

A comparison of teeth in Tithonian, Late Jurassic, predatory actinopterygian fishes from Owadów-Brzezinki Lägerstatte and its palaeoecological implications
ŁUKASZ WERYŃSKI, BŁAŻEJ BŁAŻEJOWSKI, and MARIUSZ KĘDZIERSKI
Weryński, Ł., Błażejowski, B., and Kędzierski, M. 2023. A comparison of teeth in Tithonian, Late Jurassic, predatory actinopterygian fishes from Owadów-Brzezinki Lägerstatte and its palaeoecological implications. Acta Palaeontologica Polonica 68 (3): 493–512.
The Owadów-Brzezinki palaeontological site is known for its very well-preserved fossils of Late Jurassic vertebrates, such as numerous fossil fish teeth and occasional dental bones. Some of these represent well-studied taxa, including the most common large predatory fish, with notable examples of caturoids (such as Strobilodus sp.) and pachycormids (Orthocormus teyleri). The current study presents the microstructure and histological features of the teeth of the selected specimens of the above taxa. They are determined through examinations of tooth cross-sections under thin microscopic observations and by the usage of scanning electron microscopy (SEM). The above inspections, combined with aspects of external tooth morphology, allowed us to determine the palaeoecology of the aforementioned taxa of large predatory fish. It is concluded that examined Caturoidea displayed a rather homogenous dentition belonging to the intermediate cut/slash guild, characterized by an internal orthodentin histology with prominent incremental Andresen growth lines of differing form, indicating living in a highly variable, unstable environment. The teeth of pachycormid specimen (O. teyleri) can be characterized as having denteon-based orthodentin histology, with a rapid rate of tooth eruption and a heterodont, elongated specialist dentition of the piercing guild. The observed structural differences in the teeth suggest a different niche distribution between the taxa studied. They help to explain how these predatory ray-finned fishes may have coexisted both in the local environment of the Owadów-Brzezinki and in the wider, more global context of Late Jurassic shallow marine environments. In addition, the tooth samples are characterised by pronounced surface bioerosion with traces of Mycellites ossifragus durophagous fungal activity, indicating an intense bioerosion caused by these microorganisms after the death of the fish.
Key words: Actinopterygii, Caturoidea, teeth, predatory, microstructure, histology, niches, Late Jurassic, Poland, Owadów-Brzezinki Quarry.
Łukasz Weryński [lukaszwerynski@doctoral.uj.pl; ORCID: https://orcid.org/0000-0001-7036-0368 ], Doctoral School of Exact and Natural Sciences, Institute of Geological Sciences, Jagiellonian University in Kraków, Gronostajowa 3a, 30-387 Kraków, Poland.
Błażej Błażejowski [bblazej@twarda.pan.pl; ORCID: https://orcid.org/0000-0001-6320-9870 ], Institute of Paleobiology, Polish Academy of Sciences, Twarda 51/55, 00-818 Warsaw, Poland.
Mariusz Kędzierski [mariusz.kedzierski@uj.edu.pl; ORCID: https://orcid.org/0000-0002-1793-3160 ], Institute of Geological Sciences, Jagiellonian University in Kraków, Gronostajowa 3a, 30-387 Kraków, Poland.
Received 23 January 2023, accepted 24 July 2023, available online 11 September 2023.
Copyright © 2023 Ł. Weryński et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The study of fossil teeth is one of the most engaging fields of paleobiology, due to the multiplicity and quality of information they provide regarding the ecology of the vertebrates from which they came. In addition, their mechanically resistant structure and chemical composition allow them to be preserved often in an almost unchanged state, creating some of the most promising objects for paleontological research. Generally, the most common fossil material through which the Actinopterygii clade is represented, comes from the teeth that ray-finned fish usually replace during their lifetime (Berkovitz and Shellis 2017). The teeth of predatory fishes are found particularly often, since they are both very numerous in each individual and frequently exchanged. The Late Jurassic period can be especially promising when studying predatory Actinopterygii, as we have exquisitely preserved fossils from this time that can be closely compared with living ecologically equivalent species (Tyborowski 2017). The fossils of Late Jurassic ray-finned fishes are therefore valuable in reconstructing ancient food chains. Understanding the similarities in the ecologies of modern and Late Jurassic animals can add value to the study of present day/extant marine settings by providing a perspective on how evolutionary pressures force similar adaptations in different groups of Actinopterygii.
The first part of the material analysed in this study belongs to the superfamily Caturoidea, included in the clade Halecomorphi belonging to the infraclade Holostei which is widely distributed in both the Cenozoic and Mesozoic (Lambers 1994, 1998; Grande and Bemis 1998). Halecomorphi, due to their placement in the Holostei, are characterized by a more basal cranial bone arrangement (Grande and Bemis 1998). Caturoidea were widespread macropredatory hunters of Jurassic and Early Cretaceous (López-Arbarello and Ebert 2023). Although the specimens of the Caturoidea are globally distributed (Schaeffer and Patterson 1984), they are most often found in the Upper Jurassic sediments of Europe (Woodward 1893; Lambers 1994, 1999; López-Arbarello and Ebert 2023).
Another family, Pachycormidae, represented here by Orthocormus tayleri, also includes prominent macro-predators (Arratia 2004; Arratia and Schultze 2013). This family is known from Jurassic and Cretaceous sediments (Friedman et al. 2010; Maxwell et al. 2020; Cooper et al. 2022) and besides predators includes filter-feeders i.e., the famous filter-feeding Leedsichtys problematicus (Liston 2004). The predatory members of Pachycormidae family are characterized by an elongated, toothed rostrum, scythe-shaped pectoral fins, reduced pelvic fins and a prominent, nearly symmetrical caudal fin (Liston et al. 2019). They are recognised as fast-moving, open-water predators (Arratia and Schultze 2013).
In this research, optical and transmitted electron microscopy were used to study the dental microstructure and histology of two actinopterygian families with prominent Late Jurassic predatory taxa: Caturoidea (Strobilodus sp. and Caturoidea indet.) and Pachycormidae (Orthocormus teyleri). These ray-finned fish fossils come from strata exposed in the Owadów-Brzezinki quarry, which is considered a counterpart (Kin et al. 2012) to the famous Solenhofen Plattenkalk (Lambers 1994, 1998; Arratia and Schultze 2013), belonging to the distinctive facies of the Solenhofen Archipelago paleogeographic region. While pachycormids genus of Orthocormus is known mainly from this paleogeographic European region (Lambers 1988; Arratia and Schultze 2013), fossils of caturoids are observed in numerous places around the world (Woodward 1893; Müller 2011; Bogdan et al. 2013; López-Arbarello and Ebert 2023). Along with assessing the overall tooth morphology and microstructure, observations are being made to clarify how these large predatory ray-finned fishes of similar size coexisted. In addition, the analysis may indicate a link between tooth characteristics and the feeding strategy of the predators studied.
Institutional abbreviations.—ZPAL, Institute of Palaeobiology, Polish Academy of Sciences, Warsaw, Poland.
Geological setting
Since becoming a well-known attraction in recent years, Owadów-Brzezinki quarry (51o22’27’’ N, 20 o8’11’’ E; Fig. 1), first described by Kin et al. (2013), has been established as one of the richest Late Jurassic (Tithonian) fossil sites in Central-Eastern Europe and one of the most impressive Lägerstatten in Poland, bearing a wide spectrum of well-preserved taxa, including a significant number of vertebrate fossils, some of which were recognized as macro predatorial.
The site is located in the north-western margin of the Holy Cross Mountains, in the north-eastern limb of the Tomaszów Syncline. The uppermost part of the Brzostówka Marl Member of the Pałuki Formation and the overlying limestone of the Kcynia Formation, including the Sławno Limestone Member, Corbulomima Limestone and a fragment of “serpulid” beds, are exposed in the Owadów-Brzezinki section (Kutek 1994; Błażejowski et al. 2016; Fig. 1). The section shows a gradual marine regression revealed by a transition from offshore to coastal and lagoonal settings, but its uppermost part was deposited during a short-term marine transgression and the re-appearance of coastal environments (Błażejowski et al. 2016, 2023; Wierzbowski et al. 2016).
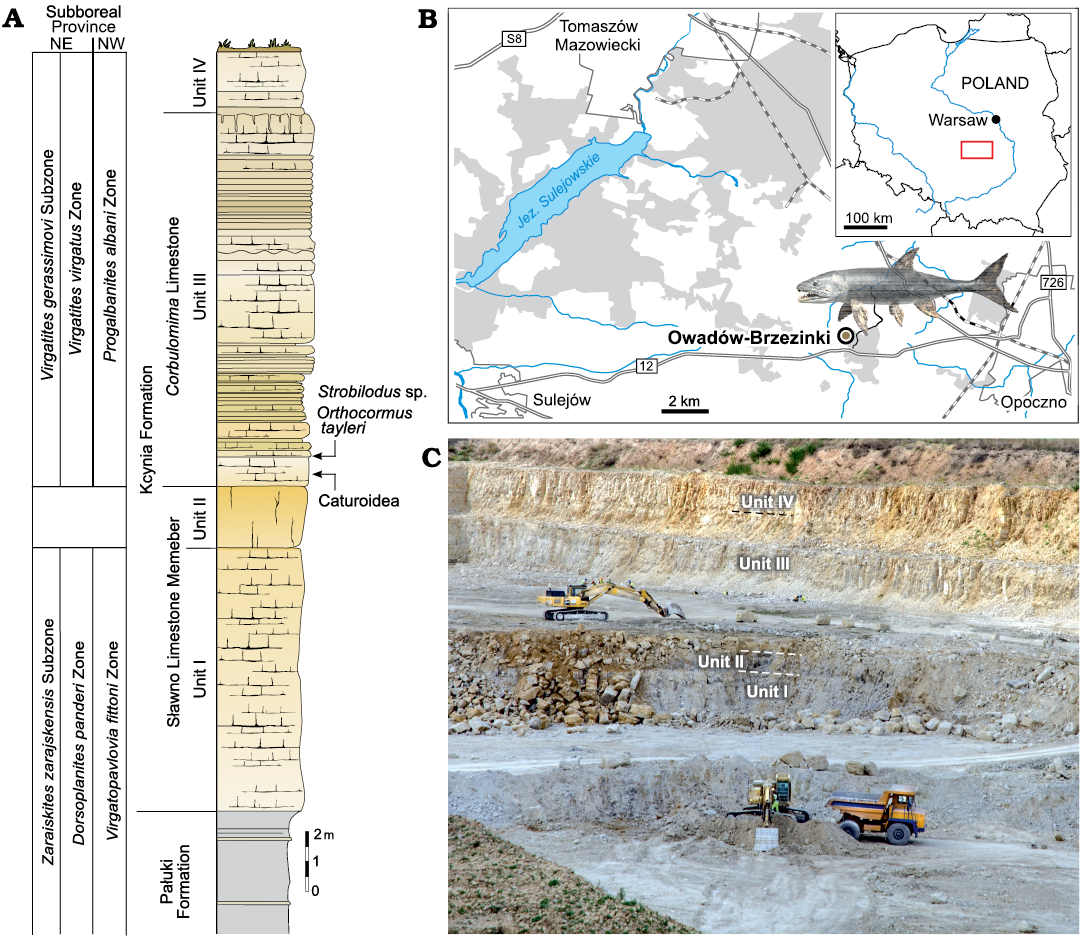
Fig. 1. A. Lithological succession and biostratigraphy of the Owadów-Brzezinki Quarry. The topmost part of the Pałuki Formation and overlying limestone of the Kcynia Formation (Units I–IV). B. Road map with the location of the Owadów-Brzezinki site and its proximity to Tomaszów Mazowiecki in Central Poland. C. General view of the Owadów-Brzezinki section (i.e., Unit III and most fossiliferous Corbulomima Limestone occurring in the middle of the quarry wall).
