaetes is low, possibly due to higher abrasion levels and Quaternary periglacial events, which may have reworked the sediments (Mangold et al. 1996), affecting the preservation of the fossils.
Any reciprocal interactions (see Taylor 2016) were very rare, or even absent in Bolęcin; therefore, most of them might have simply resulted from random settling of worms in close proximity and are not an evidence of spatial competition (Zatoń et al. 2011b; Taylor 2016). Most often, tube-dwelling polychaetes are overgrown by bryozoans and other polychaetes, both of the same and different genera, supporting an explanation of random and post-mortem overgrowth on a small surface of substrate (Taylor and Wilson 2003). Anyhow, a scarce number of mutual (reciprocal) overgrowths and intraspecific stand-offs (“when growth of both interacting individuals is halted at their junction”; see Taylor 2016) may suggest that at least some sclerobionts, notably serpulids, were actively competing for available substrate. Some individuals tended to grow towards the deflections between Ctenostreon proboscideum (Sowerby and Sowerby, 1820) shell ribs or on the underside of lower valves indicating the preferences of inhabiting cryptic niches.
Oyster shell beds.—In the shell beds with Actinostreon gregareum (Sowerby, 1815) within lower Kimmeridgian (Upper Jurassic) deposits of Małogoszcz quarry, many oyster shells which served as substrate for serpulids and sabellids are disarticulated. The oysters set up parautochtonous accumulations, resulting from storm events in a relatively shallow-marine environment (Seilacher et al. 1985; Machalski 1998; Zatoń and Machalski 2013). In spite of the high substrate availability for these polychaetes, their colonization was there impeded by mode of life of the oysters. These bivalves displayed different kinds of ecophenotypic adjustments, such as mud-sticker mode of life populating the sediment in a vertical position, sometimes cementing to other individuals; and recliners, which lay flat on the sediment and might have cemented to various hard objects, as e.g., fragmented rocks, shells or oncoids (Machalski 1998). While the flat-lying shells provided convenient settling conditions and relatively much space, in the vertically arranged ones, it was highly limited, especially when only an anteriormost part of the shell protruded from the sediment.
Possibly, a part of the shells forming small clusters of cemented valves, which displayed a three-dimensional shape might have been occasionally overturned. Such a situation occurred in slightly younger shell beds with Nanogyra nana (Sowerby, 1822) from the lower Kimmeridgian (Upper Jurassic) of Małogoszcz, which contributed to the formation of ostreoliths (Zatoń and Machalski 2013). Such large, spherical objects were prone to overturning due to hydrodynamic and biological agents (Zatoń and Machalski 2013). However, even isolated shells or shell clusters exhibiting rather flat morphology presumably might have been occasionally overturned due to their smaller sizes and a relatively shallow marine palaeoenvironment with episodic storm events (Machalski 1998; Radwańska and Radwański 2003).
The diversity of serpulid and sabellid fauna from these deposits is among the lowest of all the sites investigated (in total four species, Table 1). On the ostreoliths mentioned above, Zatoń and Machalski (2013) noted only two sessile polychaete species. Interestingly, similarily low sessile polychaete diversity was noted on lower Kimmeridgian carbonate cobbles from nearby Sobków locality by Krajewski et al. (2017). Presumably, such low diversity might have been governed by salinity fluctuations in shallow water settings, as evidenced by stable isotopes (Krajewski et al. 2017).
Nonetheless, oysters provided a range of places to be colonized, offering many cryptic and upward-facing habitats, what may reveal encrusters’ polarization. Although the slight majority of tube-dwelling polychaetes inhabited the exterior surfaces of the valves (Szewczuk 2010), many of them settled on the interiors, evidencing that encrustation of the oyster shells also took place post-mortem (e.g., McKinney 1995; Fagerstrom et al. 2000). Clustered oyster valves also could have acted as a good cryptic habitat due to an increased accessibility of fissures and crevices (e.g., Kidwell 1986; Zuschin et al. 1999; Coen and Grizzle 2007; Zatoń and Machalski 2013).
Soft muddy substrates.—Serpulids and sabellids derived from the middle Bathonian (Middle Jurassic) of Gnaszyn Dolny and lower Bathonian (Middle Jurassic) of Kawodrza Górna inhabited mainly oyster shells and belemnite rostra, as well as wood-falls (see Kaim 2011), scattered over the soft muddy seafloor. Thus, biogenic substrates suitable for sclerobiont colonization were very patchy. Tube-dwelling polychaetes on such benthic islands frequently occur crowded, forming dense aggregations, and exhibit the highest diversity among the all studied sites (in total eleven species, see Table 1).
In contrast to other kinds of substrates, oyster shells provided here a sufficiently stable habitat for the encrusters. The sediments are interpreted to have been deposited in a relatively deep, calm, oxygenated environment below the storm-wave base (Marynowski et al. 2007; Zatoń et al. 2009; Gedl and Kaim 2012; Gedl et al. 2012). However, colonization might have been intermittent as presumably a bulk of larvae did not even have a chance to settle on a convenient hard substrate. In the case of successful colonization, subsequent larvae possibly had a greater chance to be recruited in the direct vicinity because of available adjacent space. Even though serpulid larvae exhibit either lecithotrophic or planktotrophic larval development strategies (Kupriyanova et al. 2001; Rouse and Pleijel 2001), many serpulids settle non-randomly (Kupriyanova et al. 2019), which may result in a relatively distant dispersal (Andrews and Anderson 1962; Dirnberger 1993; Kupriyanova et al. 2001). The larvae may develop into dense monospecific assemblages with regard to attached individuals of their own species (Scheltema et al. 1981). To encourage gregarious settling, chemical signals associated with living adults may be used (Pawlik 1992; Toonen and Pawlik 1996; Bryan et al. 1997). On the other hand, the absence of a mature source population in close proximity to an accessible hard substrate will highly reduce the chance of recruitment (see Taylor and Wilson 2003).
Restriction of the surface may have resulted in occasional competitive interactions. Despite difficulties in a clear designation between syn vivo and post-mortem interactions (see Fagerstrom et al. 2000), possibly at least a part of overgrowths between epibionts did not result from overgrowing dead skeletons. Although clear reciprocal overgrowths are absent, a part of intraspecific interactions resulted in stand-offs (see Taylor 2016). Such an outcome may support the interpretation that encounters of the same species acted syn vivo (Taylor 2016). It is evident that some sclerobionts colonized the interiors of bivalve valves, being a proof of post-mortem colonization (e.g., McKinney 1995; Fagerstrom et al. 2000). Some tubes of Propomatoceros lumbricalis also acted as hosts for in vivo bioclaustrating hydroids; however, such interactions were extremely rare (Słowiński et al. 2020).
Large, flat-lying oyster valves were highly resilient against physical agents, especially in calm settings as in the present case, offering a stable substrate, where irregularly, slowly growing, large Propomatoceros lumbricalis constituted the basis of the community. Smaller, compact and faster-growing tube-dwellers were outcompeted. Nonetheless, because sclerobionts were substrate-restricted in settlement and colonization, they were forced to settle together with more opportunistic and thus dominating polychaetes, sometimes displaying cryptic behavior, encrusting e.g., the deflections between the oyster shell ribs. A different situation occurred on the substrate provided by belemnite rostra. Regardless of the calm palaeoenvironment, they were less stable on the seabed, being much more susceptible to any disturbances. Despite scarce exceptions, robust, slowly growing species were unable to successfully colonize the rostra, whereas smaller species like species of Nogrobs showed higher plasticity enabling them to adjust to such small, conical/cylindrical substrates. Yet another ecological adjustment was performed by flat, free-living Nogrobs aff. tetragona, which was favored by a slow sedimentation rate. Possibly, juvenile representative first encrusted a small surface of any hard substrate available, subsequently detaching, and terminating as a free-lying on the soft sediment (Sanfilippo 2009). Rare, curved tube portions may have resulted from shifting due to a response to a temporary instability within a sediment, showing an attempt to avoid ecologically unpleasant conditions (Fig. 9H; see also Sanfilippo 2009: fig. 6A, C, L). Alternatively, they may have lived embedded within the sediment with only their apertures protruding and lying upon the sea floor, as do the Recent soft bottom-inhabiting species Ditrupa arietina (Hove and Smith 1990; Vinn et al. 2008b).
Due to a stable palaeoenvironment with low hydrodynamics (Gedl et al. 2012), most encrusters were not intensively abraded, which attests that negative taphonomic processes affecting the fossil assemblages were insignificant.
