

New species of mirid insects and their importance for the higher classification of plant bugs
JUNGGON KIM, ARTUR TASZAKOWSKI, ALEKSANDER HERCZEK, MARZENA ZMARZŁY, and SUNGHOON JUNG
Kim, J., Taszakowski, A., Herczek, A., Zmarzły, M., and Jung, S. 2023. New species of mirid insects and their importance for the higher classification of plant bugs. Acta Palaeontologica Polonica 68 (1): 75–83.
The detailed morphological study based on the findings of well-preserved fossil specimens in Eocene Baltic amber revealed two new species, Metoisops michalskii Kim, Taszakowski, and Herczek sp. nov. and Metoisops popovi Kim, Taszakowski, and Jung sp. nov. The morphological information of the extinct genus Metoisops including diagnoses, descriptions of new species, and a species key are provided. The divided fourth antennal segment is depicted. The tribal transfer of Metoisops from Electromyiommini to Gigantometopini is proposed based on major morphological characters, five-six femoral trichobothria, presence of a deep incision between calli, and the structure of parameres. The need for a phylogenetic revision of the internal classification within Isometopinae is also discussed.
Key words: Hemiptera, Heteroptera, Cimicomorpha, Miridae, Isometopinae, classification, jumping tree bugs, Baltic amber.
Junggon Kim [thesv12@gmail.com; ORCID: https://orcid.org/0000-0003-0594-7618 ], 1719 Gyebaek-ro, Jung-gu, Daejeon, Korea.
Artur Taszakowski [artur.taszakowski@us.edu.pl; ORCID: https://orcid.org/0000-0002-0885-353X ], Aleksander Herczek [aleksander.herczek@us.edu.pl; ORCID: https://orcid.org/0000-0001-6047-5268 ], and Marzena Zmarzły [marzena.zmarzly@us.edu.pl; ORCID: https://orcid.org/0000-0002-0631-6079 ], Institute of Biology, Biotechnology and Environmental Protection, Faculty of Natural Sciences, University of Silesia in Katowice, Bankowa 9, 40-007 Katowice, Poland.
Sunghoon Jung [jung@cnu.ac.kr; ORCID: https://orcid.org/0000-0001-6086-0326 ] (corresponding author), Laboratory of Systematic Entomology, Department of Applied Biology, College of Agriculture and life Sciences, Chungnam National University, 99, Daehak-ro, Daejeon, Korea and Department of Smart Agriculture Systems, College of Agriculture and Life Sciences, Chungnam National University, 99, Daehak-ro, Daejeon, Korea.
Received 8 March 2022, accepted 12 December 2022, available online 10 February 2023.
Copyright © 2023 J. Kim et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Fossil specimens are important to explore the ancient characters, and it is especially the case for fossil insects included in amber. Baltic amber is one of the important materials for historical studies such as biogeography and palaeoclimate reconstructions because it usually provides well-preserved insect inclusions from the Eocene when significant climatic changes presumably formed the backbone for Recent biota (Zachos et al. 2001; Bogri et al. 2018). However, it is sometimes not easy to properly examine the important characters due to the fossil condition (e.g., transparency, impurities), which can lead the systematic position to be unclear or temporary.
The subfamily Isometopinae (Insecta: Hemiptera: Cimicomorpha) is one of the small groups in the large family Miridae, with six tribes and 279 described species worldwide (Herczek 1993; Schuh 2002–2013; Yasunaga et al. 2017). This subfamily is currently considered monophyletic (Schuh et al. 2009; Jung and Lee 2012), and is considered a sister-group to the remaining groups of Miridae, characterized by autapomorphic characters, such as possessing paired ocelli (Cassis and Schuh 2012). Interestingly, this group contains a large proportion of fossil species (approximately 30%) (Schuh and Weirauch 2020), and the fossils represent majority of the tribes of the current classification by Herczek (1993) based on the comprehensive morphological study of the extant and extinct taxa. The members of this subfamily are also called “jumping tree bugs”, which reflects their behavior: the isometopines live on bark and feed on scale insects (Schuh and Weirauch 2020), unlike members of other mirid subfamilies. The biological characteristic of isometopines may predispose them to be more easily fossilized in tree resins than the most other groups of Miridae.
The genus Metoisops was established in 1992 with the description of the type species Metoisops kerzhneri Popov and Herczek, 1992. A year later, Metoisops punctatus Popov and Herczek, 1993, was described, and then seven more in 2014 from the late Eocene Baltic, Ukrainian (Rovno), and Saxonian (Bitterfeld) ambers (Herczek and Popov 2014). An analysis of all studied specimens referring to Metoisops from different Eocene European ambers demonstrates the great variability of their features, and allows for differentiation of species (Popov and Herczek 1992, 1993; Herczek and Popov 2014), which makes Metoisops the most speciose extinct genus in Isometopinae.
Up to date, Metoisops has been placed in the tribe Electromyiommini. The characters of this tribe are: body elongated; fronto-clypeal part of head not expanded dorso-ventrally or laterally; clypeus well-marked, not shifted from the frons; eyes enlarged, separated from antero-lateral margins of pronotum; ocelli in a distance from the posterior head margin; pronotum with a marked, narrow and flattened collar (the collar differs morphologically from that in Deraeocorinae and Mirinae); calli usually distinct, divided by a median groove; and vein R+M in hind wings not shortened, running parallelly to the anterior wing margin. The tribe consist of six fossil genera described so far: Electromyiomma Popov and Herczek, 1992; Clavimyiomma Popov and Herczek, 1992; Metoisops Popov and Herczek, 1992; Archemyiomma Herczek, 1993; Electroisops Herczek and Popov, 1997, and Hoffeinsoria Herczek and Popov, 2012. However, those genera need to be reviewed for the tribal placement, as most were provisionally assigned. One of them (Hoffeinsoria) was already reconsidered as incertae sedis by Schuh and Weirauch (2020).