At the moment, the Owadów-Brzezinki Quarry is the only place in extra-Carpathian Poland where upper Tithonian strata are available for study. The exposed carbonate sequence belongs to the Kcynia Formation and can be divided into four successive units (Kin et al. 2013; Błażejowski et al. 2016). Thick-bedded chalky and micritic limestone of the unit I and unit II, which contain marine fauna, were included into the Sławno Limestone Member by Matyja and Wierzbowski (2016). Overlying well-bedded micritic limestone of unit III, which contain common Corbulomima bivalves and were formed in lagoonal environment, are, informally, called the Corbulomima Limestone (Fig. 1) according to the earlier classification (cf. Kutek 1994). Unit III is highly fossiliferous and has yielded the specimens that are the subject of this study (Fig. 1). The occasional anaerobic conditions and the accompanying set of biochemical processes were of particular importance for the preservation of the extraordinary paleontological discoveries (Wierzbowski et al. 2016; Błażejowski et al. 2019, 2020). As a result, the remains of organisms accumulated in the lagoon waters and were perfectly preserved in the fossil state. A narrow interval of unit IV from the uppermost part of the Owadów-Brzezinki section consists of oyster-bryozoan-serpulid organodetrital limestone assigned to the “serpulid” beds, which were formed in coastal settings (cf. Matyja and Wierzbowski 2016; Wierzbowski et al. 2016).
According to the stratigraphical studies of Kutek (1994) and Matyja and Wierzbowski (2016) based on ammonite fauna (Fig. 1), the lower part of the Owadów-Brzezinki deposits is dated to the Zaraiskites regularis horizon (the uppermost part of the Brzostówka Marl Member of the Pałuki Formation) and Zaraiskites zarajskensis horizon (unit I of the Sławno Limestone Member of the lowermost part of the Kcynia Formation) of the Zarajskensis Subzone of the Dorsoplanites panderi Zone of the Middle Volgian, as well as to the Virgatopavlovia fittoni Zone from the “Bolonian” zonation of England (Matyja and Wierzbowski 2016; Błażejowski et al. 2023). The upper part of the Owadów-Brzezinki section (units III and IV belonging to the Corbulomima Limestone and “serpulid” beds, respectively) has, in turn, been assigned to the both Virgatites gerassimovi Subzone of the Virgatites virgatus Zone of the Middle Volgian and the Progalbanites albani Zone of the “Portlandian” (Matyja and Wierzbowski 2016; Błażejowski et al. 2023).
Bony fish remains are one of the most common fossils found at the site (Kin et al. 2013), and they are particularly represented by numerous teeth found in the limestone of Kcynia Formation, being often present in the sediments of the normal marine setting of Unit I. However, they are exceptionally abundant in the sediments of the shallower settings of Unit III; in most cases, these teeth are almost microscopic in scale, barely noticeable with the naked eye, but alongside these tiny specimens there are also larger teeth ranging from a few millimetres to a few centimetres in length. Those are sometimes found still intact in the jawbones of fossilized fish (Błażejowski et al. 2015; Tyborowski 2017).
Material and methods
The material for the study (Fig. 2) consists of fossil fragments of jaw bones representing two important groups of predatory bony fishes: Caturoidea (Strobilodus sp.) and Pachycormidae (Orthocormus teyleri), and in the case of indetermined caturoids also three isolated teeth found in the sediments. The collected material is housed at the Institute of Palaeobiology, Polish Academy of Sciences in Warsaw (ZPAL P. 16/O-B).
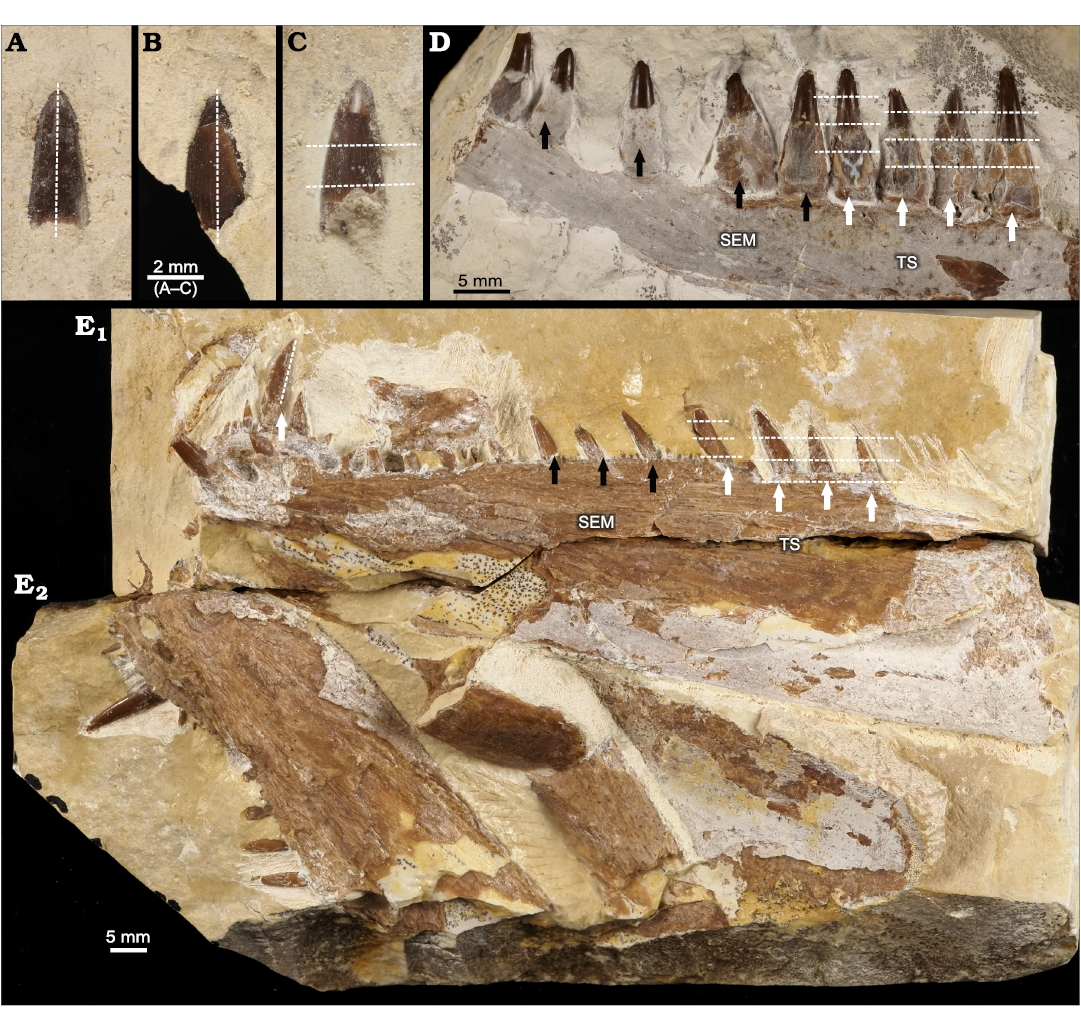
Fig. 2. Studied specimens of actinopterygian fishes from Tithonian, Upper Jurassic Kcynia Formation, Corbulomima Limestone (Unit III), Owadów-Brzezinki, Poland. A–C. Loose teeth of Caturoidea indet. A. ZPAL P. 16/O-B/FT1. B. ZPAL P. 16/O-B/FT2. C. ZPAL P. 16/O-B/FT3. D. Caturoid Strobilodus sp., ZPAL P. 16/O-B/2, right dentary in lateral view. E. Pachycormid Orthocormus teyleri Lambers, 1988, ZPAL P. 16/O-B/3, left dentary (E1) and right dentary (E2) in lateral view. Dashed white lines indicate thin-sectioning plane; white arrows indicate which teeth were sampled for thin-sectioning (TS), black arrows indicate which samples were chosen for SEM analysis.
The preparation of the material was done at the Institute of Geological Sciences of Jagiellonian University in Kraków. The teeth were cut in the vertical and horizontal planes, then cleaned and polished. Three isolated teeth from indetermined caturoids and four from the anterior region of the dentary fragment of Strobilodus sp. (ZPAL P. 16/O-B/FT1–FT3), were selected for thin sections. Two of the isolated teeth were cut in a vertical plane, while the dentary teeth and one isolated teeth specimen were cut in horizontal planes. In the case of maxillary teeth, the cuts were made in the basal, mid-crown and apical planes. Horizontal thin sections of the O. teyleri teeth were sampled from the marginal row of dentary teeth in the posterior section of the left dentary (ZPAL P.16./O-B/3). The vertical thin section was sampled from the symphyseal tusk. The fragments were polished and impregnated with resin. The samples were examined at 10×, 25× and 100× magnifications using a transmitted light microscope equipped with a Nikon Eclipse E600-POL camera. The thin sections are currently housed at the Institute of Geological Sciences of Jagiellonian University, with collection numbers NG/PAL/VERT/OB/C1–C7 for Caturoidea (ZPAL P. 16/O-B/FT1–FT3) and NG/PAL/VERT/OB/O1–O4 for Pachycormidae (ZPAL P. 16/O-B/3).
Scanning electron microscopy was realized using a HITACHI S-4700 instrument fitted with the NORAN VANTAGE microanalysis system. Four Strobilodus sp. (ZPAL P.16./O-B/2) teeth from the posterior section of the preserved fragment of dentary bone and three O. teyleri dentary (ZPAL P.16./O-B/3) teeth coming from the central part of the marginal tooth row were selected for SEM examination. These teeth were etched in a 10% acetic acid solution for ten minutes to accentuate the fine enameloid and dentin structures.
The terminology for tooth description is based on the Berkvitz and Shellis (2017) methodology used in describing fish teeth, using the following terms: apical, towards the apex of the tooth crown; basal, towards the tooth crown base; mid-crown, approximately centrally between base and apex of teeth; mesial, towards the anterior direction in animals’ mouth; distal, inwards the animals’ mouth; labial, in direction of animals’ lips; lingual, towards the tongue.
Systematic palaeontology
Class Actinopterygii Klein, 1885
Infraclass Holostei Müller, 1846
Clade Halecomorphi Cope, 1872
Order Amiiformes O.P. Hay, 1929
Superfamily Caturoidea Owen, 1860
Genus Strobilodus Wagner, 1851
Type species: Strobilodus giganteus Wagner, 1851 (sensu López-Arbarello Ebert 2023), Painten (Germany), Kimmeridgian.
Strobilodus sp.
Fig. 2D.
Material.—Right dentary, ZPAL P.16./O-B/2 from Tithonian, Upper Jurassic Kcynia Formation, Corbulomima Limestone (Unit III), Owadów-Brzezinki, Poland.
Description.—The study material consists of an incomplete right dentary bone, with preserved medial and partially preserved posterior section, with nine intact teeth. This bone measures 59 mm in the anteroposterior plane, 11 mm in the dorsoventral plane and 6 mm in the labiolingual plane. Its shape can generally be characterized as elongated and moderately gracile. In the anterior region, the bone is rectangular, while in the medial plane it is slightly curved dorsally. The robustness of the bone gradually decreases in the ventral direction. There is a prominent indentation along the ventral margin of the bone in the central region, identified as ventral grove housing sensory canal. The whole bone texture is rather rough and compact. Two teeth are missing from the alveoli of the described specimen (Fig. 2D).
The teeth of the specimen can be characterized as conical, with a thick basal part and with a diameter that drastically decreases at the mid-crown. In the mid-crown and apical parts, the shape is much more gracile and elongated. In the apical plane, the cross section can be characterized as rounded with slight labiolingual compression, characterized by carinae present from the mid crown to the apex, while the basal cross section is rather round. The length of the teeth varies slightly in the described dentary, with larger and more robust teeth located more anteriorly and the teeth are also better preserved in this part of the bone. Generally, the teeth can be described as roughly homodont in form and are characterized by a symmetrical shape, along with slight lingual curvature. The intact teeth studied herein have an average ratio of apicobasal height/mesiodistal length of 2.29 (Table 1).