Sponge build-ups.—The substrate for the tube-dwelling polychaetes from the Oxfordian (Upper Jurassic) of Zalas was provided by lithistid sponges (Trammer 1982), that formed biohermal structures (Trammer 1982, 1985; Ostrowski 2005; Matyszkiewicz et al. 2012). Such sponge mounds provided “live” substratum for sclerozoans. With respect to taxonomic composition (in total five species, Table 1), sessile polychaetes are here completely different from the all other sites, as the species composition was presumably strongly influenced by substrate-specific preferences (see Kupriyanova et al. 2001, 2019; Ippolitov 2010).
Relatively calm environment, quite low sedimentation rates, and high nutrient availability (Matyszkiewicz et al. 2012) probably escalated spatial and resource competition (Palmer and Fürsich 1981). Faster calcification rates seem to have been favored in the environment of the reefal substrate, which promoted polychaetes, which were easily adaptable to the prevailing conditions by a higher ecophenotypic plasticity. It may explain the absence of any species of Propomatoceros within sponge build-ups of Zalas. Conspicuous in this population is also the relative scarcity of Glomerula gordialis. Being rather easy-adjustable to different conditions (e.g., Parsch 1956; Ippolitov 2010; Vinn and Wilson 2010; Breton et al. 2020), this sabellid is here dominated by much more abundant and possibly more opportunistic Cementula spirolinites, which tended to occur in the advantageous conspecific aggregations (e.g., Palmer and Palmer 1977; Palmer and Fürsich 1981; Schlögl et al. 2008).
Tube-dwelling polychaetes from Zalas were attached to both sides of the sponges, being slightly more numerous on the exterior (33% compared to 22% on the interior, as calculated from Kuziomko-Szewczuk 2010). Such a polarization of space occupation could have arisen from relatively equal preferences towards certain sides. Anyway, it appears that more serpulids and sabellids inhabited the external sides of sponges. With their often irregular shapes, these principal frame-builders provided a variety of microhabitats where tubeworms might have led a cryptic mode of life (Riding 2002). Outwardly projected growth of sponges, laterally widening to the top possibly provided shaded, cryptic niches on the undersides of the mushroom-shaped sponge skeleton (Palmer and Fürsich 1981; Wilson et al. 2008).
Many sponges are fragmented, limiting an insight into actual shape of the entire organism. Such fragments possibly constituted a biohermal talus. Nevertheless, the differences in the level of tube-dwelling polychaete encrustation on both sides seem to be small. Presumably, they were able to settle on any available hard substrate provided by sponges surrounded by soft sediment (see Trammer 1982, 1989).
Remarks on serpulid and sabellid evolution during the Middle and Late Jurassic.—Following the radiation that commenced in the aftermath of the Triassic/Jurassic mass extinction, significant diversification of tube-dwelling polychaetes occurred during the Early and Middle Jurassic, when the total number of known morphotypes increased greatly. However, the Middle Jurassic was also a time of a relative stagnation within the already established clades, such as e.g., the sabellid Glomerula and serpulids Filograna, three-keeled Metavermilia, or Propomatoceros (see Ippolitov et al. 2014, for a review). Among the new evolutionary clades that appeared is Metavermilia striatissima (Fürsich et al. 1994), regarded as a possibly separate minor lineage within the genus Metavermilia, and Genicularia (Quenstedt 1856: 589), a genus which has not been reported in the Polish Basin.
Biostratigraphy of serpulids is highly constrained (but see Macellari 1984; Tapaswi 1988) and specific morphotypes most often do not correspond to certain stratigraphic intervals. It is further complicated by slow intrageneric radiation during the Middle Jurassic and an increase in tube disparity within particular species, which possibly may be an outcome of the evolution of the group, but also a result of some local, ecophenotypic adjustments. These make taxonomic attribution of many Jurassic serpulids and sabellids problematic, and there is still no widely acknowledged current scheme of species and morphological or stratigraphical borders between species in most of these clades.
The serpulid fauna became more diversified with the emergence of sponge and microbial facies. The advent of some new forms occurred during Oxfordian (Late Jurassic), when such build-ups became widespread in Europe (e.g., Goldfuss 1831; Parsch 1956; Trammer 1982; Pisera 1991; Radwańska 2004; Matyszkiewicz et al. 2012), and locally being present even in the Bathonian (Middle Jurassic; Palmer and Fürsich 1981). Such new forms include here “Serpula cingulata”, Cementula spirolinites, Placostegus planorbiformis, and Filogranula spongiophila sp. nov. However, tube-dwelling polychaete faunas during the Oxfordian and Kimmeridgian beyond these reefal deposits still remained rather “old-fashioned” (e.g., Wignall 1990; this study). Such distribution supports an explanation for high substrate-dependent serpulid and sabellid settlement (Ippolitov 2010).
Apart from the serpulid genera of clade BII represented by monophyletic Spirorbinae, members of all the informally established clades (see Kupriyanova et al. 2009) are present in our material. Some of them, like e.g., Filogranula, which possibly is a polyphyletic taxon (see Ippolitov et al. 2014; Kočí and Jäger 2015a) need further investigation. However, such considerations, although essential, are beyond the scope of the present study.
Comparisons with other Middle and Upper Jurassic tube-dwelling polychaete assemblages.—The great majority of all known Middle and Late Jurassic tube-dwelling polychaete communities are described from Europe, including the European part of Russia (among more recent publications e.g., Pugaczewska 1970; Jäger et al. 2001; Radwańska 2004; Ippolitov 2007a, b; Kočí et al. 2019; Breton et al. 2020). Except of some clearly outdated studies (e.g., Parsch 1956), the majority of these reports dealt with assemblages coming from single stratigraphic intervals (e.g. Ippolitov 2007a, b), or with assemblages which were not the main objective of the investigation (e.g., Zatoń et al. 2011a). Thus, this research dealing with fossil material spanning the upper Bajocian to lower Kimmeridgian, representing a variety of palaeoenvironments, may serve as a potential reference point for future investigations.
Relatively little data is available on Jurassic serpulids and sabellids settling on mobile rockgrounds. Investigations by Kaźmierczak (1974), Chudzikiewicz and Wieczorek (1985), Fürsich et al. (1992), Zatoń et al. (2011a), and Krajewski et al. (2014, 2017) did not deal with tube-dwelling polychaete assemblages as the major scope. The total number of the taxa reported in above mentioned studies was rather low, comprising two, or three species compared to five noted during the present study. Despite different stratigraphic intervals, the taxonomic composition of the assemblages indicated above was very similar to the hiatus concretions described here, dominated by the genera Glomerula and Propomatoceros. The quantitative data also seem to be comparable, with polychaetes being at least not abundant.
In comparison to hiatus concretions, oncoids are significantly more heavily encrusted. Tube-dwelling polychaetes preserved on this kind of substrate have been mentioned from, e.g., the Bajocian (Middle Jurassic) of England (Gatrall et al. 1972; Palmer and Wilson 1990) and France (Palmer and Wilson 1990), Bathonian (Middle Jurassic) of Poland (Zatoń and Taylor 2009a; Zatoń et al. 2012), or Oxfordian (Upper Jurassic) of Switzerland (Védrine et al. 2007). The record of serpulid and sabellid taxa present on the oncoids in the current study (six taxa) is comparable to those in the studies listed above, reaching seven (Palmer and Wilson 1990) to nine species (Zatoń et al. 2012). These assemblages are dominated by species of Glomerula, followed by those of Propomatoceros. It has to be noted that Zatoń et al. (2011a) and Zatoń et al. (2012) used most of the same research material as in the current study revealing eight and nine tubeworm species, respectively. However, presumably due to overinterpretations of certain morphotypes, which rather represented ecophenotypic variations of the same species, the number of serpulid and sabellid species is lower, comparable to the present study. Although the species richness is not significantly higher as compared to hiatus concretions, the overall number of polychaete tubes is substantially larger. In spite of the fact that at least a part of the investigations listed above (e.g., Gatrall et al. 1972; Védrine et al. 2007) might have not been sufficiently focused on serpulids and sabellids, their abundance has usually been noted.