The Oriental (ranging from India to Southeast Asia) tribe Gigantometopini is a small tribe in Isometopinae with 16 described species (Taszakowski et al. 2020, 2021a, 2022; Kim et al. 2021; Yeshwanth et al. 2021). It displays unique morphological characters such as strongly elongate large body, five and six femoral trichobothria, and two cells in the membrane (Herczek 1993). After new research on Gigantometopini (e.g., Herczek et al. 2018), additional species with interesting morphological characters have been revealed.
Herein, the tenth and eleventh Metoisops species, Metoisops michalskii Kim, Taszakowski, and Herczek sp. nov. and Metoisops popovi Kim, Taszakowski, and Jung sp. nov. are described from Eocene Baltic amber. Morphological information including a key to the species of the genus is also provided. Additionally, the tribal transfer of Metoisops to Gigantometopini and the need for revision of the isometopine tribes are discussed based on morphological characters and the number of trichobothria in particular.
Institutional abbreviations.—CEHI, Collection of Ernst Heiss, Tiroler Landesmuseum (Tyrolean State Museum), Innsbruck, Austria; CNU, Laboratory of Systematic Entomology, Chungnam National University, Daejeon, Korea; DZUS, Institute of Biology, Biotechnology and Environmental Protection collections, Faculty of Natural Sciences, University of Silesia in Katowice, Poland.
Other abbreviations.—R+M, radial+medial vein.
Nomenclatural acts.—This work and the nomenclatural acts it contains, have been registered in ZooBank: urn:lsid:zoobank.org:pub:C0D2C032-4B59-4193-89EF-C67D470763AA
Material and methods
The amber with a specimen of Metoisops michalskii Kim, Taszakowski, and Herczek sp. nov. was found in the Vistula Spit (Gdańsk Bay, Poland) (Fig. 1A). The new fossil species, Metoisops popovi Kim, Taszakowski, and Jung sp. nov. is preserved in a yellow piece of the Baltic amber from unknown locality (Fig. 1B); this amber was probably from Gdańsk Bay (Chłapowo, district Gdańsk, Poland) or Kaliningrad area (Sambian Peninsula, Russia). See Bogri et al. (2018) for general information on the place of origin and the age of Baltic amber. The term of Baltic amber is collective term therefore the reader is referred to Szwedo and Sontag (2009) and Bogri et al. (2018) for more detailed information on particular deposits included in it. The age of Baltic amber remains still controversial (Shavrin and Yamamoto 2019), in spite of the studies on the amber source (e.g., Bogri et al. 2018; Bukejs et al. 2019), therefore, we tentatively accept here the age of Baltic amber, as mid-Eocene (44.1±1.1 Ma) based on the most recently used estimation method by the absolute dating analyses of glauconites from Sambia Peninsula (Wappler 2005).
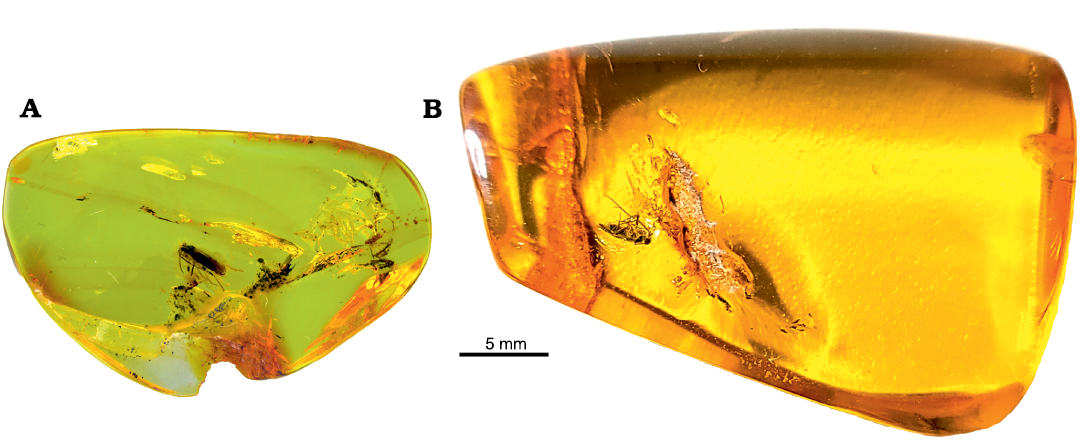
Fig. 1. Baltic ambers with specimens of mirid insects. A. Metoisops michalskii Kim, Taszakowski, and Herczek sp. nov., holotype male, DZUS HE44-451-1-001, from the Baltic Amber (Vistula Spit, Gdańsk Bay, Poland), mid-Eocene. B. Metoisops popovi Kim, Taszakowski, and Jung sp. nov., holotype female, CNU CNUHHMF005, from the Baltic Amber (unknown locality on Baltic Sea Coast), mid-Eocene.
The type specimens of the new species were photographed in the Laboratory of Insects Anatomy and Morphology of the Institute of Biology, Biotechnology and Environmental Protection, University of Silesia in Katowice (Katowice, Poland) using the following equipment: Nikon Eclipse E-600 upright light microscope with a Nikon DS-Fi2 digital camera; Nikon SMZ 1500 stereo microscope with a fiber optic illuminator SCHOTT KL 300 LED, ring light SCHOTT EasyLED and Nikon DS-Fi3 digital camera; NIS Elements 4.10 software, CorelDraw X6 2012 graphic editor with Intuos 4 Wacom Graphics tablet. All measurements are given in millimeters (mm). The tribal classification follows mainly Herczek (1993), and the new subsequent tribe erected by Yasunaga et al. (2017) is also considered, in agreement with the treatment of isometopine classification in Schuh and Weirauch (2020). The morphological terminology follows Herczek (1993) and Schuh and Weirauch (2020).