Table 1. Teeth crown lengths and width (in mm). Tooth row of Strobilodus sp.: A. Teeth rows of Orthocormus teyleri: P, parasymphyseal and symphyseal; M, mariginal; (d), damaged.
| |
Strobilodus sp. |
Orthocormus teyleri |
|
|
right dentary |
left dentary |
right dentary |
|
|
Crown apicobasal length |
A1: 8 A2: 11 A3: 10 A4: 11 A5: 11 A6: 10 A7: 9 A8: 8 A9: 8 |
P1: 2 P2: 2 P3: 14 P4: 6 P5: 2 P6: (d) M1: 8 M2: 3 M3–M7: (d) M8: 7 M9: (d) M10: 9 M11, M12: (d) M13: 11 M14: 8 M15: 6 |
P1: 4 P2: 14 P3: 3 P4: 1 M1: 4 M2: (d) M3: 7 M4: 7 |
|
Crown mesiodistal width |
A1: 5 A2: 4 A3:4.5 A4: 4.5 A5: 5 A6: 5.5 A7:3 A8: 3 A9: 3 |
P1: 0.7 P2: 0.8 P3: 1.5 P4: 4 P5: 1 P6: 3 M1: 4 M2: 3 M3: 1.5 M4: 2 M5: 2 M6: 2 M7: 2 M8: 2 M9: 2 M10: 3 M11: 4 M12: 1 M13: 4 M14: 2 M15: 1.5 |
P1: 1 P2: 4 P3: 1 P4: 0.5 M1: 2 M2: 2.3 M3: 2.2 M4: 2.1 |
|
Crown apicobasal height/mesiodistal length ratio |
2.29 |
2.76 |
|
Remarks.—The relatively narrow and anteriorly tapering dentary; robust teeth with conical acrodin caps. This specimen was first described by Błażejowski et al. (2012) as dentary, belonging to the carnivorous Caturus sp., and was later redescribed as left maxilla (Błażejowski et al. 2015). In current light we consider this specimen to belong to the Strobilodus sp., based on the latest López-Arbarello and Ebert (2023) study and interpret this specimen as dentary.
Caturoidea indet.
Fig. 2A–C.
Material.—Three partial crowns of isolated teeth (ZPAL P. 16/O-B/FT1–FT3) from Upper Jurassic Kcynia Formation, Corbulomima Limestone (Unit III), Owadów-Brzezinki, Poland.
Description.—Loose teeth: the crowns of teeth, found loosely in the sedimentary rocks of Kcynia Formation. These conical-shaped teeth are slightly mesiodistally compressed with carinae placed along the mesial and distal planes of the crown. The T1 and T2 teeth consist of the apical and mid-crown section of the teeth, while the T3 tooth consists of the mid-crown section, with part of the acrodin cap preserved (Fig. 2A–C).
Remarks.—Carinae present along the mesial and distal planes of acrodin tooth cap.
Clade Teleosteomorpha Arratia, 2004
Order Pachycormiformes Berg, 1937
Family Pachycormidae Woodward, 1895
Genus Orthocormus Weitzel, 1930
Type species: Orthocormus cornutus Weitzel, 1930, Langenaltheim (Germany), Tithonian.
Orthocormus teyleri Lambers, 1988
Fig. 2E.
Material.—Left and right dentaries ZPAL P.16./O-B/3, from Upper Jurassic Kcynia Formation, Corbulomima Limestone (Unit III), Owadów-Brzezinki, Poland.
Description.—Left dentary: the specimen under study has been previously described and identified by Tyborowski (2017: figs. 3, 5, 6) as belonging to Orthocormus teyleri. The bone, which consists of the left dentary, is excellently preserved in its (or the) dorsal part with only the very posterior part of the bone missing. Measuring 109 mm in the anterior-dorsal plane, 11 mm in the dorsoventral plane in the middle section and 6 mm in the labiolingual plane, the bone has a well-preserved row of teeth. The anteroventral part of the bone is incomplete, making this part much less robust than better preserved part of the anterior right dentary. The overall shape can be described as moderately gracile in the anterior part and robust in the mesial and posterior parts, with a maximum dorsoventral height of 21 mm in this part, but this gracility of the bone, as noted by Tyborowski (2017), can be attributed to missing antero-ventral part of the bone. The anterior part is characterized by the presence of a tooth rosette with marginal and parasymphyseal tooth rows, with a prominent and strong dental battery in the row of parasymphyseal teeth, crowned with a prominent 14 mm high tusk in the apicobasal crown. The anterior rosette can be characterized as ovaloid in shape in its anterior region and elongated and rectangular in its posterior part, with the anterior part curved anterodorsally and abruptly ending with a keel on which the horizontal branch begins. The anterior part of the horizontal branch is rather straight and gracile in shape, with small peg-shaped mesial teeth. The posterior part of the horizontal branch shows distinct conical teeth in the marginal tooth row, with miniscule peg-like teeth in the ventral tooth row aligned in between.
Right dentary: The bone measures 70 mm in the anterior-posterior plane, 23 mm in the dorsoventral plane and 7 mm in the labiolingual plane. It is a very robust bone, particularly deep in the dorsoventral plane. This right part of the dentary has preserved the anterior and mesial part, and the posterior part is missing. In the anterior part of the bone, two rows of teeth are visible, parasymphyseal and marginal, forming a tooth rosette with a prominent canine-like tusk measuring 14 mm at the apicobasal crown height. This bone can be characterized by the indentation present near the parasymphyseal region, where the parasymphyseal and marginal teeth rows meet. The teeth in the row of parasymhyseal teeth row are much more robust. The bone is slightly curved in the dorsal plane, with the anterior part rounded and robust in form, while the horizontal branch is rectangular.
The shape of the teeth can be described as strongly conical and elongated in proportion, with an average ratio of apicobasal height/mesiodistal length of the complete crown of 2.76 (Table 1). Teeth can be characterized as heterodont, with a wide variation in size and morphology, with the largest teeth developed as labiolingually curved tusks and the smallest as straight-peg-shaped structures. The teeth are rather simple in their form, without any ornamentation, with carinae present only on the mesial surfaces of large tusks. The posterior teeth are mostly mesially inclined, while the symphyseal and parasymphyseal teeth are perpendicular to bone. For research purpose, only the left dental bone was sampled.
Remarks.—Some dentary teeth perpendicularly directed, but most of them inclined forward; some dentary teeth lingually curved; dentary bone with at least one, conical and slightly procumbent tooth; closely arranged dentary teeth; crown of dentary teeth with a circular base and mesiodistal oval section.
Results
Observations of microstructure in thin sections
Caturoidea.—The vertical cross section (Fig. 3A) shows two distinct layers: an inner tubular orthodentin and an outer enameloid layer. In the apical part, the teeth consist of large caps of acrodentin, which form a very thick coating on the tops of the teeth. The ortodentin layer is tubular in structure, with slightly oblique alignment of tubules, generally perpendicular to the tooth surface. The collar enameloid layer is rather thin, consisting of a layer of about 20–30 μm thick, and is characterized by a prominent prismatic structure, while the acrodentin has a structure more similar to orthodentin, with a slightly tubular structure, forming a massive cap enameloid at the apex. Vertical-transverse cross sections show pronounced diagenetic alteration in the centre, resulting in a reduction in the prominence of dentin tubules. The horizontal cross section (Fig. 3B) can be characterized as rounded with slight protuberances in the mesiodistal plane.
The internal structure of the teeth composed mainly of the orthodentin, which accounts for most of the cross section. The orthodentin can be described as a highly regular structure, in the form of parallel tubules radially radiating from the centre. The observed collar enameloid layer is rather thin, measuring 30 μm at the apical cross section and about 20 μm in basal cross section. The examined teeth are characterized by a gradual increase in the diameter of the tooth in the basal direction (Fig. 4A1, B1, C). The basal plane surface exhibits superficial and subsurface dentin boreholes in many places (Fig. 4B2, B3). In the mid-crown part of the teeth, there are distinct incremental growth lines in the orthodentin layer (Fig. 4A2, B2). The growth lines appear either as a more prominent band dividing orthodentin into two distinct layers, as in Fig. 4A2 or very lightly accentuated structures regularly becoming more expansive as shown in Fig. 4B2.
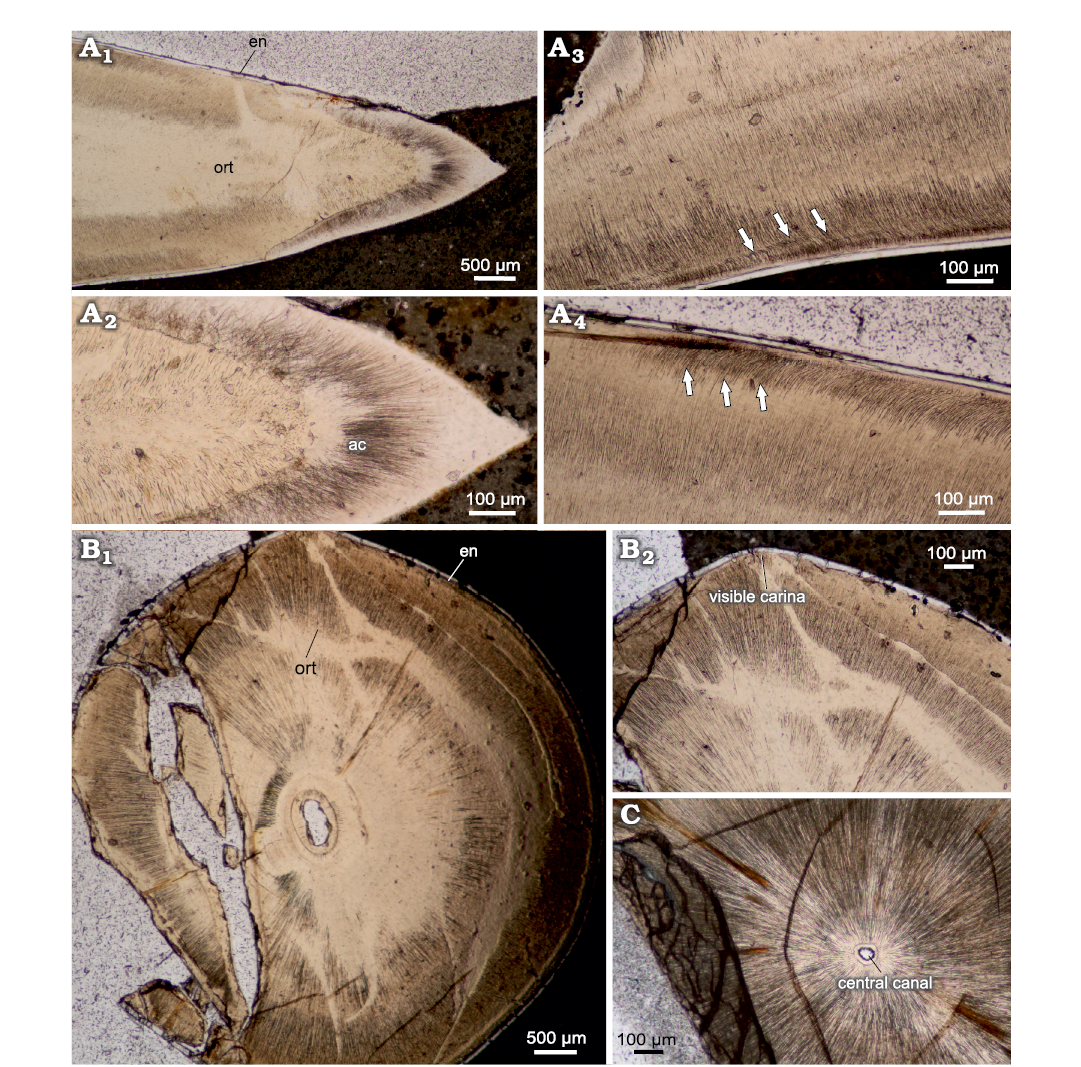
Fig. 3. Thin sections of teeth of caturoidean fishes Caturoidea indet. (A) and Strobilodus sp. (B, C) from Tithonian, Upper Jurassic Kcynia Formation, Corbulomima Limestone (Unit III), Owadów-Brzezinki, Poland. A. NG/PAL/VERT/OB/C5 thin section from ZPAL P.16./O-B/FT1. A1, general view of vertical thin section with orthodentin histology (ort) and thin enameloid layer (en); A2, close view of apical section, with prominent acrodin cap (ac); A3, A4, crown mid-section with angled orthodentin tubules (highlighted) present. B, C. Horizontal cross section of Strobilodus sp. teeth in apical plane. Arrows indicate phenomena. B. NG/PAL/VERT/OB/C7 thin section from ZPAL P.16./O-B/FT3. B1, overview of teeth structure, with slight mesiodistal compression and mostly solid structure; B2, carinae present as a perturbances in mesial and distal planes of teeth. C. NG/PAL/VERT/OB/C1 thin section from ZPAL P.16./O-B/O2, small central canal surrounded by prominent dental tubules of orthodentin.