In comparison to various mobile rockgrounds, more is known about tube-dwelling polychaetes from metazoan build-ups and a variety of hardgrounds. Serpulids and sabellids preserved on Jurassic reefal structures extending across Europe have been mentioned many times (e.g., Goldfuss 1831; Parsch 1956; Flügel and Steiger 1981; Palmer and Fürsich 1981; Pisera 1991; Radwańska 2004; Matyszkiewicz et al. 2012; Pleş et al. 2013). However, more recent investigations focusing on sessile polychaetes in more detail have been performed only by Radwańska (2004) and to a lesser extent by Palmer and Fürsich (1981). Compared to only five species from the Oxfordian (Upper Jurassic) of Zalas, Radwańska (2004) reported 14 polychaete taxa occurring in Kimmeridgian sponge buildups of Wapienno/Bielawy in Kuyavia region (see also Loba and Radwańska 2022). In spite of striking differences in species numbers, the serpulid and sabellid fauna from Zalas appears to be more abundant than that of Kuyavia (Radwańska 2004). The Wapienno/Bielawy quarries (Radwańska 2004) contain the majority of the taxa found in Zalas, including Glomerula gordialis, Cementula spirolinites, and the genera Placostegus and Filogranula. They are also present in the Oxfordian (Upper Jurassic) sponge facies of southern Germany (Parsch 1956). Upper Bathonian (Middle Jurassic) tube-dwelling polychaetes described by Palmer and Fürsich (1981) consist of seven species. However, “Spirorbula sp.”, which has been described as the most abundant tubeworm within the upper Bathonian sclerobiont assemblage (Palmer and Fürsich 1981), has been proven to actually represent a microconchid (Vinn and Taylor 2007). Other species may also require systematic reinvestigation, although the genera Glomerula, Propomatoceros and Cementula are likely to be represented.
Serpulid and sabellid communities inhabiting lithic substrates (e.g., hardgrounds) and carbonate skeletal remains of various organisms seem to be more diverse due to a wider range of substrate types and prevailing conditions (up to eleven taxa in the present study). Breton et al. (2020) described tube-dwelling polychaetes among other sclerobionts from the Bajocian (Middle Jurassic) ferruginous oolithic facies of France. They are mostly preserved on mollusk shells, with diversity reaching nine species, where Glomerula gordialis and Propomatoceros lumbricalis dominate. Krajewski et al. (2017) mentioned only one serpulid species, Tetraserpula sp. (possibly representing Nogrobs); and two sabellid species represented by Cycloserpula sp. and Glomerula gordialis (presumably representing a single species) from the Kimmeridgian (Upper Jurassic) carbonate cobbles from the Mesozoic margin of the Holy Cross Mountains.
Serpulid and sabellid assemblages preserved on marl nodules and invertebrate skeletons from the Callovian (Middle Jurassic) of Russia described by Ippolitov (2007a, b) are represented by eight species. Interestingly, in contrast to the majority of reports, sabellids (e.g., Glomerula) are a minor component there, with a predominance of Propomatoceros lumbricalis constituting hundreds of specimens.
Outside of Europe, Middle Jurassic serpulid and sabellid fauna has also been described from the Matmor Formation in Israel, being the closest to the equator assemblage during the Middle Jurassic (Vinn and Wilson 2010). It differs in the high dominance of the species of sabellid genus Glomerula and the presence of the genus Vermiliopsis, which seems to be uniformly absent in the Jurassic of Europe. Such differences might have resulted from a domination of the species of opportunistic Glomerula; however, Vermiliopsis might have originated in the warm equatorial, shallow seas before its further dispersal towards higher latitudes (Vinn and Wilson 2010).
Kočí et al. (2019) described nine serpulid and sabellid species from the Oxfordian (Upper Jurassic) of the Czech Republic, mainly encrusting brachiopod shells and sponge remains. However, all the taxa are represented by only a few individuals limiting an insight into the community. Even though the species composition differs from site to site, diversity remains relatively similar, often displaying a pattern of biodiversity increase through time (see Ippolitov 2010). Moreover, tube-dwelling polychaete assemblages are frequently dominated by a single, possibly most opportunistic species. Finally and crucially, true sabellid and serpulid diversity was likely higher in all studied Jurassic sites. This is because the taxonomy of fossil tube-dwelling polychaetes is based exclusively on the morphology of their tubes, while many different modern serpulid species produce similar or identical tubes (Hove and Kupriyanova 2009).
Conclusions
The first comprehensive investigation dealing with the Middle and Late Jurassic tube-dwelling polychaetes (sabellids and serpulids) from the Polish Basin reveals the presence of 24 species, of which Filogranula spongiophila sp. nov. and Cementula radwanskae sp. nov. are considered new. Certain species (e.g., Cementula spirolinites, Placostegus planorbiformis, Filogranula spongiophila sp. nov.) are clearly associated with specific kinds of substrate, while stratigraphic interval plays a secondary role in determining patterns of distribution. At the majority of locations and substrates, however, the stratigraphically and geographically widespread sabellid species Glomerula gordialis dominates over the serpulids and very often seems to be the most opportunistic tubeworm species. The second most abundant species is Propomatoceros lumbricalis, although it is uniformly absent within the sponge build-ups of the Polish Jura.
Taking the distribution patterns and abundance of serpulids and sabellids into account, it is evident that prevailing conditions had a significant impact on the composition of assemblages. Aside from substrate type, food availability, hydrodynamism of the palaeoenvironment and sedimentation rate were among the most important factors influencing colonization and subsequent development of serpulids and sabellids. Biodiversity indices show, that the most diverse tube-dwelling polychaete faunas are those inhabiting skeletal remains derived from soft muddy bottoms, as well as those inhabiting hardgrounds and large oncoids, which resulted from highly suitable conditions at these sites. The lowest biodiversity occurs in communities colonizing hiatus concretions, which possibly was an effect of their repeated overturning due to external agents, such as bottom-currents and/or animal activity. Many of the tube-dwelling polychaetes display similar distributional patterns featuring e.g., a cryptic lifestyle, or competition for space with both other polychaetes and other epibionts.
Acknowledgements
We would like to thank the Chrzanów Forest Inspectorate, in particular Roman Babicki, Henryk Gawron, and Witold Bogacz for their help in field work aimed at collecting the fossil material from Bolęcin. Our colleague Marek Trzepizur (Częstochowa, Poland) is warmly thanked for donation of some of the middle Bathonian encrusted shells from Gnaszyn Dolny. Wojciech Krawczyński (Institute of Earth Sciences, Sosnowiec, Poland) is cordially thanked for his assistance in photographing the specimens. Rossana Sanfilippo (University of Catania, Italy) and an anonymous reviewer provided numerous constructive comments and remarks which are greatly appreciated.
References
Andrews, J.C. and Anderson, D.T. 1962. The development of the polychaete Galeolaria caespitosa Lamarck (Fam. Serpulidae). Proceedings of the Linnean Society of New South Wales 87: 185–188.
Assmann, P. 1937. Revision der Fauna der Wirbellosen der oberschlesischen Trias. Abhandlungen der Preußischen Geologischen Landesanstalt 170: 1–134.
Barnes, D.K.A. and Kuklinski, P. 2005. Bipolar patterns of intraspecific competition in bryozoans. Marine Ecology Progress Series 285: 75–87. Crossref
Berra, F. and Jadoul, F. 1996. Norian serpulid and microbial bioconstructions: implications for the platform evolution in the Lombardy Basin (Southern Alps, Italy). Facies 35: 143–162. Crossref
Bosák, P., Mihevc, A., and Pruner, P. 2004. Geomorphological evolution of the Podgorski Karst, SW Slovenia: contribution of magnetostratigraphic research of the Črnotiče II site with Marifugia sp. Acta Carsologica 33: 175–204. Crossref
Breton, G., Jäger, M., and Kočí, T. 2020. The sclerobionts of the Bajocian Oolithe ferrugineuse de Bayeux Formation from Calvados (Paris Basin, Normandy, France). Annales de Paléontologie 106: 102361. Crossref
Brünnich Nielsen, K. 1931. Serpulidae from the Senonian and Danian deposits of Denmark. Meddelelser fra Dansk geologisk Forening 8: 71–113.
Bryan, P.J., Qian, P.Y., Kreider, J.L., and Chia, F.S. 1997. Induction of larval settlement and metamorphosis by pharmacological and conspecific associated compounds in the serpulid polychaete Hydroides elegans. Marine Ecology Progress Series 146: 81–90. Crossref
Bush, K.J. 1905. Tubicolous annelids of the tribes Sabellides and Serpulides from the Pacific Ocean. Harriman Alaska Expedition 12: 169–355. Crossref
Bush, K.J. 1907. Descriptions of the two genera of tubicolous annelids, Paravermilia and Pseudovermilia, with species from Bermuda referable to them. American Journal of Science, New Haven 23: 131–136. Crossref
Chamberlin, R.V. 1919. The Annelida Polychaeta [Albatross Expeditions]. Memoirs of the Museum of Comparative Zoölogy at Harvard College 48: 1–514.