Systematic palaeontology
Class Insecta Linnaeus, 1758
Order Hemiptera Linnaeus, 1758
Family Miridae Hahn, 1833
Subfamily Isometopinae McAtee and Malloch, 1924
Tribe Gigantometopini Herczek, 1993
Genus Metoisops Popov and Herczek, 1992
Type species: Metoisops kerzhneri Popov and Herczek, 1992; Poland, Baltic amber, mid-Eocene (ca. 44.1±1.1 Ma according to Wappler 2005).
Emended diagnosis.—Differs from other extinct genera in the subfamily Isometopinae by body elongated oval, small to moderate, approximately 2.4–3.3 mm; dorsum deeply punctate, covered with densely long and golden pubescence; compound eye slightly prominent; ocelli large or medium sized; length of second antennal segment almost same as third and fourth segments combined; in some species fourth antennal segment subdivided; vertex wide; labium exceeding hind coxae, reaching first or third abdominal segment; posterior margin of pronotum straight or slightly concave in middle; pronotal collar narrow; calli weakly swollen; propleuron distinctly punctate (Figs. 2A2, 3A2, A3); mesoscutum narrowly or normally developed; hemelytral membrane with two cells; tarsi two segmented.
Description.—See Herczek and Popov (2014) for detailed redescription.
Remarks.—We propose herein the tribal replacement of Metoisops to the Gigantometopini based on the major morphological characters. See the discussion section below for details.
Herczek and Popov (2014) provided illustrations of each fossil specimen classified to Metoisops to date. In some species (e.g., Metoisops intergerivus Herczek and Popov, 2014, and Metoisops punctatus Popov and Herczek, 1993), the fourth antennal segment is subdivided, reminiscent of the antennae with five or six segments, what is unique in Miridae. Nonetheless, the species possessing the subdivided fourth antennal segment are currently affiliated in the same genus. At this point, these species remain in Metoisops pending fourth segment evaluation and confirmation of the monophyly based on the phylogenetic approach. The state of fourth antennal segment subdivided may be used as a diagnostic character for the subgeneric subdivision in accordance to the phylogenetic result.
Metoisops michalskii Kim, Taszakowski, and Herczek sp. nov.
Figs. 1A, 2.
Zoobank LCID: urn:lsid:zoobank.org:act:D378FF45-A830-4D05-B346-6FAAC446208A
Etymology: Named after Artur Michalski, a collector of the new fossil; a noun in the genitive case.
Holotype: DZUS HE44-451-1-001, male in 23×14 ×4 mm in piece of Baltic amber, with the curatorial museum code.
Type locality: Vistula Spit, Gdańsk Bay, Poland.
Type horizon: Baltic amber, mid-Eocene (ca. 44.1±1.1 Ma according to Wappler 2005).
Material.—Holotype only.
Diagnosis.—Differs from other congeners by the following combination of characters: body relatively small, deeply punctate, covered with densely golden pubescence (Fig. 2A1); head extremely transverse, wider than 3× longitudinal length; vertex distinctly narrow; compound eye wide, single compound eye as wide as 3× vertex; second antennal segment thick, slightly clavate; third segment shorter than fourth segment; fourth segment subdivided (Fig. 2A3); second labial segment shortest; third labial segment longest; posterior pronotal width shorter than 2 times longitudinal length; embolium impunctate; basal and apical parts of femur dark brown.
Description.—Male body elongate-oval, length 2.69 mm. Coloration mostly dark brown, partly pale brown. Head mostly dark brown; vertex and frons dark; clypeus and juga dark brown; antennae mostly dark brown; apical part of first segment, second segment and apical part of first subdivision of fourth segment somewhat paler; labium mostly pale brown, apex of fourth labial segment dark brown. Thorax pronotum entirely dark brown; scutellum pale brown; hemelytron mostly dark brown; embolial margin paler; membrane grayish, subhyaline; legs pale brown with dark bands; basal and apical parts of femur dark brown. Abdomen entirely dark brown.
Body generally glossy, mostly punctate, densely covered with golden pubescence; frons and clypeus impunctate, covered with relatively short pubescence; antennae with short and dense erect setae; pronotum with large and dense punctures except for calli region, densely covered with golden pubescence; scutellum with small punctures and with somewhat short pubescence; mesoscutum impunctate; hemelytra with large punctures, covered with dense golden pubescence; embolial area impunctate; cuneus impunctate.
Head hypognathous, elongate in lateral view, anterior margin almost straight in dorsal view, less than pronotum height in lateral view; vertex narrow, approximately 3 times as wide as single compound eye; compound eye slightly prominent in frontal view; ocelli large, almost touching compound eye, not close to each other; fovea antennalis positioned below compound eyes; antennae shorter than the body, four segmented including subdivided fourth segment, cylindrical; first segment slightly longer than the third segment; second segment longest and thickest, longer than the third and fourth segments combined; third segment shorter than fourth segment; proportion of first to fourth antennal segments 0.20: 0.81: 0.26: 0.39 (first subsegment: 0.20; second subsegment 0.19); frontal-clypeal part rather elongate; labium somewhat thick, exceeding hind coxae, reaching third abdominal segment. Thorax pronotum trapezoid, midline length longer than anterior width and 1/2 posterior width respectively, posterior margin weakly convex in middle, lateral margin straight, posterior angle slightly carinate; pronotal collar thin; calli region weakly swollen; scutellum large, midline as same as pronotal midline length, width more than anterior pronotal width; mesoscutum narrowly developed; lateral margin of hemelytra rounded; commissure as long as scutellum length; cuneus broad, inner margin straight, cuneal fracture weakly developed; membrane with two cells (large and very small); legs moderately long; hindfemur not reaching the apex of abdomen; tarsus two segmented (Fig. 2A5). Abdomen elongate, exceeding apex of cuneus. Genital capsule with a pair of relatively large parameres, left paramere subequal to right paramere in length; left paramere scythe-shaped, hypophysis broad; right paramere elongate and curved, hypophysis tapered to apex (Fig. 2A6).