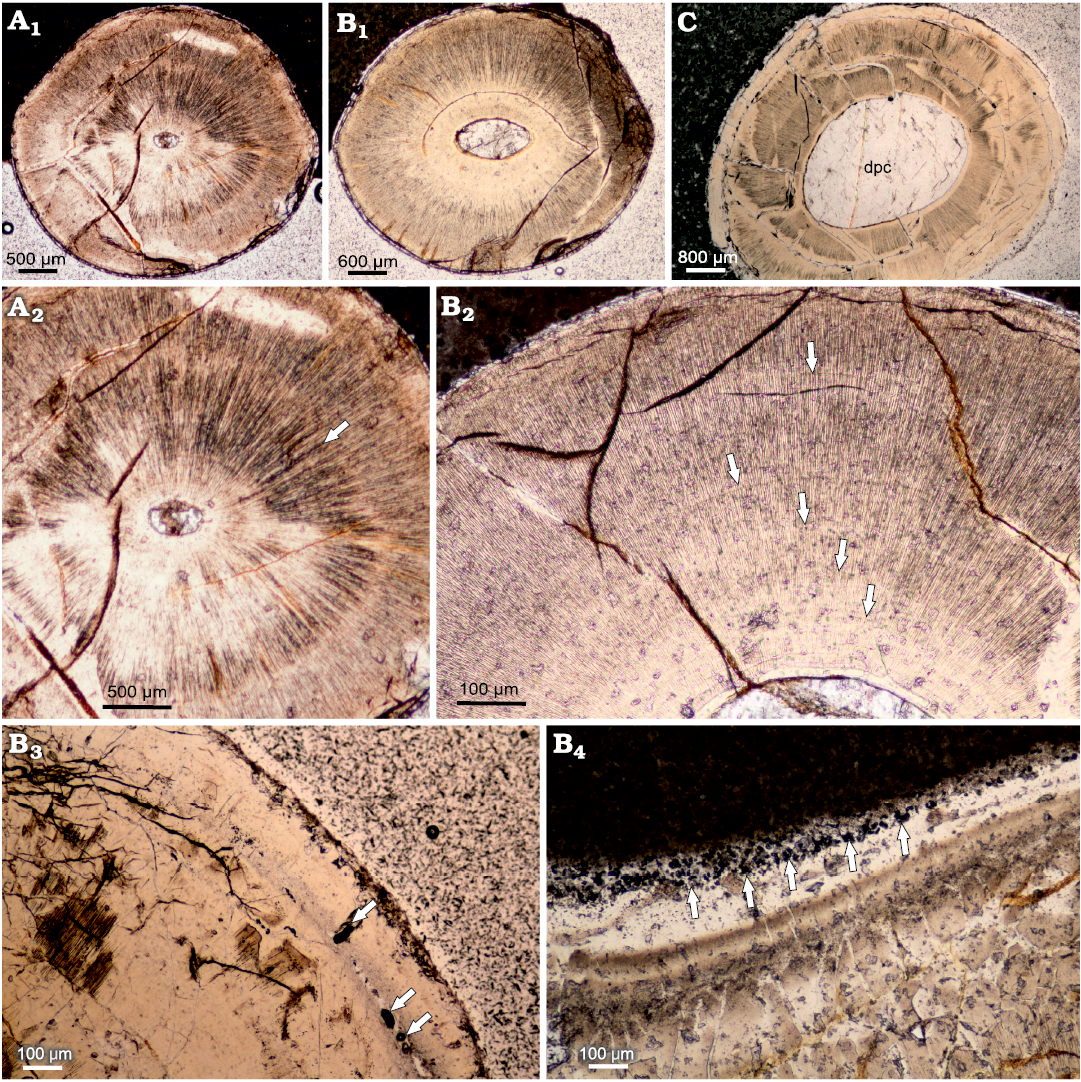
Fig. 4. Cross-sections of teeth caturoidean fishes Strobilodus sp. from Tithonian, Upper Jurassic Kcynia Formation, Corbulomima Limestone (Unit III), Owadów-Brzezinki, Poland. A–C. NG/PAL/VERT/OB/C1–C3, respectively, thin sections from ZPAL P.16./O-B/2, in apical (A1), mid-crown (B1), and basal (C) planes, documenting relative increase in dental pulp cavity diameter in basal direction. Incremental growth lines: irregular (A2) and more regular (B2) patterns of growth are documented. Dentin drillings (arrows) present sub-superficially (B3) and superficially (B4).
Orthocormus teyleri.—In vertical cross section, the teeth can be characterized as elongated and conical, having multiple internal capillary cavities, which become more numerous basally (Fig. 5). Larger capillaries are connected by an extensive network of smaller and more abundant capillaries. The examined sample shows a moderately thin acrodentin cap in apical section. The teeth are composed of three layers. The innermost osteodentin is filled with pores identified as dentoeonal canals (Thangadurai et al. 2022). The next is the denteons, which are divided by inter-denteonal dentin; the orthodentin layer and the outermost layer is a very thin enameloid, about 20 μm thick.
The horizontal cross section can be characterized as ovaloid, with a slight mesiodistal compression (Fig. 6). In horizontal cross section, denteons polygonal structure is clearly visible, with visible internal vasularization in series of small capillaries diverging from denteonal canals. Separation of denteons by inter-denteonal dentin is distinct. Below the orthodentin layer, a system of small capillaries surrounds inner osteodentin, exhibited as radial bundled structures. The osteodentin in basal teeth sections (Fig. 6C2), exhibits especially prominent denteons, characterized by prominent diagenetic alteration, which highlights denteon structure. Enameloid layer is very thin in all horizontal sections, ranging on average 20 μm. The alveolar bone is visible in one of the basal thin sections, with multiple additional teeth erupting (Fig. 7) and cases of individual teeth arrested eruption on underlying specimens.

Fig. 5. Vertical cross-section of tooth of pachycormid fish Orthocormus teyleri Lambers, 1988, NG/PAL/VERT/OB/O1 thin section from ZPAL P. 16/O-B/3 from Tithonian, Upper Jurassic Kcynia Formation, Corbulomima Limestone (Unit III), Owadów-Brzezinki, Poland. Basal (A1), mid-crown (A2), and apical (A3) planes, with visible acrodin cap. Internal structure (A4), with visible denteonal canals (white arrows) and inter-denteonal dentin (black arrows).
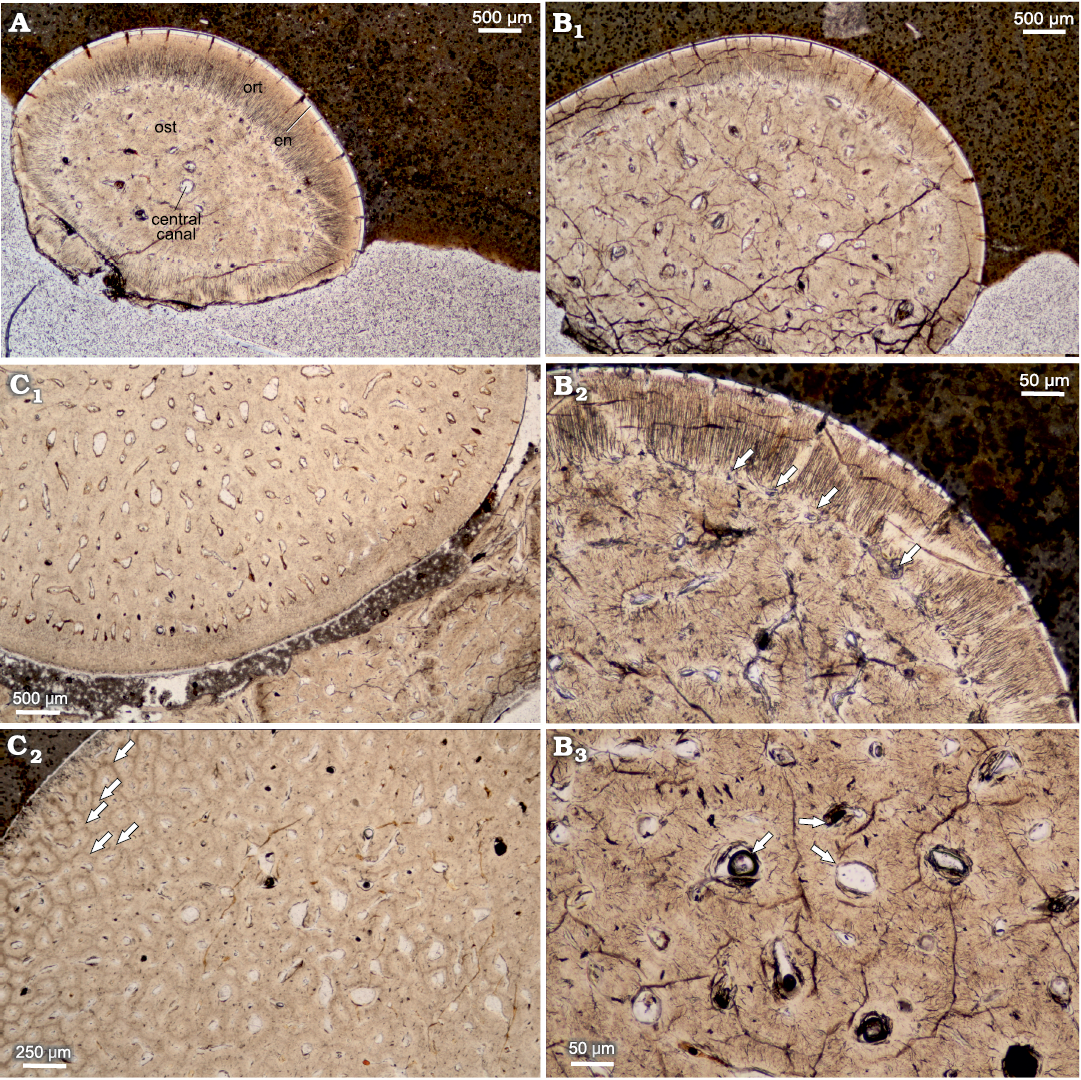
Fig. 6. Horizontal cross sections of teeth of pachycormid fish Orthocormus teyleri Lambers, 1988. A–C. NG/PAL/VERT/OB/O2–4, respectively, thin sections from ZPAL P. 16/O-B/3 from Tithonian, Upper Jurassic Kcynia Formation, Corbulomima Limestone (Unit III), Owadów-Brzezinki, Poland. A. Apical plane with visible osteodentin (ost), surrounded by orthodentin (ort), and outermost enameloid (en). B. Mid-crown plane. B1, general view; B2, osteodentin layer encircled by marginal capillaries (arrows), which contact with orthodentin; B3, osteodentin densely packed with denteons with central denteonal canals (arrows). C. Basal plane. C1, general view; C2, individual denteons pronounced due to diagenetic processes (arrows).
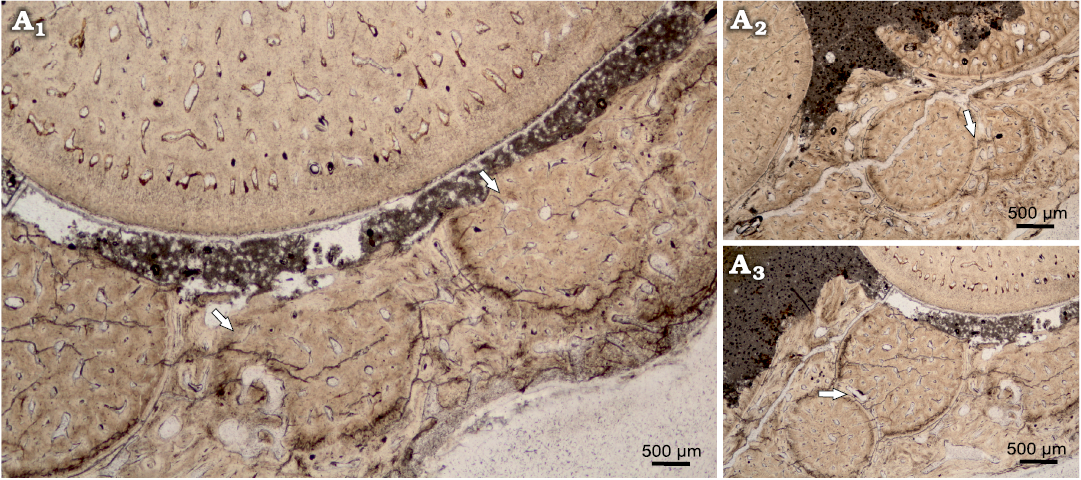
Fig. 7. Teeth eruption from alveolar bone of Orthocormus teyleri Lambers, 1988, NG/PAL/VERT/OB/O4 thin section from ZPAL P. 16/O-B/3 from Tithonian, Upper Jurassic Kcynia Formation, Corbulomima Limestone (Unit III), Owadów-Brzezinki, Poland. A1, numerous teeth erupting in posterior tooth row; A2, A3, case of rapid teeth eruption, evidence of odontogenesis of one teeth specimen atop on the another tooth. Arrows indicate the observed phenomenon.