Chiplonkar, G.W. and Tapaswi, P.M. 1973. Fossil polychaetes from the Upper Cretaceous rock formations of South India. Part I. Proceedings of the Indian Academy of Science, Section B Biological Science 77: 116–130. Crossref
Chudzikiewicz, L. and Wieczorek, J. 1985. Bored and encrusted clasts in the lower Kimmeridgian carbonates at Sobków (SW margin of the Holy Cross Mts., Poland). Annales Societatis Geologorum Poloniae 55: 295–306.
Cirilli, S., Iannace, A., Jadoul, F., and Zamparelli, V. 1999. Microbial-serpulid build-ups in the Norian–Rhaetian of the western Mediterranean area: ecological response of shelf margin communities to stressed environments. Terra Nova 11: 195–202. Crossref
Coen, L. and Grizzle, R. 2007. The importance of habitat created by molluscan shellfish to managed species along the Atlantic Coast of the United States. In: J. Thomas and J. Nygard (eds.), Atlantic States Marine Fisheries Commission Habitat Management. Atlantic States Marine Fisheries Commission, Washington, DC, USA 8: 1–108.
Dayczak-Calikowska, K. and Moryc, W. 1988. Evolution of sedimentary basin and palaeotectonics of the Middle Jurassic in Poland. Kwartalnik Geologiczny 32: 117–136.
Dayczak-Calikowska, K., Kopik, J., and Marcinkiewicz, T. 1997. Middle Jurassic. In: S. Marek and M. Pajchlowa (eds.), The Epicontinental Permian and Mesozoic in Poland. Prace Państwowego Instytutu Geologicznego 153: 236–282.
Dembicz, K. and Praszkier, T. 2007. Kelowej południowo-wschodnie części Jury Krakowsko-Częstochowskiej. Tomy Jurajskie 4: 71–76.
Dirnberger, J.M. 1993. Dispersal of larvae with a short planktonic phase in the polychaete Spirorbis spirillum (Linnaeus). Bulletin of Marine Science 52: 898–910.
Fabricius, O. 1779. Reise nach Norwegen mit Bemerkungen aus der Naturhistorie und Oekonomie. 388 pp. Carl Ernst Bohn, Hamburg.
Fagerstrom, J.A., West, R.R., Kershaw, S., and Cossey, P.J. 2000. Spatial competition among clonal organisms in extant and selected Paleozoic reef communities. Facies 42: 1–24. Crossref
Feldman, H.R. and Brett, C.E., 1998. Epi- and endobiontic organisms on Late Jurassic crinoid columns from the Negev Desert, Israel: implications for co-evolution. Lethaia 31: 57–71. Crossref
Feldman-Olszewska, A. 1997. Depositional architecture of the Polish epicontinental Middle Jurassic basin. Geological Quarterly 41: 491–508.
Fischer, R., Galli Oliver, C., and Reitner, J. 1989. Skeletal structure, growth, and paleoecology of the patch reef-building polychaete worm Diplochaetetes mexicanus Wilson, 1986 from the Oligocene of Baja California (Mexico). Geobios 22: 761–775. Crossref
Fischer, R., Pernet, B., and Reitner, J. 2000. Organomineralization of cirratulid tubes—fossil and Recent examples. Facies 42: 35–50. Crossref
Flügel, E. and Steiger, T. 1981. An Upper Jurassic sponge-algal buildup from the northern Frankenalb, West Germany. In: D.F. Toomey (ed.), European Fossil Reef Models. The Society of Economic Paleontologists and Mineralogists, Special Publication 30: 371–397. Crossref
Flügel, E., Flügel-Kahler, E., Martin, J.M., and Martin-Algarra, A. 1984. Middle Triassic reefs from Southern Spain. Facies 11: 173–218. Crossref
Fürsich, F.T., Oschmann, W., Singh, I.B., and Jaitly, A.K. 1992. Hardgrounds, reworked concretion levels and condensed horizons in the Jurassic of western India: their significance for basin analysis. Journal of the Geological Society 149: 313–331. Crossref
Fürsich, F.T., Palmer, T.J., and Goodyear, K.L. 1994. Growth and disintegration of bivalve-dominated patch-reefs in the Portlandian (Upper Jurassic) of southern England. Palaeontology 37: 131–171.
Gatrall, M., Jenkyns, H.C., and Parsons C.F. 1972. Limonitic concretions from the European Jurassic, with particular reference to the “snuff-boxes” of southern England. Sedimentology 18: 79–103. Crossref
Gedl, P. and Kaim, A. 2012. An introduction to the palaeoenvironmental reconstruction of the Bathonian (Middle Jurassic) ore-bearing clays at Gnaszyn, Kraków-Silesia Homocline, Poland. Acta Geologica Polonica 62: 267–280. Crossref
Gedl, P., Kaim, A., Leonowicz, P., Boczarowski, A., Dudek, T., Kędzierski, M., Rees, J., Smoleń, J., Szczepanik, P., Sztajner, P., Witkowska, M., and Ziaja, J. 2012. Palaeoenvironmental reconstruction of Bathonian (Middle Jurassic) ore-bearing clays at Gnaszyn, Kraków-Silesia Homocline, Poland. Acta Geologica Polonica 62: 463–484. Crossref
Gerasimov, P.A. 1955. Rukovodâŝie iskopaemye mezozoâ central’nyh oblastej evropejskoj časti SSSR. Čast II: Iglokožie, rakoobraznye, červi, mšanki i korally ûrskih otloženij. 92 pp. Gosgeoltehizdat, Moskva.
Giżejewska, M. and Wieczorek, J. 1977. Remarks on the Callovian and lower Oxfordian of the Zalas Area (Cracow Upland, Southern Poland). Bulletin de l’Académie Polonaise des Sciences, Série des Sciences de la Terre 24: 167–175.
Goldfuss, A. (1826–1844). Petrefacta Germaniae: tam ea, quae in Museo Universitatis Regiae Borussicae Fridericiae Wilhelmiae Rhenanae servantur quam aliaquae cunque in Museis Hoeninghusiano Muensteriano aliisque extant; iconibus et descriptionibus illustrata. 1 (1): i–viii + 1–76 (1826); 1 (2): 77–164 (1829); 1 (3): 165–240 (1831); 1 (4): 241–252 (1833); 2 (1): 1–68 (1834); 2 (2): 69–140 (1835); 2 (3): 141–224 (1837); 2 (4): i–iii + 225–312 (1840); 3 (1): 1–20 (1841); 3 (2): 21–28 (1844); 3 (3): i–iv + 29–128 (1844). Arnz and Co., Düsseldorf.
Gosselin, L.A. and Sewell, M.A. 2013. Reproduction, larval development and settlement of the intertidal serpulid polychaete Spirobranchus cariniferus. Journal of the Marine Biological Association of the United Kingdom 93: 1249–1256. Crossref
Grube, A.E. 1850. Die Familien der Anneliden. Archiv für Naturgeschichte 16: 249–364.
Gutowski, J., Popadyuk, I.V., and Olszewska, B. 2005. Late Jurassic–earliest Cretaceous evolution of the epicontinental sedimentary basin of southeastern Poland and western Ukraine. Geological Quarterly 49: 31–44.
Hammer, Ø., Harper, D.A.T., and Ryan, P.D. 2001. PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4: 1–9.
Hesselbo, S.P. and Palmer, T.J. 1992. Reworked early diagenetic concretions and the bioerosional origin of a regional discontinuity within British Jurassic marine mudstones. Sedimentology 39: 1045–1065. Crossref
Hove, H.A. ten 1975. Serpulinae (Polychaeta) from the Caribbean. III. The genus Pseudovermilia. Studies on the Fauna of Curaçao and other Caribbean Islands 47: 46–101.
Hove, H.A. ten and Kupriyanova, E.K. 2009. Taxonomy of Serpulidae (Annelida, Polychaeta): the state of affairs. Zootaxa 2036: 1–126. Crossref
Hove, H.A. ten and Smith, R.S. 1990. A re-description of Ditrupa gracillima Grube, 1878 (Polychaeta, Serpulidae) from the Indo-Pacific, with a discussion of the genus. Records of the Australian Museum 42: 101–118. Crossref
Ippolitov, A.P. 2007a. Contribution to the revision of some late Callovian serpulids (Annelida, Polychaeta) of central Russia: Part 1. Paleontological Journal 41: 260–267. Crossref
Ippolitov, A.P. 2007b. Contribution to the revision of some late Callovian serpulids (Annelida, Polychaeta) of central Russia: Part 2. Paleontological Journal 41: 429–436. Crossref
Ippolitov, A.P. 2010. Serpulid (Annelida, Polychaeta) evolution and ecological diversification patterns during Middle–Late Jurassic. Earth Science Frontiers 17: 207–208.