Fig. 2. Mirid insect Metoisops michalskii Kim, Taszakowski, and Herczek sp. nov., holotype male, DZUS HE44-451-1-001, from the Baltic Amber (Gdańsk Bay, Poland), mid-Eocene. Dorsal habitus (A1), lateral habitus (A2), head and thorax in lateral view and antennal structure (A3), abdomen and legs in lateral view (A4), hindtarsus (A5), genital segment with parameres (A6). Abbreviations: i, first antennal segment; ii, second antennal segment; iii, third antennal segment; iv, fourth antennal segment; iv-1, first subsegment of fourth antennal segment; iv-2, second subsegment of fourth antennal segment.
Measurements (in mm).—Body length 2.69; head length 0.18; head width including compound eyes 0.54; vertex width 0.08; first antennal segment 0.20; second antennal segment 0.81; third antennal segment 0.26; fourth antennal segment 0.39 (subsegments; first 0.20; second 0.19); first labial segment 0.27; second labial segment 0.32; third labial segment 0.29; fourth labial segment 0.34; pronotal midline length 0.48; basal pronotal maximal width (straight) 0.90; anterior scutellar width 0.52; scutellar midline length 0.37; commissure length 0.46; outer embolial margin length (straight) 1.24; maximal width across hemelytron 0.42; hindleg (femur: tibia: tarsus) 0.92: 1.20: 0.33.
Remarks.—The subequal parameres in length and the structure of apices of parameres (broad hypophysis in left paramere and tapered hypophysis in right paramere) are similar to those found in Gigantometopini. The paramere morphology of fossil in Isometopinae is the first finding.
Stratigraphic and geographic range.—Type horizon and locality only.
Metoisops popovi Kim,
Taszakowski, and Jung
sp. nov.
Figs. 1B, 3.
Zoobank LCID: urn:lsid:zoobank.org:act:9143B0B3-926B-469E-A372-E8491E48C37B
Etymology: Named after the late Professor Yuri A. Popov, a specialist of fossil taxa and the one of the authors of the genus Metoisops; a noun in the genitive case.
Holotype: CNU CNUHHMF005, female in a 25×39 ×15 mm in piece of Baltic amber, with the curatorial museum code.
Type locality: Unknown locality on Baltic Sea Coast, Poland or Russia.
Type horizon: Baltic amber, mid-Eocene (ca. 44.1±1.1 Ma according to Wappler 2005).
Material.—Holotype only.
Diagnosis.—Differs from other congeners by the following combination of characters: body size large, more than 3 mm, deeply punctate, covered with densely golden pubescence (Fig. 3A1); head extremely transverse, wider than 3× longitudinal length; vertex wide, as wide as 1/4 head; compound eye as wide as 1.6× vertex; second antennal segment as thick as first segment; third segment longer than fourth segment; fourth antennal segment not divided; second labial segment shortest; third labial segment longest (Fig. 3A1–A4); posterior pronotal width shorter than 2 times longitudinal length; mesoscutum narrow (Fig. 3A5); embolium impunctate.
Description.—Female body elongate-oval, length 3.31 mm. Mostly dark brown, partly pale brown. Head mostly pale brown; vertex and frons dark; clypeus and juga dark brown; antennae mostly dark brown; fourth antennal segment somewhat paler; labium mostly brown, apex of fourth labial segment dark brown. Thorax pronotum entirely dark brown; scutellum paler, brown; hemelytron mostly dark brown; embolial margin paler; membrane grayish, subhyaline; legs entirely pale brown. Abdomen entirely dark brown.
Body generally glossy, mostly punctate, densely covered with golden pubescence; frons and clypeus impunctate, covered with relatively short pubescence; antennae with short and dense erect setae; pronotum with large and dense punctures except for calli region, densely covered with golden pubescence; scutellum with small punctures and with somewhat short pubescence; mesoscutum impunctate; hemelytra with large punctures, covered with dense golden pubescence; embolial area impunctate; cuneus impunctate.
Head hypognathous, elongate in lateral view, anterior margin straight in dorsal view, less than pronotum height in lateral view; vertex relatively wide, 1.68 times as wide as single compound eye; compound eye slightly prominent in frontal view; ocelli large, positioned near compound eye, not close to each other; fovea antennalis positioned below compound eyes; antennae shorter than the body, four segmented, cylindrical; first segment shorter than the fourth segment, as thick as second segment; second segment longest, shorter than the third and fourth segments combined; third segment longer than 2 times fourth segment; proportion of first to fourth antennal segments 0.27: 1.01: 0.77: 0.3; frontal-clypeal part rather elongate; labium somewhat thick, exceeding hind coxae, reaching third abdominal segment. Thorax pronotum trapezoid, midline length longer than anterior width and 1/2 posterior width respectively, posterior margin weakly convex in middle, lateral margin straight, posterior angle slightly carinate; pronotal collar narrow; calli region weakly swollen; scutellum large, midline the same as pronotal midline length, width more than anterior pronotal width; mesoscutum narrowly developed; lateral margin of hemelytra rounded; commissure as long as scutellum length; cuneus broad, inner margin straight, cuneal fracture weakly developed; membrane with two cells (large and very small); legs moderately long; hindfemur not reaching the apex of abdomen; tarsus two segmented. Abdomen elongate, exceeding apex of cuneus. Genitalia not examined.