Observation of microstructure using scanning electron microscopy
Caturoidea.—The tooth enameloid of this taxa has fine small-scale longitudinal ridges (Fig. 8A, B). These form a very complex structure (Fig. 9C–H), which results in smooth ridges in the midsection of the teeth but, closer to the base of the crown, changes to a winding, more densely packed form, which then turns into fine-grained structures at the base of the teeth. The dentin layer examined the in horizontal view appears as an irregular nodular structure, with small capillaries lining the layers (Fig. 10). The observed teeth in the vertical plane have a granular layered structure, where each irregular dentin layer is stacked on top of the other. In the exposed orthodentin layer there are clearly visible microboring structures, forming winding, extensive surface channel systems, with visible bifurcations (Fig. 9).
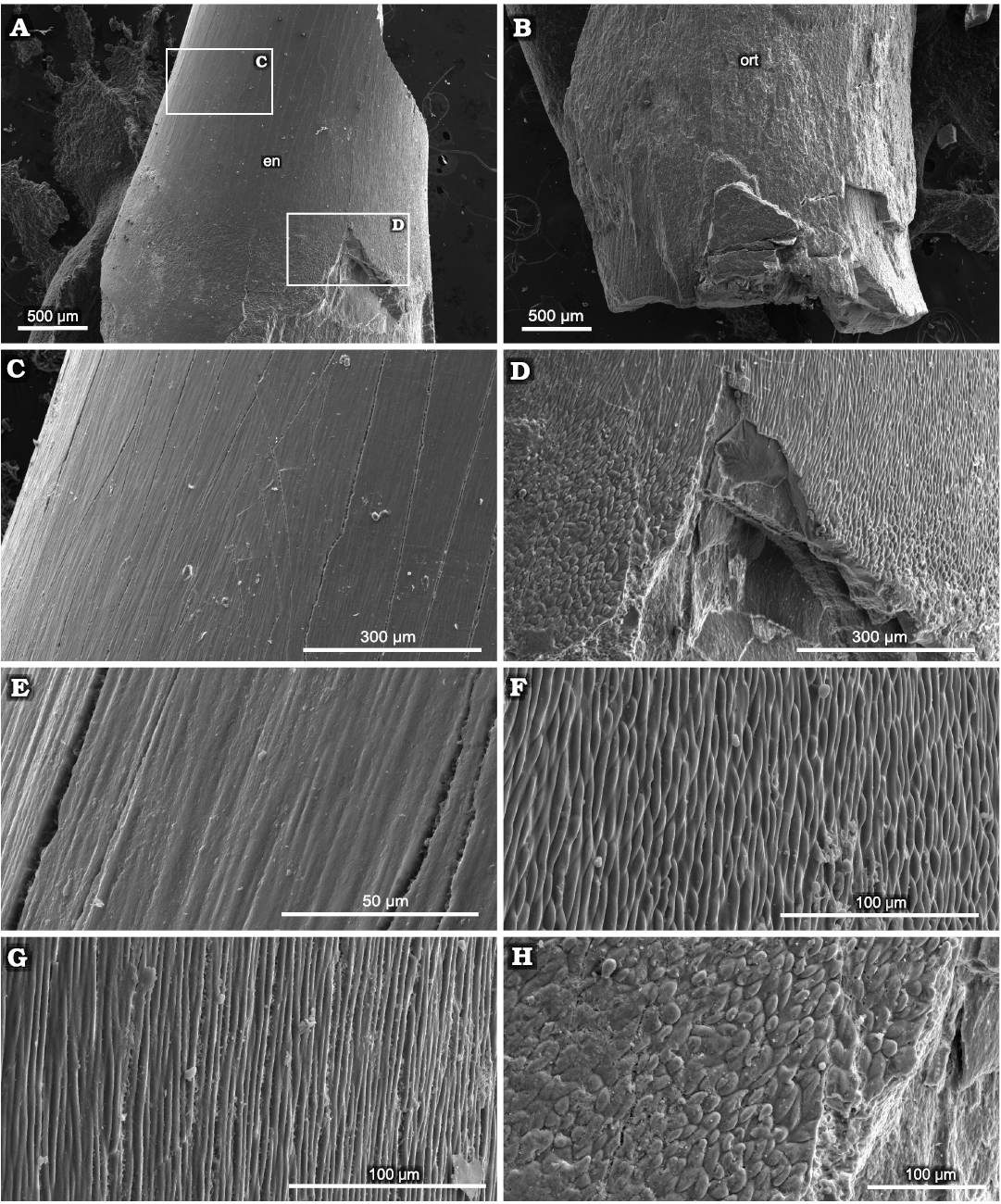
Fig. 8. SEM overview of tooth of caturoid fish Strobilodus sp. ZPAL P. 16/O-B/2 from Tithonian, Upper Jurassic Kcynia Formation, Corbulomima Limestone (Unit III), Owadów-Brzezinki, Poland. A. Mid-crown section with visible layer of enameloid (en). B. Basal section with exposed orthodentin (ort). C, D. Overview in apical-mid-crown surface. E–H. Changes in enameloid structure from linear through irregular to scale-like in basal direction.
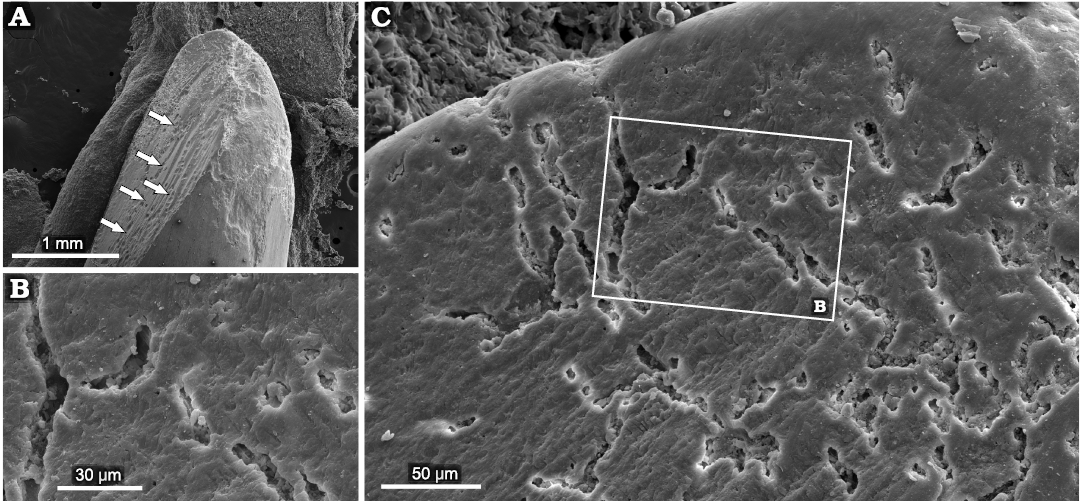
Fig. 9. Examples of superficial penetrations present in orthodentin of caturoid fish Strobilodus sp. ZPAL P. 16/O-B/2 from Tithonian, Upper Jurassic Kcynia Formation, Corbulomima Limestone (Unit III), Owadów-Brzezinki, Poland. A. Overview of penetrated teeth with exposed dentin (borings marked by arrows). B. Close view of structure with visible bifurcating canals. C. Example of advanced bioerosion, with substantial surface area of orthodentin penetrated.
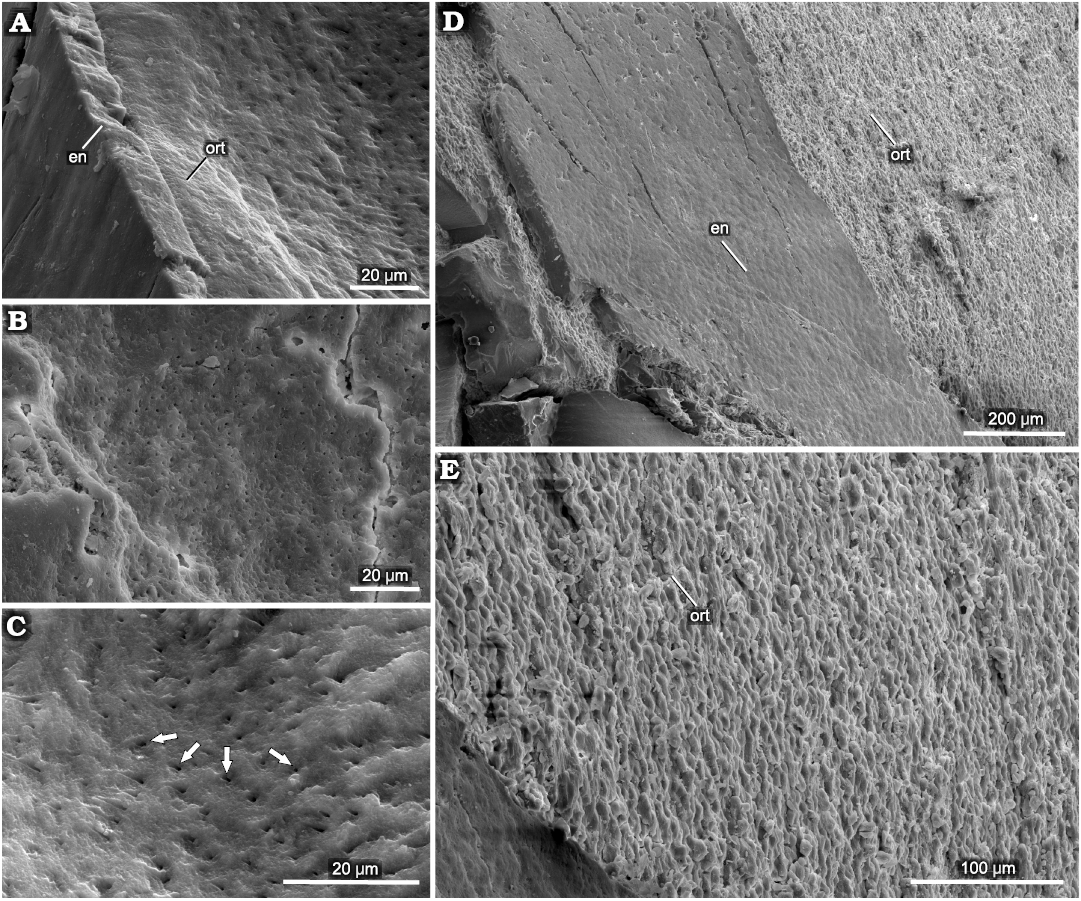
Fig. 10. Structure of orthodentin of tooth of caturoid fish Strobilodus sp. ZPAL P. 16/O-B/2 from Tithonian, Upper Jurassic Kcynia Formation, Corbulomima Limestone (Unit III), Owadów-Brzezinki, Poland. A. Boundary between orthodentin (ort) and enameloid (en). B, C. Overview of horizontal surface of orthodentin with dental tubuli (arrows). D. Vertical view of boundary between enameloid and orthodentin, with compact, regular enameloid and (E) irregular, rugose, porous orthodentin surface.
Orthocormus teyleri.—The surface of the enameloid in this species can be characterized by the presence of prominent longitudinal ridges (Fig. 11), which appear as bump-like structures arranged linearly in the apicobasal plane. These structures are divided by visible fractures, probably of diagenetic origin. The base of the teeth is devoid of these structures, with a non-ridged granular enameloid with a dentin-like structure. The inner dentin is strongly perforated by numerous capillary dentinal canals present in cross section in horizontal view (Fig. 11D, H). These canals appear mostly smooth internally (Fig. 11D), with only minor horizontal disturbances. In the horizontal view, the dentin shows an elaborate winding structure, with horizontal ridges perpendicular to the tooth surface, which form an irregular canal-shaped surface (Fig. 11E–G).