Ippolitov, A.P., Vinn, O., Kupriyanova, E., and Jäger, M. 2014. Written in stone: history of serpulid polychaetes through time. Memoirs of Museum Victoria 71: 123–159. Crossref
Jäger, M. 1983. Serpulidae (Polychaeta sedentaria) aus der norddeutschen höheren Oberkreide – Systematik, Stratigraphie, Ökologie. Geologisches Jahrbuch, Reihe A 68: 3–219.
Jäger, M. 2005. Serpulidae und Spirorbidae (Polychaeta sedentaria) aus Campan und Maastricht von Norddeutschland, den Niederlanden, Belgien und angrenzenden Gebieten. Geologisches Jahrbuch A 157: 121–249.
Jäger, M. 2011. Sabellidae, Serpulidae and Spirorbinae (Polychaeta sedentaria) from the Barremian (Lower Cretaceous) of the Serre de Bleyton (Drôme, SE France). Annalen des Naturhistorischen Museums in Wien, Serie A 113: 675–733.
Jäger, M. and Lang, F. 2017. Serpuliden und Sabelliden aus dem oberen Kimmeridgium von Saal bei Kelheim. In: F. Lang and S. Simonsen (eds.), Fossilien aus dem Riffschuttkalk des Kimmeridgium (Oberjura) von Saal a. d. Donau bei Kelheim (Bayern). Der Steinkern 2017 (30): 76–83.
Jäger, M. and Schubert, S. 2008. Das Ober-Pliensbachium (Domerium) der Herforder Liasmulde; Teil 2, Serpuliden (Kalkröhrenwürmer). Geologie und Paläontologie in Westfalen 71: 47–75.
Jäger, M., Kapitzke, M., and Rieter, M. 2001. Neufunde von Pannoserpula pannosa (Quenstedt, 1857) (Polychaeta, Serpulidae) aus den Korallenkalken (Ober-Kimmeridgium) von Nattheim und Gerstetten (Schwäbische Alb). Stuttgarter Beiträge zur Naturkunde, Serie B 308: 1–17.
Kaim, A. 2011. Non-actualistic wood-fall associations from Middle Jurassic of Poland. Lethaia 44: 109–124. Crossref
Kaźmierczak, J. 1974. Crustacean associated hiatus concretions and eogenetic cementation in the Upper Jurassic of central Poland. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen 147: 329–342.
Kidwell, S.M. 1986. Taphonomic feedback in Miocene assemblages: testing the role of dead hardparts in benthic communities. Palaios 1: 239–255. Crossref
Kobluk, D.R. 1988. Pre-Cenozoic fossil record of cryptobionts and their presence in early reefs and mounds. Palaios 3: 243–250. Crossref
Kočí, T. and Jäger, M. 2015a. Filogranula cincta (Goldfuss, 1831), a serpulid worm (Polychaeta, Sedentaria, Serpulidae) from the Bohemian Cretaceous Basin. Sborník Národního Muzea v Praze, řada B, Přírodní védy. Acta Musei Nationalis Pragae, Series B, Historia Naturalis 71: 293–300.
Kočí, T. and Jäger, M. 2015b. Sabellid and serpulid worms (Polychaeta, Canalipalpata, Sabellida, Sabellidae, Serpulidae) from the rocky coast facies (Late Cenomanian) at Předboj near Prague. Sborník Národního Muzea v Praze, řada B, Přírodní védy. Acta Musei Nationalis Pragae, Series B, Historia Naturalis 71: 31–50. Crossref
Kočí, T., Bosio, G., Collareta, A., Sanfilippo, R., Ekrt, B., Urbina, M., and Malinverno, E. 2021. First report on the cirratulid (Annelida, Polychaeta) reefs from the Miocene Chilcatay and Pisco Formations (East Pisco Basin, Peru). Journal of South American Earth Sciences 107: 103042. Crossref
Kočí, T., Jäger, M., and Morel, N. 2017. Sabellid and serpulid worm tubes (Polychaeta, Canalipalpata, Sabellida) from the historical stratotype of the Cenomanian (Late Cretaceous; Le Mans region, Sarthe, France). Annales de Paléontologie 103: 45–80. Crossref
Kočí, T., Jäger, M., Šamánek, J., and Hykš, P. 2019. Tube dwelling polychaetes from the Oxfordian (Late Jurassic) of Hády Quarry at Brno (Moravia, Czech Republic). Neues Jahrbuch für Geologie und Paläontologie 294: 311–332. Crossref
Kosenko, I.N. and Ippolitov, A.P. 2020. Encrusters and borings on Gryphaea shells from the Middle Callovian of the Fokino section (Bryansk region): taphonomical and palaeoecological interpretation. In: V.A. Zakharov, M.A. Rogov, E.V. Shchepetova, and A.P. Ippolitov (eds.), Jurassic system of Russia: problems of stratigraphy and paleogeography. Proceedings of the VIIIth All-Russian Meeting with international participation, online-conference, September, 7–10, 2020, 117–121. Institute of Geology Komi, Syktyvkar.
Krajewski, M., Matyszkiewicz, J., Król, K., and Olszewska, B. 2011. Facies of the Upper Jurassic–Lower Cretaceous deposits from the southern part of the Carpathian Foredeep basement in the Kraków-Rzeszów area (southern Poland). Annales Societatis Geologorum Poloniae 81: 269–290.
Krajewski, M., Olchowy, P., and Felisiak, I. 2014. Lower Kimmeridgian layer with bored and encrusted hiatus concretions (Upper Jurassic, Central Poland): implications for stratigraphy and basin evolution. Annales Societatis Geologorum Poloniae 84: 113–129.
Krajewski, M., Olchowy, P., and Felisiak, I. 2016. Late Jurassic facies architecture of the Zloczew Graben: implications for evolution of the tectonic-controlled northern peri-Tethyan shelf (Upper Oxfordian–Lower Kimmeridgian, Poland). Facies 62: 4. Crossref
Krajewski, M., Olchowy, P., Zatoń, M., and Bajda, T. 2017. Kimmeridgian hardground-sequence boundary from the Mesozoic margin of the Holy Cross Mountains (central Poland): implications for the evolution of the northern Tethyan carbonate shelf. Facies 63: 15. Crossref
Kuklinski, P. 2009. Ecology of stone-encrusting organisms in the Greenland Sea—a review. Polar Research 28: 222–237. Crossref
Kupriyanova, E.K. 1993. A new species, Metavermilia arctica (Polychaeta, Serpulidae), from the Arctic Ocean. Sarsia 78: 155–157. Crossref
Kupriyanova, E.K., Hove, H.A. ten, Sket, B., Trontelj, P., Zakšek, V., and Rouse, G.W. 2009. Evolution of a unique freshwater cave-dwelling serpulid polychaete Marifugia cavatica Absolon and Hrabĕ, 1930. Systematics and Biodiversity 7: 389–401. Crossref
Kupriyanova, E.K., Nishi, E., Hove, H.A. ten, and Rzhavsky, A.V. 2001. A review of life history patterns in serpulimorph polychaetes: ecological and evolutionary perspectives. Oceanography and Marine Biology: an Annual Review 39: 1–101.
Kupriyanova, E.K., Rzhavsky, A.V., and Hove, H.A. ten 2019. Serpulidae Rafinesque, 1815. In: G. Purschke, M. Böggemann, and W. Westheide (eds.), Handbook of Zoology. Annelida, Volume 2: Pleistoannelida, Sedentaria II, 213–275. De Gruyter, Berlin. Crossref
Kutek, J. 1994. Jurassic tectonic events in south-eastern cratonic Poland. Acta Geologica Polonica 44: 167–222.
Kuziomko-Szewczuk, M. 2010. Jurajskie epibionty z kamieniołomu Zalas. 43 pp. Unpublished M.Sc. Thesis, University of Silesia, Sosnowiec.
Lamarck, J.B. de 1802. La nouvelle classes des Annélides. Bulletin du Muséum d’Histoire Naturelle 27: 483–517.
Lamarck, J.B. de 1818. Histoire Naturelle des animaux sans vertèbres. 612 pp. Deterville and Verdiere, Paris.