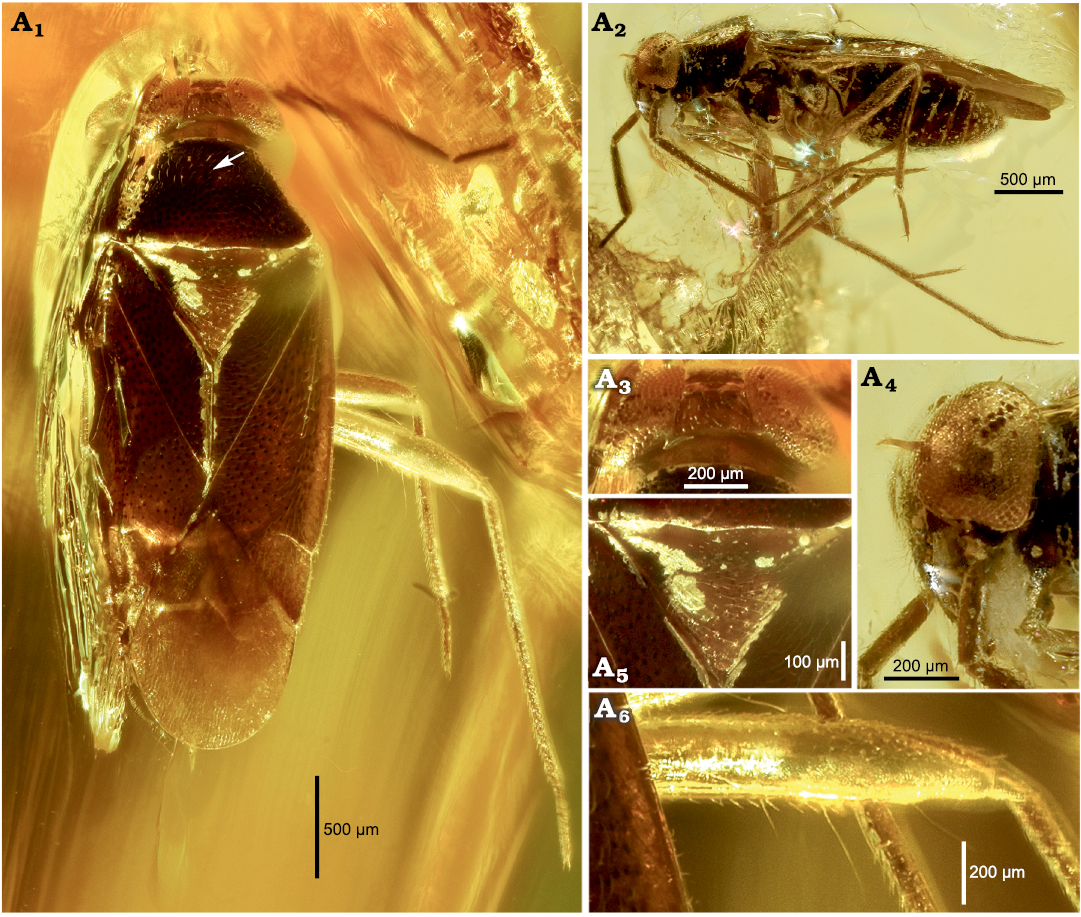
Fig. 3. Mirid insect Metoisops popovi Kim, Taszakowski, and Jung, sp. nov., holotype female, CNU CNUHHMF005, from the Baltic Amber (unknown locality on Baltic Sea Coast), mid-Eocene. Dorsal habitus. Arrow points to deep incision between calli (A1), lateral habitus (A2), head in dorsal view (A3), head in lateral view (A4), scutellum (A5), hindfemur with trichobothria (A6).
Measurements (in mm).—Body length 3.31; head length 0.18; head width including compound eyes 0.65; vertex width 0.16; first antennal segment 0.27; second antennal segment 1.01; third antennal segment 0.77; fourth antennal segment 0.3; first labial segment 0.56; second labial segment 0.21; third labial segment 0.6; fourth labial segment 0.33; pronotal midline length 0.52; basal pronotal maximal width (straight) 0.96; anterior scutellar width 0.80; scutellar midline length 0.60; commissure length 0.61; outer embolial margin length (straight) 1.47; maximal width across hemelytron 0.64; hindleg (femur: tibia: tarsus) 1.28: 1.44: 0.35.
Stratigraphic and geographic range.—Type horizon and locality only.
Discussion
We propose herein to transfer Metoisops from Electromyiommini to Gigantometopini based on three characters. The first is the undoubted presence of five meso- and six metafemoral trichobothria found in Metoisops akingbohungbei (Fig. 4) and Metoisops popovi Kim, Taszakowski, and Jung sp. nov. (Fig. 3A6; three of six trichobothria are only visible in the photograph, but all are visible under the microscope). This feature is considered to be one of the determining factors of the Gigantometopini, although this should be evaluated based on the cladistics analysis. The second feature is the presence of a deep incision (pit) between calli (arrow in Fig. 3A1). While it was impossible to determine the number of trichobotria in other described species of the Metoisops (the technical condition or the arrangement of the specimen in amber does not allow it), all the described species have a deep groove in the middle of the calli which occurs in the members of Gigantometopini (see Taszakowski et al. 2020, 2021a; Kim et al. 2021 for the structure within Gigantometopini). The paramere structure is one of the pieces of evidence. In most cases of the other isometopine tribes, right paramere is distinctly smaller than left paramere in length (for Diphlebini, see Henry 1977: figs. 6, 7; for Myiommini, see Yasunaga et al. 2017: fig. 5; Namyatova and Cassis 2016: fig. 7; for Isometopini, see Kim and Jung 2015: fig. 2; Yeshwanth et al. 2021: fig. 12; and for Sophianini, see Taszakowski et al. 2021b: fig. 4; Yeshwanth et al. 2021: fig. 16). This similar length of parameres is usually found in the most gigantometopine groups (e.g., Gigantometopus Schwartz and Schuh, 1990, and Megalofaciatus Taszakowski, Kim, and Herczek, 2021). In addition, this proposal was also based on the very high similarity (especially the head structure) of Metoisops species to the extant gigantometopine taxa such as Isometopidea Poppius, 1913, and Kohnometopus Yasunaga, 2005, although these taxa are in need of the evaluation of their placement based on the cladistics approach.