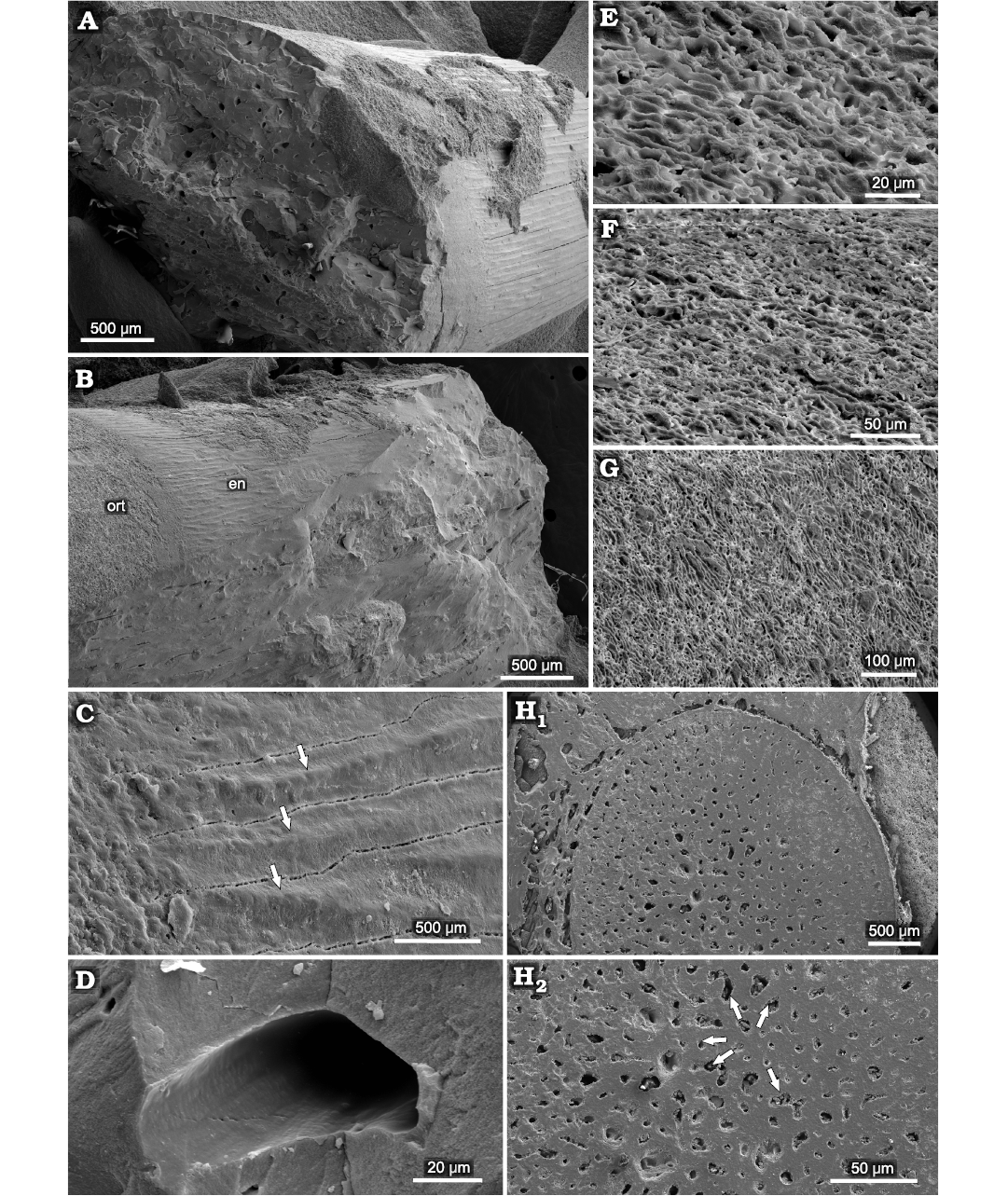
Fig. 11. Microstructure of teeth of pachycormid fish Orthocormus teyleri Lambers, 1988, ZPAL P. 16/O-B/3 from Tithonian, Upper Jurassic Kcynia Formation, Corbulomima Limestone (Unit III), Owadów-Brzezinki, Poland. A, B. General view. C. Enameloid (en) and orthodentin (ort) surface with visible longitudinal ridging (marked by arrows). D. View of singular denteonal canal of orthodentin. E–G. Visible structure of orthodentin layer in vertical view, developed as series of winding, ridged irregular cannals. H. Basal cross section in transverse plane, with visible osteodentin (H1), arrows indicating denteonal canals (H2).
Discussion
Microstructural and histological comparison.—Typical teeth of ray-finned fish consist of a core, dentin and is hardened on the outer surface by enameloid. The core, filled with dental pulp during life is preserved in the fossil record as a hollow pulp cavity, which often recrystallises as in the specimens presented herein. The cavity is surrounded by dentin, which can be either in form of tubular orthodentin or vascularised osteodentin, also called vasodentin, due to the vast internal network of canals which is more similar in structure to the bone (Ørvig 1976), with the possibility of only one or both types of dentin being present (Sasagawa et al. 2009; Berkovitz and Shellis 2017; Kawasaki et al. 2021; Thangadurai et al. 2022). The outermost layer of enameloid is more similar in structure to dentin as opposed to the typical enamel of tetrapods (Sire et al. 2021), being less mineralized. In addition, in primitive actinopterygians, such as those examined in the present study, enameloid caps, also designated as acrodin, are very prominent (Shellis and Miles 1974), forming the apexes and upper regions of the teeth. The enameloid is thus formed as a cap enameloid at the apical part and a collar enameloid on the labial, lingual, distal and mesial surfaces (Kawasaki et al. 2021).
The examined caturoids teeth show a largely solid internal structure with a small central canal in the apical part, while a moderately large pulp cavity is present in the basal part. They display orthodentin histology, with the presence of strongly developed dental tubules arranged radially around the pulp cavity. Their internal structure can be described as generally similar to teeth of many predatory fish (Berkovitz and Shellis 2017), exhibiting a structure also similar to other macropredatory aquatic taxa such as marine reptiles or crocodilians (e.g., Berkovitz and Shellis 2017; Owocki and Madzia 2020), with orthodentin building up the inner structure of the teeth, together with a prominent dental pulp cavity in the basal part. The pattern of dentin development can be described as rhythmic, since teeth show incremental growth lines.
Based on X-ray tomography of the sampled left dentary, Tyborowski (2017) discerned three histotypes present along the tooth row of O. tayleri: (i) “osteodentin type” with an internal structure composed of osteodentin, and no pulpal cavity, present in symphyseal and parasymphyseal teeth; (ii) “transitional type” with a noticeable pulpal cavity, surrounded by a thin layer of osteodentin, present in central part of marginal tooth row; (iii) “orthodentin type” with a well-defined pulpal cavity, and an internal structure filled with orthodentin, without visible osteodentin, located in posterior part of marginal tooth row.
The teeth of O. teyleri were sampled from the posterior part of the maxilla, so they should not have an osteodentin pulp cavity filling. Intriguingly, however, it can be observed that most of the internal tooth structure consists of osteodentin, and only a very small cavity in the dental pulp was observed (Fig. 6A1). This can be interpreted as an internal reinforcement of the medial teeth in the row, which means that not only the symphyseal teeth are braced, but this condition also partly corresponds to the more posterior teeth.
The histology of the O. tayleri teeth, as seen in the horizontal thin sections closely corresponds to the “osteodentin type”, with minor characteristics of the “transitional type”. However, it should be noted that these teeth were sampled from the marginal tooth row in distal parts of the maxilla. This could mean that the characteristic histology pattern observed by Tyborowski (2017) using X-ray tomography does not coincide with microscopic observations, as this type of histology would be expected from teeth located only in the symphyseal and parasymphyseal planes of the tooth row. Thus, it should be concluded that not only the parasymphyseal and symphyseal teeth show internal osteodentin histology, but so do the posterior teeth. The above conclusion also indicates that observation of traditional thin sections under light microscope is proving to be a more nuanced method of histological examination. However, the disadvantage of this technique is the destruction of the specimen during thin-section preparation.
Finally, a striking feature of the teeth of O. teyleri is the polygonal structure of the osteodentin, characterized by numerous denteonal canals that serve as ossification centres for odontoblasts spreading radially to form characteristic denteons. The individual canals can pass nutrients through a series of smaller capillaries diverging from a central capillary. This system was vital for providing the nutrients needed for the rapid growth of odontoblasts. It can be suspected that the pronounced nature of the denteons in the basal parts may be influenced by diagenetic processes that highlighted the individual denteons. A very similar osteodentin structure can be observed in contemporary Atlantic wolfish (see Thangadurai et al. 2022: figs. 2, 9), with the internal vascularization occurring as multiple pores rather than as a single dental pulp cavity with a central canal.
Micro-borings present in the structure of the teeth.—Numerous pits are visible on the surfaces of many of the observed specimens. This feature is particularly evident on SEM examination, where it can be seen that they are instead developed in the form of superficial canals. These structures only appear on surfaces with exposed dentin. Enamel in general is devoid of such structures, probably due to its higher mechanical resistance in comparison to dentin. The observed canals can be interpreted as post-mortem structures of possible fungal origin. Similar structures have been observed in the teeth of Jurassic neoselachians (Martill 1989). These micro-borings can probably be attributed to the ichnospecies Mycellites ossifragus Roux, 1887, known to occur on the teeth of fossil fishes (Underwood et al. 1999), also in orthodentin. Although apatite is one of the most physically and chemically resistant biominerals, it is believed that a group of fungi have developed the ability to use this phosphate source (Martill 1989), enabling them to colonise vertebrate skeletal elements. Underwood et al. (1999) believe that the teeth and roots of bony fish such as Caturoidea often display these types of fungal origin structures, even though they are more commonly found in selachian teeth. In the tooth specimens studied, the structures observed often show the typical form of an advanced stage of colonisation, with a bifurcated, irregular branching structure on the tooth surface, sometimes involving large areas of exposed dentin. These observations suggest that many of the teeth studied were intensively colonised after the death of fish. The lagoonal environment of Unit III, from the sediments of which the specimens studied were derived, was characterised by the presence of microbial mats that enabled the preservation of horseshoe crabs (Kin and Błażejowski 2014). Possibly, this type of environment was also favourable for the development of bone-consuming fungi, which would explain the extent of colonisation present in the samples.
Palaeoecological aspects inferred from observed macro- and microstructural differences.—The shape and proportions of teeth, on a par with their microstructure, are one of the most crucial indicators of animal ecology. For studies of fossil marine predators, a classification of ecological traits based on tooth proportions has been proposed (Massare 1987; Hornhung and Reich 2015; Foffa et al. 2018). This classification has been successfully applied to the fossil teeth of large marine reptiles, as they are often the best-preserved part of these large vertebrates and therefore provide abundant and good-quality samples to reconstruct the ecology of the organism. The palaeoecological grouping of large marine reptiles is based on the relative proportions of apicobasal, mesiodistal and labiolingual measurements along with other dental characteristics. Those include the presence of shearing carinae with three basal groups (cutting, piercing, and crushing) as well as emerging additional intermediate groups (Hornhung and Reich 2015), and for the purposes of this study similar general observations can be made.
Ecological classifications of fishes, including modern ones, are based on criteria other than tooth shape (Eliott et al. 2007; Franco et al. 2008). Nevertheless, it appears that dentary features such as shape and proportions, including those in the extinct predatory Actinopterygii studied herein, are among the common indicators of ecology for all vertebrates (Alistair 2013). That is the case since specific adaptive pressures affect morphology regardless of the taxonomic position of the organism under investigation.
The taxa studied are often found in strata of similar facies, indicating their cohabitation in comparable environments. While both caturoids and raptorial pachycormids can be described as fast-swimming predatory fishes living in open water basins, their dental characteristics (Fig. 12), presented as macro- and microstructural features, suggest that they used different hunting strategies. This allowed these large, similar-sized predators to coexist and exploit different resources of the same environment.