Langerhans, P. 1884. Die Wurmfauna von Madeira. 4. Zeitschrift fur Wissenschaftliche Zoologie 40: 247–285.
Latreille, P.A. 1825. Familles naturelles du règne animal, exposées succinctement et dans un ordre analytique avec l’indication de leurs genres. 570 pp. J.B. Baillière, Paris. Crossref
Lee, D.E., Scholz, J., and Gordon, D.P. 1997. Paleoecology of a late Eocene mobile rockground biota from North Otago, New Zealand. Palaios 12: 568–581. Crossref
Leone, D.E. 1970. The maturation of Hydroides dianthus. Biological Bulletin 138: 306–315. Crossref
Leonowicz, P. 2013. The significance of mudstone fabric combined with palaeoecological evidence in determining sedimentary processes—an example from the Middle Jurassic of southern Poland. Geological Quarterly 57: 243–260. Crossref
Leonowicz, P. 2015a. Ichnofabrics of shallow-marine mudstone, the result of changing environmental conditions: an example from the Middle Jurassic ore-bearing clay from southern Poland. Facies 61: article 11. Crossref
Leonowicz, P. 2015b. Storm-influenced deposition and cyclicity in a shallow-marine mudstone succession—example from the Middle Jurassic ore-bearing clays of the Polish Jura (southern Poland). Geological Quarterly 59: 325–344. Crossref
Leonowicz, P. 2016. Nearshore transgressive black shale from the Middle Jurassic shallow-marine succession from southern Poland. Facies 16: article 2570. Crossref
Levinsen, G.M.R. 1883. Systematisk-geografisk Oversigt over de nordiske Annulata, Gephyrea, Chaetognathi og Balanoglossi. Videnskabelige Meddelelser fra Dansk naturhistorisk Forening i Kjøbenhaven 1882: 160–251. Crossref
Linnaeus, C. 1767. Systema naturae, per regna tria naturae, secundum classes, ordines, genera, species, cum caracteribus, differentiis, synonymis, locis. Tomus I. Pars 2. Editio duodecima, reformata. 533–1327, L. Salvii, Holmiae. Crossref
Loba, M. and Radwańska, U. 2022. Ophiuroids of the Upper Jurassic of Kuyavia and the Kraków-Częstochowa Upland, Poland. Acta Geologica Polonica [published online, https://doi.org/10.24425/agp.2022.140432].
Luci, L., Garberoglio, R.M., and Lazo, R.G. 2013. Serpulids and other calcareous tube-dwelling encrusting polychaetes from the Early Cretaceous Agrio Formation (Neuquén Basin, Argentina). Geobios 46: 213–224. Crossref
Macellari, C.E. 1984. Revision of serpulids of the genus Rotularia (Annelida) at Seymour Island (Antarctic Peninsula) and their value in stratigraphy. Journal of Paleontology 58: 1098–1116.
Machalski, M. 1998. Oyster life positions and shell beds from the Upper Jurassic of Poland. Acta Palaeontologica Polonica 43: 609–634.
Majewski, W. 2000. Middle Jurassic concretions from Częstochowa (Poland) as indicators of sedimentation rates. Acta Geologica Polonica 50: 431–439.
Makowski, H. 1952. Callovian fauna from Łuków in Poland. Palaeontologia Polonica 4: 1–64.
Mallela, J. 2007. Coral reef encruster communities and carbonate production in cryptic and exposed coral reef habitats along a gradient of terrestrial disturbance. Coral Reefs 26: 775–785. Crossref
Mangold, C., Marchand, D., Thierry, J., and Tarkowski, R. 1996. Les Ammonites de l’Oolite de Balin (Pologne); nouvelles données et réinterprétation stratigraphique. Reuve de Palèobiologie 15: 55–77.
Marynowski, L., Zatoń, M., Simoneit, B.R.T., Otto, A., Jędrysek, M.O., Grelowski, C., and Kurkiewicz, S. 2007. Compositions, sources and depositional environments of organic matter from the Middle Jurassic clays of Poland. Applied Geochemistry 22: 2456–2485. Crossref
Matyja, B.A. 2006. Stop A17—Zalas Quarry. Callovian transgressive to condensed pelagic deposits, Lower to lowermost Middle Oxfordian deposits of sponge megafacies. In: A. Wierzbowski, R. Aubrecht, J. Golonka, J. Gutowski, M. Krobicki, B.A. Matyja, G. Pieńkowski, and A. Uchman (eds.), Jurassic of Poland and Adjacent Slovakian Carpathians. Field Trip Guidebook of 7th International Congress on the Jurassic System Poland, Kraków, September 6–18, 2006, 70–72. Kraków, Poland.
Matyja, B.A. and Wierzbowski, A. 1998. Palaeogeographic evolution of the Middle–Upper Jurassic of Poland. In: N.E. Poulsen, J. Bojesen-Koefoed, A. Drewniak, E. Głowniak, J. Ineson, B.A. Matyja, T. Merta, and A. Wierzbowski (eds.), Mellem-Øvre Jura i Polen.EEP-1995 projekt: Det polske Mellem-Øvre Epikratoniske Bassin, Stratigrafi, Facies og Bassin Historie. Program Østeuropa. Danmarks og Grønlands Geologiske Undersøgelse Rapport 1998/14: 161–179.
Matyja, B.A. and Wierzbowski, A. 2000. Ammonites and stratigraphy of the uppermost Bajocian and Lower Bathonian between Częstochowa and Wieluń, Central Poland. Acta Geologica Polonica 50: 191–209.
Matyja, B.A. and Wierzbowski, A. 2006. The oceanic “Metis Geotectonic Event” (Callovian/Oxfordian) and its implications for the peri-Tethyan area of Poland. Volumina Jurassica 4: 60–61.
Matyja, B.A., Wierzbowski, A., and Wright, J.K. 2006. The Sub-Boreal/Boreal ammonite succession at the Oxfordian/Kimmeridgian boundary at Flodigarry, Staffin Bay (Isle of Skye), Scotland. Transactions of the Royal Society of Edinburgh, Earth Sciences 96: 387–405. Crossref
Matyszkiewicz, J., Kochman, A., and Duś, A. 2012. Influence of local sedimentary conditions on development of microbialites in the Oxfordian carbonate buildups from the southern part of the Kraków-Częstochowa Upland (South Poland). Sedimentary Geology 263–264: 109–132. Crossref
Matyszkiewicz, J., Krajewski, M., Kochman, A., Kozłowski, A., and Duliński, M. 2016. Oxfordian neptunian dykes with brachiopods from the southern part of the Kraków-Częstochowa Upland (southern Poland) and their links to hydrothermal vents. Facies 62: 12. Crossref
Maughan, B.C. and Barnes, D.K.A. 2000. Epilithic boulder communities of Lough Hyne, Ireland: the influences of water movement and sediment. Journal of the Marine Biological Association of the United Kingdom 80: 767–776. Crossref
McIntosh, W.C. 1885. Report on the Annelida Polychaeta collected by H.M.S. Challenger during the years 1873–1876. Reports on the Scientific Results of the Voyage of H.M.S. “Challenger”. Zoology 12: 1–554.
McKinney, F.K. 1995. Taphonomic effects and preserved overgrowth relationships among encrusting marine organisms. Palaios 10: 279–282. Crossref
McKinney, F.K. 1996. Encrusting organisms on co-occurring disarticulated valves of two marine bivalves: comparison of living assemblages and skeletal residues. Paleobiology 22: 543–567. Crossref
Montfort, D. de 1808. Conchyliologie systématique. Coquilles univalves, cloisonées. 409 pp. F. Schoell, Paris.
Osman, R.W. 1977. The establishment and development of a marine epifaunal community. Ecological Monographs 47: 37–63. Crossref
Ostrowski, S. 2005. Autocykliczny rozwój oksfordzkich bioherm gąbkowo-mikrobialitowych centralnej i południowej Polski. Volumina Jurassica 3: 45–53.
Palmer, T.J. and Fürsich, F.T. 1974. The ecology of a Middle Jurassic hardground and crevice fauna. Palaeontology 17: 507–524.
Palmer, T.J. and Fürsich, F.T. 1981. Ecology of sponge reefs from Upper Bathonian of Normandy. Palaeontology 24: 1–23.