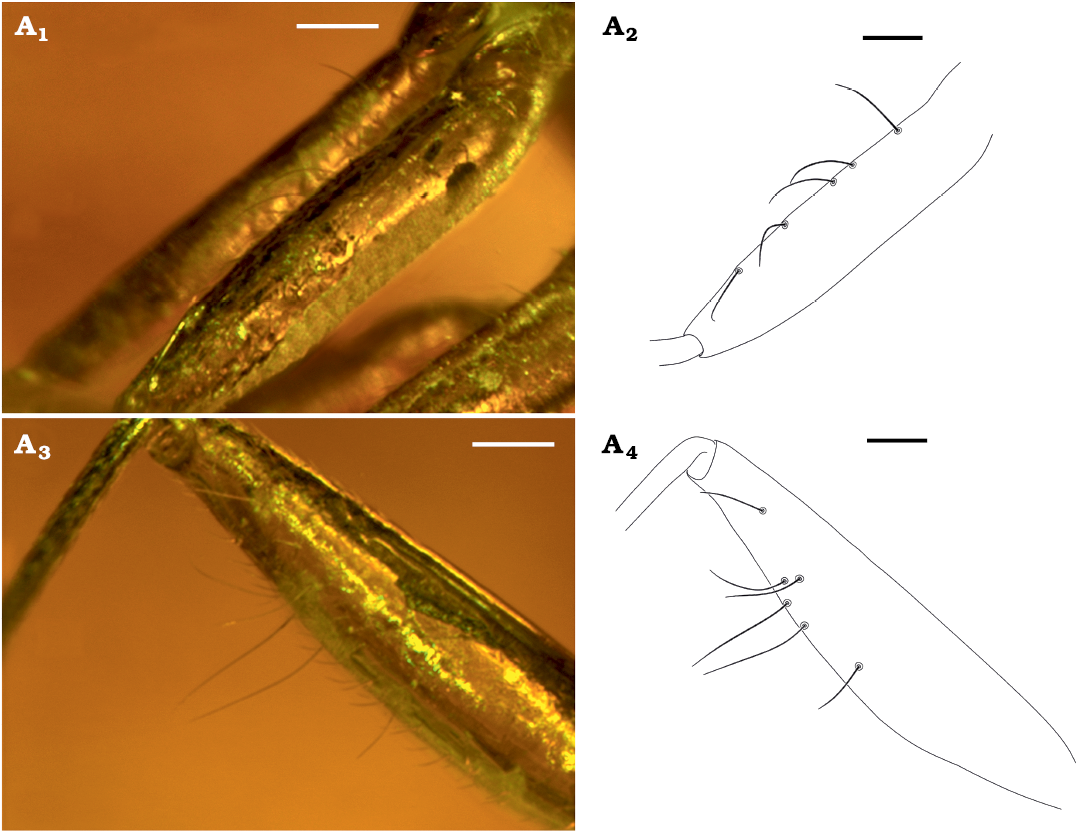
Fig. 4. Photographs (A1, A3) and drawings (A2, A4) of femoral trichobothria in mirid insect Metoisops akingbohungbei Herczek and Popov, 2014, holotype male, CEHI BB M HE 4, from the Baltic Amber (unknown locality on Baltic Sea Coast), mid-Eocene. Five mesofemoral trichobothria (A1, A2); six metafemoral trichobothria (A3, A4). Scale bars 0.1 mm.
Meanwhile, Taszakowski et al. (2021b) recently showed that the recently erected tribe Sophianini, established for taxa originally placed in the tribe Myiommini, displays the four to five trichobothria as well (see Taszakowski et al. 2021b: fig. 3). This tribe is easily distinguished from the Gigantometopini by many characters such as the antennae originating near the compound eye, the largely modified first and second antennal segments and the remarkably polished pronotum (here the major characters are only indicated; also see the diagnosis of the Sophianini in the original description [Yasunaga et al. 2017] for detailed characters); nonetheless, the cladistics analysis within Isometopinae is needed to evaluate the internal tribal classification, given that the number of the trichobothria is currently considered important to the tribal classification within Isometopinae (all tribes except for Gigantometopini and Sophianini have two or three trichobothria), and that the characters which traditionally supported the tribes have been revised by the recent studies.
The number of tarsal segmentations (3-segmented tarsi) was one of the diagnostic characters for Gigantometopini within Isometopinae; however, it is reported that the tarsal segmentations in Gigantometopini are variable in the recent studies, depending on the group (e.g., 2-segmented: Kohnometopus and Planicapitus (Yasunaga 2005; Taszakowski et al. 2020); and 3-segmented: Gigantometopus and Megalofaciatus (Kim et al. 2021; Taszakowski et al. 2021a). Considering the recent discoveries, the proposal of tribal replacement of the Metoisops possessing the two segmented tarsi to Gigantometopini is also supported.
Conclusions
The description of two new fossil species in this work has an influences the understanding of the systematics of the mirid subfamily Isometopinae, as they display morphological characters suggesting that the tribal placement of Metoisops should be reconsidered.
We believe that two new species and the morphological characters addressed above may be also considered in further isometopine phylogenetic analysis, as the Isometopinae consist of extinct tribe Electromyiommini and living Isometopinae with significantly different morphological characters pending re-evaluation of the entire group. Therefore, this study not only provides the detailed ancient morphological characters, but also may play an important role in understanding the evolution of Miridae.