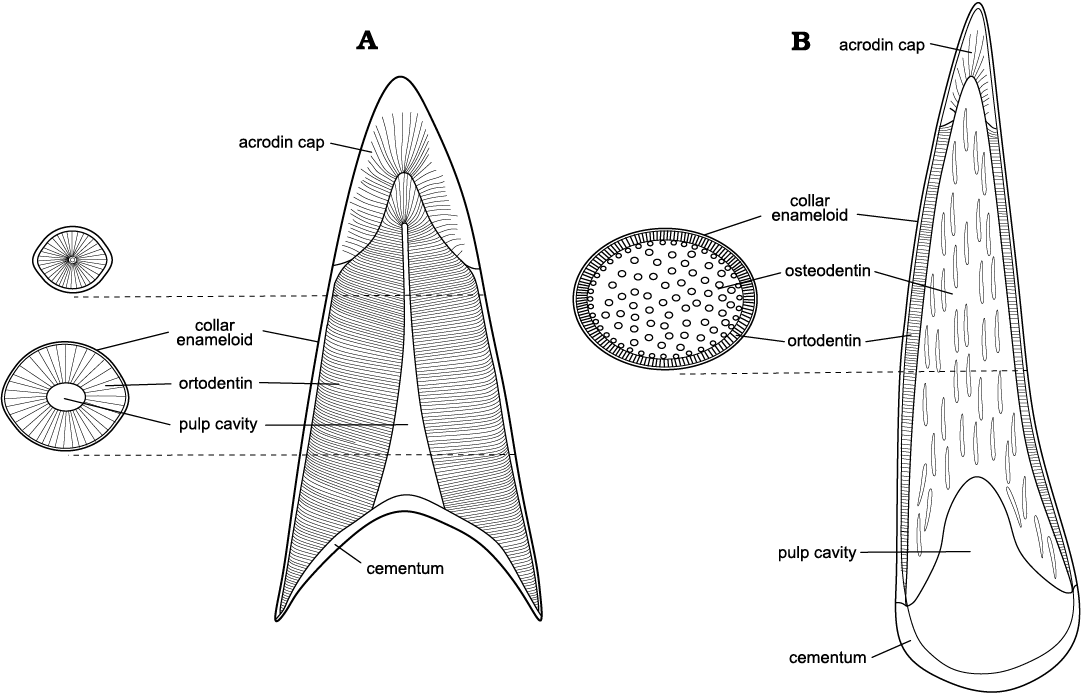
Fig. 12. Comparison of generalized teeth vertical cross sections of the studied taxa. A. Caturoidea. B. Pachycormidae.
The incremental growth lines are undoubtedly the most interesting aspect of the histology of the studied teeth of caturoids, as this type of structure is particularly prominent in animals inhabiting diverse environments or ones with seasonal variation. Caturoids can be considered a superfamily that was resistant to oxygen deficiency, as it is placed in the Amiiformes (Wierzbowski et al. 2019), which often shows such adaptations. The dentin structures observed herein are interpreted as long-term increments known as the Andresen lines (Dean 2000). The apparent pattern of dentin development can be described as long-lasting endogenous biorhythms. Such characteristic of the Andresen lines identified in this case, distinguishes it from the more widely known daily incremental growth marker—von Ebner lines, which documents longer incremental cycles seen in dentin (Dean 2000).
It should be mentioned that the observed growth patterns of successive layers in all the maxillary teeth studied appear to be very irregular. These increments probably grew at different times and, therefore, the growth of dentin was regulated by a varied supply of nutrients. Although members of Caturoidea, such as Caturus are considered marine (Wenz et al. 1994; Kriwet et al. 1997; Pouech et al. 2014), they are also found in coastal and lacustrine paleoenvironments (Lambers 1994; Bogan et al. 2013; Martín-Abad and Poyato-Ariza 2013; Woodward 2014; Poyato-Ariza and Martín-Abad 2020). It can therefore be assumed that they are the result of growth in an unstable habitat. Occurrences such as fluctuations in salinity and oxygenation levels may cause irregular nutrient replenishment, which would manifest as differential dentin growth. This is supported by the results of the isotopic study by Wierzbowski et al. (2019), in which oxygen stable isotope ratios from Caturoidea (referred in the study as Caturus sp.) teeth indicate hypersaline episodes recorded in the Unit III sediments. The observed pattern of structural growth suggests that in Caturoidea, stress conditions not only affect oxygen isotope ratios, but also control biomineralization processes. Wierzbowski et al. (2019) hypothesised that Caturoidea like modern bowfins (Amia calva), may be characterized as bimodally respiring and thus adapted to low-oxygen environments, which often occur in such shallow waters as those represented by the Plattenkalk sediments (Fürsich et al. 2007). The apparent differential growths observed in the dentition of studied caturoids support this theory, since episodes or periods of low oxygenation certainly affect the biological processes of the animals. This also backs the supposition that caturoids. were capable of inhabiting unstable and demanding environments. Such adaptations enabled them to thrive in environments in which the more pelagic species of Orthocormus could not. To some extent, it gives an explanation to the numerous finds of caturoids in the fossil record worldwide.
The teeth of caturoids examined herein are characteristic for a predator with a wide range of potential prey. The presence of cutting edges (visible in the geometry of the teeth in thin-section) in the mesiodistal plane indicates that the animal had a dentition with cutting abilities. Its hunting strategy was not restricted to grasping, but the described taxon was also capable of biting off chunks of flesh, suggesting that it could handle prey larger than itself. The ratio of apicobasal crown length to mesiodistal length of 2.29 display that teeth are robust. The teeth show some minor labiolingual compression and, more importantly, the symmetrical shape of the teeth with carinae present on both the mesial and distal surfaces indicates that shearing action played an important role in the feeding strategy.
Furthermore, the geometry of the apex of the tooth can be described as conical, hinting that puncturing was also an important aspect in prey capture, as piscivory requires a degree of puncturing and adaptation to hold prey (Cohen et al. 2020). The relatively uniform dentition of the studied Caturoidea specimens can be described as villiform (Mihalitsis and Bellwood 2019), which is characteristic of piscivorous predators adapted to prey capture with an irregular or puncture-resistant integument. These features suggest that caturoids represented a member of the intermediate slash/cut guild. In contrast to internally solid teeth of O. teyleri, the teeth of caturoids. have a rather prominent dental pulp cavity, which means that the teeth were most likely susceptible to severe trauma, even taking into account their more robust ratio of crown apicobasal height to mesiodistal length. Due to the presence of prominent carinae on both the mesial and distal sides, shearing action enabled the teeth to reduce mechanical trauma during prey capture. In this scenario, greater internal tooth strength is not required, as the priority is to tear apart prey fragments.
The most outstanding feature of O. teyleri is the presence of multiple teeth that erupt from the alveolar bone, which demonstrates a very rapid rate of tooth replacement. This trait, combined with a persistent internal osteodentin solid core present in all dentary teeth, including mesial teeth, and not only in the symphyseal and parasymphyseal region, as postulated by Tyborowski (2017), indicates multiple adaptations to a specialized predatory lifestyle, with a possible high-impact feeding pattern balanced by a fast rate of tooth replacement. The findings of present study complements the study by Tyborowski (2017) and highlights the possible additional reinforcement of all dentary teeth in the specimen studied, as opposed to only the anterior teeth. Such an arrangement would provide further mechanical reinforcement during ram feeding, which is a proposed hunting strategy by Tyborowski (2017). Tyborowski (2017) also compares O. tayleri ecology with modern day Sphyraena barracuda, and correspondingly, with a similar lifestyle to other modern predatory Actinopterygii living in open water (Harper and Blake 1991; Grubich et al. 2008). In the ram-feeding model, it should be taken into consideration that not only the anterior teeth, but also mesial teeth may come into contact with high force when ramming into prey, hence the more posterior teeth may require additional structural support. This aspect requires further biomechanical studies to assess the possible jaw dilation in this taxon. Furthermore, it is noteworthy that a high impact feeding strategy may be possible due to the fast rate of tooth emergence observed in the alveolar bone examined in this study, in which teeth lost due to ramming can be easily replaced. The pronounced internal vascularisation of the teeth researched can be explained by the need for a rapid supply of nutrients to sustain prompt tooth development.
The teeth of O. teyleri are strongly heterodont, and with an average elongation of 2.76 in the apicobasal/mesiodistal ratio, represent features belonging to a piercing guild animal. The teeth examined have some intermediate features related to cutting adaptation, such as a slight distal curvature, ovaloid cross-section with very little labiolingual compression, and above all the presence of mesial carinae in larger marginal teeth and symphyseal tusks. Despite this, the rather elongated proportions of the teeth, coupled with their marked variation in size, indicate an animal adapted primarily for grasping. The anterior rosette of teeth, which ends in an indentation located behind the plane of the jaw symphysis, also implies this ecology. Furthermore, the anterior symphyseal and parasymphyseal teeth are directed posteriorly, while the posterior teeth are directed anteriorly, forming a kind of trap directed at the symphyseal notch. Mihalitsis and Bellwood (2019) postulate that front fanged condition in piscivorous fished such as in examined O. teyleri, is an adaptation that aids in capture of fast, elusive prey. These features, combined with described tooth microstructure, allow us to conclude that this taxon, in contrast to caturoids. was probably a predator characterized by a more dynamic hunting strategy, involving the active ramming of mid-sized prey, which was retrieved by impalement through conical-shaped teeth.
Conclusions
Our observations in this study provide incentive for further microstructural analyses of fossil Actinopterygii as a tool for interpretation of their biology, especially when it comes to microstructural aspects of the predatory Jurassic fish of the families examined herein. Although both Caturoidea, and predatory pachycormids possess the elongated bodies of rapid predators that hunt from ambush (Tyborowski 2017), their dental characteristics indicate different adaptations for predation. Both tooth morphology and histology suggest niche partitioning. Due to their varied feeding strategies, these two similarly sized predatory fish taxa were able to co-exist in marine environments on a global scale, known from Late Jurassic sites such as famous Solnhofen (Germany) or, becoming increasingly important, Owadów-Brzezinki (Poland). Comparison of the microstructure and external tooth features of the aforementioned taxa allowed the following conclusions to be drawn, also concerning their palaeoecology:
(i) The examined caturoids possessed conical dentition, slightly mesiodistally compressed, with mesiodistal carinae. It is rather uniform in shape with large piercing-shearing teeth, capable not only of grasping but also of cutting chunks of flesh to retrieve a wider range of prey, representing an intermediate piercing/cutting guild. The internal tooth structure visible in cross-section consists of solid orthodentin tubules, with a moderately sized dental pulp cavity present in the basal plane and very small one in more apical plane. The specimen of Strobilodus sp. whose dentary was examined here probably lived in a variable environment, as reflected in the fluctuating rate of dentin growth line pattern. This was due to changes in salinity and oxygen levels, indicating a high environmental tolerance of members of the family Caturoidea. Such characteristic enabled Caturoidea to thrive in demanding environments, which may explain their worldwide presence in the fossil record;
(ii) The dentition of predatory pachycormids can be characterized as conical, slightly mesiodistally compressed, with distal curvature of the larger teeth in the parasymphaseal tooth row, along with the general anterior alignment of the posterior teeth. The elongated, conical shape of the teeth and heterodont condition suggest that this species belongs to the guild of piercing fishes. Internally, the majority of the examined internal tooth volume in the dentary consists of a small-diameter porous osteodentin structure that forms denteons with central canals to create a characteristic polygon-like structure. Their internal structure can be described as highly vascularized. Numerous growing teeth are visible in alveolar bone, indicating a very rapid rate of tooth replacement. Considering the proposed high-powered ram-type feeding strategy, these physiological features could help to replace teeth that are often lost during attacks on prey.
Acknowledgements
We would like to express our gratitude to the reviewers for providing valuable suggestions: an anonymous referee and especially to Adriana López-Arbarello (Ludwig-Maximilians-University of Munich, Germany) for helping with establishing the taxonomic position Caturoidea material and other very helpful comments. This work was supported by the Polish National Science Centre (grant no. 2020/39/B/ST10/01489). The research in 2022 was supported by the Priority Research Area (WSPR.WGiG.1.5.2022.2) under the Strategic Programme Excellence Initiative at Jagiellonian University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding was received for this study.
References
Alistair, R.E. 2013. Shape descriptors as ecometrics in dental ecology. Virtual Morphology and Evolutionary Morphometrics in the New Millenium 24: 133–140.
Arratia, G. 2004. Mesozoic halecostomes and the early radiation of teleosts. In: G. Arratia and A. Tintori (eds.), Mesozoic Fishes 3—Systematics, Paleoenvironments and Biodiversity, 279–315. Verlag Dr. Friedrich Pfeil, München.
Arratia, G. and Schultze, H.P. 2013. Outstanding features of a new Late Jurassic pachycormiform fish from the Kimmeridgian of Brunn, Germany and comments on current understanding of pachycormiforms In: G. Arratia, H.-P. Schultze, and M.V.H. Wilson (eds.), Mesozoic Fishes—Global Diversity and Evolution V, 87–120. Verlag Dr. Friedrich Pfeil, München.