Palmer, T.J. and Palmer, C.D. 1977. Faunal distribution and colonization strategy in a Middle Ordovician hardground community. Lethaia 10: 179–199. Crossref
Palmer, T.J. and Wilson, M.A. 1990. Growth of ferruginous oncoliths in the Bajocian (Middle Jurassic) of Europe. Terra Nova 2: 142–147. Crossref
Parsch, K.O.A. 1956. Die Serpuliden-Fauna des südwestdeutschen Jura. Palaeontographica A, Paläozoologie, Stratigraphie 107: 211–240.
Pawlik, J.R. 1992. Chemical ecology of the settlement of benthic marine invertebrates. Oceanography and Marine Biology: an Annual Review 30: 273–335.
Perkins, T.H. 1991. Calcisabella piloseta, a new genus and species of Sabellinae (Polychaeta: Sabellidae). Bulletin of Marine Science 48: 261–267.
Philippi, A. 1844. Einige Bemerkungen über die Gattung Serpula, nebst Aufzählung der von mir im Mittelmeer mit dem Thier beobachteten Arten. Archiv für Naturgeschichte, Berlin 10: 186–198. Crossref
Pillai, T.G. 1993. A review of some Cretaceous and Tertiary serpulid polychaetes of the genera Cementula and Spiraserpula Regenhardt 1961, Laqueoserpula Lommerzheim 1979 and Protectoconorca Jäger 1983. Paläontologische Zeitschrift 67: 69–88. Crossref
Pillai, T.G. and Hove, H.A. ten 1994. On recent species of Spiraserpula Regenhardt, 1961, a serpulid polychaete genus hitherto known only from Cretaceous and Tertiary fossils. Bulletin of the Natural History Museum, London (Zoology) 60: 39–104.
Pisera, A. 1991. Upper Jurassic Sponge Megafacies in Spain: Preliminary Report. In: J. Reitner and H. Keupp (eds.), Fossil and Recent Sponges, 486–497. Springer, Berlin. Crossref
Pleş, G., Mircescu, C.V., Bucur, I.I. and Săsăran, E. 2013. Encrusting micro-organisms and microbial structures in Upper Jurassic limestones from the Southern Carpathians (Romania). Facies 59: 19–48. Crossref
Pugaczewska, H. 1967. Serpulidae from the Dano-Montian bore-hole at Boryszew, Poland. Acta Palaeontologica Polonica 12: 179–197.
Pugaczewska, H. 1970. Traces of the activity of bottom organisms on the shells of the Jurassic ostreiform pelecypods of Poland. Acta Palaeontologica Polonica 15: 425–440.
Quenstedt, F.A. 1856–1858. Der Jura. 842 pp. H. Laupp, Tübingen.
Radwańska, U. 1996. Tube-dwelling polychaetes from some Upper Cretaceous sequences of Poland. Acta Geologica Polonica 46: 61–80.
Radwańska, U. 2003. Eko-tafonomia serpulitów tytonu Brzostówki i Sławna na tle badań wieloszczetów dzisiejszych. Tomy Jurajskie 1: 99–104.
Radwańska, U. 2004. Tube-dwelling polychaetes from the Upper Oxfordian of Wapienno/Bielawy, Couiavia region, north-central Poland. Acta Geologica Polonica 54: 35–52.
Radwańska, U. and Radwański, A. 2003. Siedlisko życia, miejsce pogrzebania oraz epibionty ramienionogów Sellithyris subsella (Leymerie, 1846) z muszlowców dolnego kimerydu Małogoszcza. Volumina Jurassica 1: 85–92.
Rafinesque, C.S. 1815. Analyse de la nature ou tableau de l‘univers et des corps organisés. 224 pp. Aux dépens de l’Auteur, Palerme. Crossref
Ramsdale, R. 2021. A possible serpulid tube worm of the genus Filograna from the upper Permian Cadeby Formation of South Yorkshire, UK. Proceedings of the Yorkshire Geological Society 63 (3): pygs2020-016. Crossref
Regenhardt, H. 1961. Serpulidae (Polychaeta sedentaria) aus der Kreide Mitteleuropas, ihre ökologische, taxionomische und stratigraphische Bewertung. Mitteilungen aus dem Geologischen Staatsinstitut in Hamburg 30: 5–115.
Riding, R. 2002. Structure and composition of organic reefs and carbonate mud mounds: concepts and categories. Earth-Science Reviews 58: 163–231. Crossref
Rouse, G.W. and Fauchald, K. 1997. Cladistics and polychaetes. Zoologica Scripta 26: 139–204. Crossref
Rouse, G.W. and Pleijel, F. 2001. Polychaetes. 354 pp. Oxford University Press, Oxford.
Rovereto, G. 1901. Briozoi, anellidi e spugne perforanti del Neogene Ligure. Palaeontographica Italiana 7: 219–234.
Sadlok, G. and Zatoń, M. 2020. Ichnology of the Middle Jurassic hiatus concretions from Poland: implications for their formation, exhumation, and palaeoenvironment. Palaeobiodiversity and Palaeoenvironments 100: 757–771. Crossref
Sanfilippo, R. 2009. Systematics and life habit in Serpula israelitica Amoureux, 1977 (Polychaeta Serpulidae) from the Mediterranean with remarks on other soft-bottom serpulids. Journal of Natural History 43: 2009–2025. Crossref
Sanfilippo, R., Rosso, A., Reitano, A., and Insacco, G. 2017. First record of sabellid and serpulid polychaetes from the Permian of Sicily. Acta Palaeontologica Polonica 62: 25–38. Crossref
Sanfilippo, R., Rosso, A., Reitano, A., Viola, A., and Insacco, G. 2018. New serpulid polychaetes from the Permian of western Sicily. Acta Palaeontologica Polonica 63: 579–584. Crossref
Scheltema, R.S., Williams, I.P., Shaw, M.A., and Loudon, C. 1981. Gregarious settlement by the larvae of Hydroides dianthus (Polychaeta: Serpulidae). Marine Ecology Progress Series 5: 69–74. Crossref
Schlotheim, E.F. von 1820. Die Petrefactenkunde auf ihrem jetzigen Standpunkte. 437 pp. Becker, Gotha.
Schlögl, J., Michalík, J., Zágoršek, K., and Atrops, F. 2008. Early Tithonian serpulid-dominated cavity-dwelling fauna, and the recruitment pattern of the serpulid larvae. Journal of Paleontology 82: 382–392. Crossref
Seilacher, A., Matyja, B.A., and Wierzbowski, A. 1985. Oyster beds: morphologic response to changing substrate conditions. In: U. Bayer and A. Seilacher (eds.), Sedimentary and Evolutionary Cycles, Lecture Notes in Earth Sciences, 421–435. Springer, Berlin. Crossref
Senowbari-Daryan, B., Link, M., and Işintek, I. 2007. Filograna minor nov. sp. (Worm Tube) from the Middle Triassic (Anisian) reef boulders of the Karaburun Peninsula, Western Turkey. Turkish Journal of Earth Sciences 16: 1–9.
Słowiński, J. 2019. Środkowojurajskie onkoidy z okolic Ogrodzieńca. 57 pp. Unpublished M.Sc. Thesis, University of Silesia, Sosnowiec.
Słowiński, J., Surmik, D., Duda, P., and Zatoń, M. 2020. Assessment of serpulid-hydroid association through the Jurassic: A case study from the Polish Basin. PLoS ONE 15: e0242924. Crossref
Sousa, W.P. 1979. Disturbance in marine intertidal boulder fields: the nonequilibrium maintenance of species diversity. Ecology 60: 1225–1239. Crossref
Sowerby, J. 1815. The Mineral Conchology of Great Britain. 234 pp. The Author, London.
Sowerby, J. 1822. The Genera of Recent and Fossil Shells. 301 pp. The Author, London.
Sowerby, J. de C. 1829. The Mineral Conchology of Great Britain. 230 pp. The Author, London.
Sowerby, J. de C. 1844. The Mineral Conchology of Great Britain. 91 pp. The Author, London.
Sowerby, J. and Sowerby, G.B. 1820. The Genera of Recent and Fossil Shells, For the Use of Students in Conchology and Geology. 275 pp. G.B. Sowerby, London. Crossref
Stiller, F. 2000. Polychaeta (Annelida) from the Upper Anisian (Middle Triassic) of Qingyan, south-western China. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen 217: 245–266. Crossref
Szewczuk, A. 2010. Sklerobionty z kimerydzkich ławic ostrygowych Małogoszczy. 44 pp. Unpublished M.Sc. Thesis, University of Silesia, Sosnowiec.
Tapaswi, P.M. 1988. Role of serpulids in the biostratigraphy of the Upper Cretaceous rocks from Tiruchirapally District, South India. Recent Researches in Geology 12: 183–187.