Key to the species of extinct genus Metoisops (updated from Herczek and Popov 2014)
1. Pronotum very broad, posterior width not less than 2.4× midline length . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
– Pronotum not very broad, posterior width less than 2.4× midline length . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .. . . . . . . . . . . . . . . . . . . . . . 3
2. Pronotum wider than 3× midline length; head width subequal to 3× head length . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . M. akingbohungbei
– Pronotum subequal to 2.4× midline length; head width subequal to 2.5× head length . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . M. groehni
3. Head transverse, width more than 2.6× length . . . . . . . . . . . . . . . . . 4
– Head width not more than 2.6× length . . . . . . . . . . . . . . . . . . . . . . . . 7
4. Dorsal width of single compound eye more than 2× vertex width . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
– Dorsal width of single compound eye not more than 2× vertex width . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
5. Dorsal width of single compound eye subequal 2× vertex width; pronotum posterior width subequal to 2.1× midline length . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . M. kerzhneri
– Dorsal width of single compound eye subequal 2.9× vertex width; pronotum posterior width subequal to 1.9 midline length . . . . . . . . . . . . . . . . . M. michalskii Kim, Taszakowski, and Herczek sp. nov.
6. Body large, more than 3 mm; ratio of scutellum and commissure lengths = ca. 1 . . M. popovi Kim, Taszakowski, and Jung sp. nov.
– Body less than 3 mm; ratio of scutellum and commissure lengths = ca. 1.2 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .M. grabenhorsti
7. Head width subequal to 2.2–2.35× midline length . . . . . . . . . . . . . 8
– Head width subequal to 2.5× midline length . . . . . . . . . . . . . . . . . . 9
8. Ratio of pronotum anterior and posterior widths 1.85–2.0; claval commissure shorter, ratio of claval commissure and mesoscutum + scutellum lengths ca. 1.25–1.27 . . . . . . . . . . . . . . . M. punctatus
– Ratio of pronotum anterior and posterior widths ca. 1.65; claval commissure shorter, ratio of claval commissure and mesoscutum + scutellum lengths more than 1.5 . . . . . . . . . . . . . . . . . M. consimilis
9. Mesoscutum broad, 1.8× length subequal to scutellum length; hemelytra with scattered punctuate corium and clavus and delicate pubescence . . . . . . . . . . . . . . . . . . . . . . . . . M. punctatodiffusus
– Mesoscutum distinctly narrow, 4× length subequal to scutellum length . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10
10. Dorsal width of single compound eye less than ca. 1.65× vertex width; scutellum slightly wrinkled; cuneus coloration uniform . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . M. variabilis
– Dorsal width of single compound eye more than ca. 1.65× vertex width; scutellum shiny and impunctate; cuneus coloration not uniform, its apex dark brown . . . . . . . . . . . . . . . . . . . . M. intergerivus
Authors’ contributions
This study is based on the collection of fossils by SJ and AT, with the contribution of all co-authors. JK and AT wrote the main script and their contributions are equal. MZ prepared the images. AH and SJ reviewed and supervised the manuscript. All authors have read and agreed to the published version of the manuscript.
Acknowledgements
We would like to thank Artur Michalski (Wrocław, Poland) for donation of the valuable specimen. We also would like to thank Jacek Szwedo (University of Gdańsk, Poland), André Nel (Muséum national d’Histoire naturelle and Université des Antilles, Paris, France), and one anonymous reviewer for commenting up on our earlier drafts, which obviously improved this paper. This work was supported by the National Research Foundation of Korea (NRF) NRF-2021K2A9A1A06095880.
References
Bogri, A., Solodovnikov, A., and Żyła, D. 2018. Baltic amber impact on historical biogeography and palaeoclimate research: oriental rove beetle Dysanabatium found in the Eocene of Europe (Coleoptera, Staphylinidae, Paederinae). Papers in Palaeontology 4: 433–452. Crossref
Bukejs, A., Alekseev, V.I., and Pollock, D.A. 2019. Waidelotinae, a new subfamily of Pyrochroidae (Coleoptera: Tenebrionoidea) from Baltic amber of the Sambian peninsula and the interpretation of Sambian amber stratigraphy, age and location. Zootaxa 4464: 261–273. Crossref
Cassis, G. and Schuh, R.T. 2012. Systematics, biodiversity, biogeography, and host associations of the Miridae (Insecta: Hemiptera: Heteroptera: Cimicomorpha). Annual Review of Entomology 57: 377–404. Crossref
Henry, T.J. 1977. Teratodia Bergroth, new synonym of Diphleps Bergroth with descriptions of two new species (Heteroptera: Miridae: Isometopinae). The Florida Entomologist 60: 202–210. Crossref
Herczek, A. 1993. Systematic position of Isometopinae Fieb. (Miridae, Heteroptera) and their interrelationships. Prace Naukowe Uniwersytetu Śląskiego 1357: 1–86.
Herczek, A. and Popov, Y.A. 1997. New peculiar representatives of the Isometopinae from the Baltic amber (Heteroptera: Miridae). Mitteilungen aus dem Geologisch-Paläontologischen Institut der Universität Hamburg 80: 189–195.