Tarkowski, R., Thierry, J., Marchand, D., Mangold, C., Delance, J.-H., Garcia, J.-P., and Laurin, B. 1994. L’“oolithe de Balin” (Pologne méridionale). Nouvelles observations et interprétations stratigraphiques. The “Balin Oolithe” (Southern Poland). New observations and stratigraphical interpretations Geobios 27: 461–467. Crossref
Taylor, P.D. 2008. Bryozoans from the Middle Jurassic of Balin, Poland: a revision of material described by A.E. Reuss (1867). Annalen des Naturhistorischen Museums in Wien.Serie A für Mineralogie und Petrographie, Geologie und Paläontologie, Anthropologie und Prähistorie 110: 17–53.
Taylor, P.D. 2016. Competition between encrusters on marine hard substrates and its fossil record. Palaeontology 59: 481–497. Crossref
Taylor, P.D. and Wilson, M.A. 2002. A new terminology for marine organisms inhabiting hard substrates. Palaios 17: 522–525. Crossref
Taylor P.D. and Wilson, M.A. 2003. Palaeoecology and evolution of marine hard substrate communities. Earth-Scientific Reviews 62: 1–103. Crossref
Toonen, R.J. and Pawlik, J.R. 1996. Settlement of the gregarious tube worm Hydroides dianthus (Polychaeta: Serpulidae): cues for gregarious settlement. Marine Biology 126: 725–733. Crossref
Trammer, J. 1982. Lower to Middle Oxfordian sponges of the Polish Jura. Acta Geologica Polonica 32: 1–39.
Trammer, J. 1985. Sponge bioherms in the Jasna Góra Beds (Oxfordian of the Polish Jura Chain). Przegląd Geologiczny 33: 78–81.
Trammer, J. 1989. Middle to Upper Oxfordian sponges of the Polish Jura. Acta Geologica Polonica 39: 49–92.
Védrine, S., Strasser, A., and Hug, W. 2007. Oncoid growth and distribution controlled by sea-level fluctuations and climate (Late Oxfordian, Swiss Jura Mountains). Facies 53: 535–552. Crossref
Vermeij, G.J. 1977. The Mesozoic marine revolution: evidence from snails, predators and grazers. Paleobiology 3: 245–258. Crossref
Vinn, O. and Taylor, P.D. 2007. Microconchid tubeworms from the Jurassic of England and France. Acta Palaeontologica Polonica 52: 391–399.
Vinn, O. and Wilson, M.A. 2010. Sabellid-dominated shallow water calcareous polychaete tubeworm association from the equatorial Tethys Ocean (Matmor Formation, Middle Jurassic, Israel). Neues Jahrbuch für Geologie und Paläontologie Abhandlungen 258: 31–38. Crossref
Vinn, O., Hove, H.A. ten, and Mutvei, H. 2008a. On the tube ultrastructure and origin of calcification in sabellids. Palaeontology 51: 295–301. Crossref
Vinn, O., Hryniewicz, K., Little, C.T.S., and Nakrem, H.A. 2014. A Boreal serpulid fauna from Volgian–Ryazanian (latest Jurassic–earliest Cretaceous) shelf sediments and hydrocarbon seeps from Svalbard. Geodiversitas 36: 527–540. Crossref
Vinn, O., Mutvei, H., Hove, H.A. ten, and Kirsimäe, K. 2008b. Unique Mg-calcite skeletal ultrastructure in the tube of the serpulid polychaete Ditrupa. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen 248: 79–89. Crossref
Ware, S. 1975. British Lower Greensand Serpulidae. Palaeontology 18: 93–116.
Wieczorek, S.K. and Todd, C.D. 1998. Inhibition and facilitation of settlement of epifaunal marine invertebrate larvae by microbial biofilm cues. Biofouling 12: 81–118. Crossref
Wierzbowski, H., Dembicz, K., and Praszkier, T. 2009. Oxygen and carbon isotope composition of Callovian–Lower Oxfordian (Middle–Upper Jurassic) belemnite rostra from central Poland: A record of a Late Callovian global sea-level rise? Palaeogeography, Palaeoclimatology, Palaeoecology 283: 182–194. Crossref
Wierzbowski, A., Atrops, F., Grabowski, J., Hounslow, M.W., Matyja, B.A., Olóriz, F., Page, K.N., Parent, H., Rogov, M.A., Schweigert, G., Villaseñor, A.B., Wierzbowski, H., and Wright, J.K. 2016. Towards a consistent Oxfordian/Kimmeridgian global boundary: current state of knowledge. Volumina Jurassica 14: 15–50.
Wignall, P.B. 1990. Depositional history and palaeoecology of the Oxfordian/Kimmeridgian boundary beds at South Ferriby, South Humberside. Proceedings of the Yorkshire Geological Society 48: 197–208. Crossref
Wilson, M.A. 1985. Disturbance and ecologic succession in an Upper Ordovician cobble-dwelling hardground fauna. Science 228: 575–577. Crossref
Wilson, M.A. 1987. Ecological dynamics on pebbles, cobbles, and boulders. Palaios 2: 594–599. Crossref
Wilson, M.A. 1998. Succession in a Jurassic marine cavity community and the evolution of cryptic marine faunas. Geology 26: 379–381. Crossref
Wilson, M.A. and Palmer, T.J. 1990. A review of evolutionary trends in carbonate hardground communities. In: W. Miller (ed.), Paleocommunity Temporal Dynamics: the Long-Term Development of Multispecies Assemblies. The Paleontological Society, Special Publication 5: 137–152. Crossref
Wilson, M.A., Feldman, H., Bowen, J.C., and Avni, Y. 2008. A new equatorial, very shallow marine sclerozoan fauna from the Middle Jurassic (late Callovian) of southern Israel, Palaeogeography, Palaeoclimatology, Palaeoecology 263: 24–29. Crossref
Zatoń, M. and Machalski, M. 2013. Oyster-microbial rolling stones from the Upper Jurassic (Kimmeridgian) of Poland. Palaios 28: 839–850. Crossref
Zatoń, M. and Taylor, P.D. 2009a. Microconchids (Tentaculita) from the Middle Jurassic of Poland. Bulletin of Geosciences 84: 653–660. Crossref
Zatoń, M. and Taylor, P.D. 2009b. Middle Jurassic cyclostome bryozoans from the Polish Jura. Acta Palaeontologica Polonica 54: 267–288. Crossref
Zatoń, M., Kremer, B., Marynowski, L., Wilson, M.A., and Krawczyński, W. 2012. Middle Jurassic (Bathonian) encrusted oncoids from the Polish Jura, southern Poland. Facies 58: 57–77. Crossref
Zatoń, M., Machocka, S., Wilson, M. A., Marynowski, L. and Taylor, P.D. 2011a. Origin and paleoecology of Middle Jurassic hiatus concretions from Poland. Facies 57: 275–300. Crossref
Zatoń, M., Marynowski, L., and Bzowska, G. 2006. Konkrecje hiatusowe z iłów rudonśnych Wyżyny Krakowsko-Częstochowskiej. Przegląd Geologiczny 54: 131–138.
Zatoń, M., Marynowski, L., Szczepanik, P., Bond, D.P.G., and Wignall, P.B. 2009. Redox conditions during sedimentation of the Middle Jurassic (Upper Bajocian–Bathonian) clays of the Polish Jura (south-central Poland). Facies 55: 103–114. Crossref
Zatoń, M., Wilson, M.A. and Zavar, E. 2011b. Diverse sclerozoan assemblages encrusting large bivalve shells from the Callovian (Middle Jurassic) of southern Poland. Palaeogeography, Palaeoclimatology, Palaeoecology 307: 232–244. Crossref
Ziegler, V. and Michalík, J. 1980. Late Triassic serpulids (Annelida, Polychaetia, Sedentarida) in the Western Carpathians. Geologicky Zborník – Geologica Carpathica 31: 627–640.
Ziegler, P.A. 1990. Geological Atlas of Western and Central Europe. 239 pp. Shell Internationale Petroleum Maatschappij B.V., The Hague.
Zuschin, M., Stachowitsch, M., Pervesler, P. and Kollmann, H. 1999. Structural features and taphonomic pathways of a high-biomass epifauna in the northern Gulf of Trieste, Adriatic Sea. Lethaia 32: 299–317. Crossref
Acta Palaeontol. Pol. 67 (4): 827–864, 2022
https://doi.org/10.4202/app.01006.2022