Herczek, A. and Popov, Y.A. 2012. A new peculiar isometopine genus (Hemiptera: Heteroptera: Miridae) from the Eocene Baltic amber. Zootaxa 3196: 64–68. Crossref
Herczek, A. and Popov, Y.A. 2014. Revision of the genus Metoisops (Hemiptera: Heteroptera, Miridae, Isometopinae) from late Eocene European amber. Zootaxa 3887: 401–421. Crossref
Herczek, A., Gorczyca, J., and Taszakowski, A. 2018. Sulawesimetopus henryi, a new genus and species of Isometopinae (Hemiptera, Heteroptera, Miridae) from Sulawesi. ZooKeys 796: 147–161. Crossref
Jung, S. and Lee, S. 2012. Molecular phylogeny of the plant bugs (Heteroptera: Miridae) and the evolution of feeding habits. Cladistics 28: 50–79. Crossref
Kim, J. and Jung, S. 2015. Taxonomic review of the genus Isometopus (Hemiptera: Miridae: Isometopinae) from the Korean Peninsula, with description of a new species. Zootaxa 4137: 137–145. Crossref
Kim, J., Taszakowski, A., Herczek, A., and Jung, S. 2021. Gigantometopus coronobtectus sp. nov., the first Isometopinae (Hemiptera: Cimicomorpha: Miridae) from Vietnam. Zootaxa 4990: 104–116. Crossref
Namyatova, A.A. and Cassis, G. 2016. Review of the seven new species of Isometopinae (Heteroptera: Miridae) in Australia and discussion of distribution and host plant associations of the subfamily on a worldwide basis. Austral Entomology 55: 392–422. Crossref
Popov, Y.A. and Herczek, A. 1992. The first Isometopinae from Baltic Amber (Insecta: Heteroptera, Miridae). Mitteilungen aus dem Geologisch-Paläontologischen Institut der Universität Hamburg 73: 241–258.
Popov, Y.A. and Herczek, A. 1993. Metoisops punctatus sp. n., the second representative of the fossil genus Metoisops from Baltic amber (Heteroptera: Miridae: Isomteopinae). Annals of the Upper Silesian Museum, Entomology 1 (Supplement): 51–56.
Poppius, B. 1913. Zur Kenntnis der Miriden, Isometopiden, Anthocoriden, Nabiden und Schizopteriden Ceylon’s. Entomologisk Tidskrift 34: 239–260. Crossref
Schuh, R.T. 2002–2013. On-line Systematic Catalog of Plant Bugs (Insecta: Heteroptera: Miridae) [available online, https://research.amnh.org/pbi/catalog/ (accessed on 30 June 2022)]
Schuh, R.T. and Weirauch, C. 2020. True Bugs of the World (Hemiptera: Heteroptera). Classification and Natural History (Second Edition). 800 pp. Siri Scientific Press, Manchester.
Schuh, R.T., Weirauch, C., and Wheeler, W.C. 2009. Phylogenetic relationships within the Cimicomorpha (Hemiptera: Heteroptera): a total-evidence analysis. Systematic Entomology 34: 15–48. Crossref
Shavrin, A.V. and Yamamoto, S. 2019. Unexpected palaeodiversity of omaliine rove beetles in Eocene Baltic amber (Coleoptera, Staphylinidae, Omaliinae). ZooKeys 863: 35–83. Crossref
Szwedo, J. and Sontag, E. 2009. The traps of the “amber trap”. How inclusions could trap scientists with enigmas. Denisia 26: 155–169.
Taszakowski, A., Kim, J., Damken, C., Wahab, R.A., Herczek, A., and Jung, S. 2020. Two new genera and species of the Gigantometopini (Hemiptera, Heteroptera, Miridae, Isometopinae) from Borneo with remarks on the distribution of the tribe. ZooKeys 941: 71–89. Crossref
Taszakowski, A., Kim, J., Damken, C., Wahab, R.A., Herczek, A., and Jung, S. 2021a. A remarkable new genus and two new species of the Gigantometopini (Hemiptera, Heteroptera, Miridae, Isometopinae) from Brunei. Zootaxa 4970: 171–181. Crossref
Taszakowski, A., Kim, J., Herczek, A., and Jung, S. 2021b. Sophianus palawanensis sp. nov., the first Isometopinae (Hemiptera, Heteroptera, Miridae) from the Philippines. Zootaxa 4996: 392–400. Crossref
Taszakowski, A., Kim, J., Masłowski, A., Herczek, A., and Jung, S. 2022. Kohnometopus yasunagai sp. nov. (Hemiptera, Heteroptera, Miridae, Isometopinae) from Peninsular Malaysia. Zootaxa 5141: 183–191. Crossref
Wappler, T. 2005. The age of Baltic amber: Could Eckfeld resolve this problem? In: D. Brothers and M. Mostovski (eds.), Fossils X3, 3rd International Congress of Palaeoentomology with 2nd International Meeting on Palaeoarthropodology and 2nd World Congress on Amber and its Inclusions. Programme and Abstracts, 7th to 11th Feb 2005, Pretoria South Africa, 53. University of Kwazulu-Natal, South African National Biodiversity Institute, Pretoria.
Yasunaga, T. 2005. Isometopinae plant bugs (Heteroptera: Miridae) preferably inhabiting Fraxinus griffithii on Ishigaki Island of the Ryukyus, Japan. Tijdschrift voor Entomologie 148: 341–349. Crossref
Yasunaga, T., Yamada, K., and Tsai, J.-F. 2017. Taxonomic review of the plant bug subfamily Isometopinae for Taiwan and Japanese Southwest Islands, with descriptions of new taxa (Hemiptera: Heteroptera: Miridae: Isometopinae). Zootaxa 4365: 421–439.
Yeshwanth, H.M., Chérot, F., and Henry, T.J. 2021. The Isometopinae (Hemiptera: Heteroptera: Miridae) of India and Sri Lanka: A review of the subfamily, with descriptions of six new species. Zootaxa 4903: 151–193. Crossref
Zachos, J., Pagani, M., Sloan, L., Thomas, E., and Billups, K. 2001. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 292: 686–693. Crossref
Acta Palaeontol. Pol. 68 (1): 75–83, 2023
https://doi.org/10.4202/app.00991.2022