Błażejowski, B. and Wierzbowski, A. 2021. The Owadów-Brzezinki geoeducation area at Sławno. Geotourism/Geoturystyka 17 (1–2) (60–61): 39–45. Crossref
Błażejowski B., Gieszcz P., and Tyborowski, D. 2016. New finds of well-preserved Tithonian (Late Jurassic) fossils from the Owadów-Brzezinki Quarry, Central Poland: a review and perspectives. Volumina Jurassica 14: 123–132.
Błażejowski B., Gieszcz P., Shinn A.P., Feldmann R.M., and Durska, E. 2019. Environment deterioration and related fungal infection of Upper Jurassic horseshoe crabs with remarks on their exceptional preservation. Palaeogeography, Palaeoclimatology, Palaeoecology 516: 336–441. Crossref
Błażejowski, B., Lambers, P., Gieszcz, P., Tyborowski, D., and Binkowski, M. 2015. Late Jurassic jaw bones of halecomorph fish (Actinopterygii: Halecomorphi) studied with X-ray microcomputed tomography. Palaeontologia Electronica 18 (53A): 1–10. Crossref
Błażejowski, B., Pszczółkowski, A., Grabowski, J., Wierzbowski, W., Deconinck, J.-F., Olempska and E., Teodorski A., and Nawrocki, J. 2023. Integrated stratigraphy and clay mineralogy of the Owadów-Brzezinki section (Lower–Upper Tithonian transition, Central Poland): implications for correlations between the Boreal and the Tethyan domains and palaeoclimate. Journal of the Geological Society 180 (2): jgs2022-073 [published online, https://doi.org/10.1144/jgs2022-073]. Crossref
Błażejowski, B., Wierzbowski, H., and Feldmann, R.M. 2020. Reply to the comment on “No evidence for fungal infection of Upper Jurassic horseshoe crabs: A comment on Błażejowski et al. (2019)” by Zatoń 2020. Palaeogeography, Palaeoclimatology, Palaeoecology 554: 109733. Crossref
Berg, L.S. 1937. A classification of fish-like vertebrates. Bulletin del’Académie des Sciences de l’URSS 4:1277–1280.
Berkovitz, B.K.B. and Shellis, R.P. 2017. The Teeth of Non-Mammalian Vertebrates. 354 pp. Academic Press, London.
Bogan, S., Taverne, L., and Agnoli, F. 2013. First Triassic and oldest record of a South American amiiform fish: Caturus sp. from the Los Menucos Group (lower Upper Triassic), Rio Negro Province, Argentina. Geologica Belgica 16: 191–195.
Cohen, K.E., Weller, H.I., and Summers, A.P. 2020. Not your father’s homodonty-stress, tooth shape, and the functional homodont. Journal of Anatomy 237: 837–848. Crossref
Cooper, S.L., Giles, S., Young, H., and Maxwell, E.E. 2022. A new large †pachycormiform (Teleosteomorpha:†Pachycormiformes) from the Lower Jurassic of Germany, with affinities to the suspension-feeding clade, and comments on the gastrointestinal anatomy of pachycormid fishes. Diversity 14: 1026. Crossref
Cope, E.D. 1871. Observations on the systematic relations of the fishes. The American Naturalist 5 (8/9): 579–593. Crossref
Cope, E.D. 1887. General notes: geology and palaeontology. American Naturalist 21: 1016–1019. Crossref
Dean, M.C. 2000. Incremental markings in enamel and dentine: what they can tell us about the way teeth grow. In: M.F. Teaford, M.M. Smith, and M.W.J. Ferguson (eds.), Development, Function and Evolution of Teeth, 119–130. Cambridge University Press, Cambridge. Crossref
Elliott, M., Whitfield, A.K., Potter, I.C., Blaber, S.J., Cyrus, D.P., Nordlie, F.G., and Harrison, T.D. 2007. The guild approach to categorizing estuarine fish assemblages: a global review. Fish and Fisheries 8: 241–268. Crossref
Franco, A., Elliott, M., Franzoi, P., and Torricelli, P. 2008. Life strategies of fishes in European estuaries: the functional guild approach. Marine Ecology Progress Series, 354: 219–228. Crossref
Friedman, M., Shimada, K., Martin, L.D., Everhart, M.J., Liston, J., Maltese, A., and Triebold, M. 2010. 100-million-year dynasty of giant planktivorous bony fishes in the Mesozoic seas. Science 327: 990–993. Crossref
Foffa, D., Young, M.T., Stubbs, T.L., Dexter, K.G., and Brusatte, S.L. 2018. The long-term ecology and evolution of marine reptiles in a Jurassic seaway. Nature Ecology & Evolution 2: 1548–1555. Crossref
Fürsich, F.T., Werner, W. Schneider, S., and Mäuser, M. 2007. Sedimentology, taphonomy, and palaeoecology of a laminated plattenkalk from the Kimmeridgian of the northern Franconian Alb (southern Germany). Palaeogeography, Palaeoclimatology, Palaeoecology 243: 92–117. Crossref
Grande, L. and Bemis, W.E. 1998. A comprehensive phylogenetic study of amiid fishes (Amiidae) based on comparative skeletal anatomy. An empirical search for interconnected patterns of natural history. Journal of Vertebrate Paleontology, Memoirs 4 (Supplement to volume 18): i–x + 1–690. Crossref
Grubich, J.R., Rice, A.N., and Westneat, M.W. 2008. Functional morphology of bite mechanics in the great barracuda (Sphyraena barracuda). Zoology 111: 16–29. Crossref
Harper, D.G., and Blake, R.W. 1991. Prey capture and the fast-start performance of northern pike Esox lucius. Journal of Experimental Biology 155: 175–192. Crossref
Hay, O.P. 1929. Second bibliography and catalogue of the fossil vertebrata of North America. Publications of the Carnegie Institute of Washington 390: 1–2003.
Hornung, J.J. and Reich, M. 2015. Tylosaurine mosasaurs (Squamata) from the Late Cretaceous of northern Germany. Netherlands Journal of Geosciences 94: 55–71. Crossref
Kawasaki, K., Keating, J.N., Nakatomi, M., Welten, M., Mikami, M., Sasagawa, I., Puttick M.N., Donoghue P.C.J., and Ishiyama, M. 2021. Coevolution of enamel, ganoin, enameloid, and their matrix SCPP genes in osteichthyans. Iscience 24 (1): 102023. Crossref
Kin, A., Błażejowski, B., and Binkowski, M. 2012. The “Polish Solnhofen”: a long-awaited alternative? Geology Today 28: 91–94. Crossref
Kin, A., Gruszczyński, M., Martill, D., Marshall, J.D., and Błażejowski, B. 2013. Palaeoenvironment and taphonomy of a Late Jurassic (late Tithonian) Lagerstätte from central Poland. Lethaia 46: 71–81. Crossref
Kin, A. and Błażejowski, B. 2014. The horseshoe crab of the genus Limulus: living fossil or stabilomorph? PLoS One 9 (10): e108036. Crossref
Kriwet, J., Rauhut, O.W.M., and Gloy, U. 1997. Microvertebrate remains (Pisces, Archosauria) from the Middle Jurassic (Bathonian) of southern France. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen 206: 1–28. Crossref
Kutek, J. 1994. The Scythicus Zone (Middle Volgian) in Poland: its ammonites and biostratigraphic subdivision. Acta Geologica Polonica 44: 1–34.
Lambers, P.H. 1988. Orthocormus teyleri nov. sp., the 1st pachycormid (pisces, actinopterygii) from the Kimmeridge Lithographic Limestone at Cerin (Ain), France-with remarks on the genus Orthocormus Weitzel. Proceedings of the Koninklijke Nederlandse Akademie van Wetenschappen. Series B: Palaeontology Geology Physics Chemistry Anthropology 91: 369–391.
Lambers, P.H. 1994. The halecomorph fishes Caturus and Amblysemius in the lithographic limestone of Solnhofen (Tithonian), Bavaria. Geobios 27: 91–99. Crossref
Lambers, P.H. 1998. The genus Furo (Pisces, Halecomorphi) from the Upper Jurassic Plattenkalke of Germany. Oryctos 1: 23–35.
Lambers, P.H. 1999. The actinopterygian fish fauna of the Late Kimmeridgian and Early Tithonian “Plattenkalke” near Solnhofen (Bavaria, Germany: state of the art). Geologie en Mijnbouw 78: 215–229. Crossref
Liston, J.J. 2004. An overview of the pachycormiform Leedsichthys. Mesozoic Fishes 3: 379–390.
Liston, J.J., Maltese, A.E., Lambers, P.H., Delsate, D., Harcourt-Smith, W.E., and van Heteren, A.H. 2019. Scythes, sickles and other blades: defining the diversity of pectoral fin morphotypes in Pachycormiformes. PeerJ 7: e7675. Crossref
López-Arbarello, A. and Ebert, M. 2023. Taxonomic status of the caturid genera (Halecomorphi, Caturoidea) and their Late Jurassic species. Royal Society Open Science 10 (1): 221318. Crossref
Matyja, B.A. and Wierzbowski, A. 2016. Ammonites and ammonite stratigraphy of the uppermost Jurassic (Tithonian) of the Owadów-Brzezinki quarry (central Poland). Volumina Jurassica 14: 85–122.
Martill, D.M. 1989. Fungal borings in neoselachian teeth from the Lower Oxford Clay of Peterborough. Mercian Geologist 12 (1): 1–4.
Martín-Abad, H. and Poyato-Ariza, F.J. 2013. Historical patterns of distribution in pycnodontiform and amiiform fishes in the context of moving plates. Geologica Belgica 16 (4): 217–226.
Massare, J.A. 1987. Tooth morphology and prey preference of Mesozoic marine reptiles. Journal of Vertebrate Paleontology 7: 121–137. Crossref
Maxwell, E.E., Lambers, P.H., Lopez-Arabello, A., and Schweigert, G. 2020. Re-evaluation of pachycormid fishes from the Late Jurassic of Southwestern Germany. Acta Palaeontologica Polonica 65: 429–453. Crossref
Mihalitsis, M. and Bellwood, D. 2019. Functional implications of dentition-based morphotypes in piscivorous fishes. Royal Society Open Science 6 (9): 190040. Crossref
Müller, J. 1845. Über den Bau und die Grenzen der Ganoiden, und über das natürliche System der Fische. Königlichen Akademie der Wissenschaften, Berlin 11: 91–141.
Müller, M.K. 2011. The fish fauna of the Late Jurassic Solothurn Turtle Limestone (NW Switzerland). Swiss Journal of Geosciences 104: 133–146. Crossref
Owen, R.B. 1860. Palaeontology or a Systematic Summary of Extinct Animals and Their Geological Relations. 420 pp. Adam and Black, Edinburgh. Crossref
Owocki, K. and Madzia, D. 2020. Predatory behaviour in mosasaurid squamates inferred from tooth microstructure and mineralogy. Cretaceous Research 111: 104430. Crossref
Ørvig, T. 1976. Palaeohistological notes. 4. The interpretation of osteodentine,with remarks on the dentition in the Devonian dipnoan Griphognathus. Zoologica Scripta 5: 79–96. Crossref
Pouech, J., Amiot, R., Le´Cuyer, C., Mazin, J.-M., Martineau, F., and Fourel, F. 2014. Oxygen isotope composition of vertebrate phosphates from Cherves-de-Cognac (Berriasian, France): environmental and ecological significance. Palaeogeography, Palaeoclimatology, Palaeoecology 410: 290–299. Crossref
Poyato-Ariza, F.J. and Martín-Abad, H. 2020. History of two lineages: Comparative analysis of the fossil record in Amiiformes and Pycnodontiformes (Osteischtyes, Actinopterygii). Spanish Journal of Palaeontology 28: 79–90. Crossref
Roux, W. 1887. Über eine im Knochen lebende Gruppe von Fadenpilzen (Mycelites ossifragus). Zeitschrift der wissenschaftlichen Zoologie 45: 227–254.
Sasagawa, I., Ishiyama, M., Yokosuka, H., Mikami, M., and Uchida, T. 2009. Tooth enamel and enameloid in actinopterygian fish. Frontiers of Materials Science in China 3 (2): 174–182. Crossref
Schaeffer, B. and Patterson, C. 1984. Jurassic fishes from the western United States, with comments on Jurassic fish distribution. American Museum Novitates 2796: 1–86.
Shellis, R.P. and Miles, A.E.W. 1974. Autoradiographic study of the formation of enameloid and dentine matrices in teleost fishes using tritiated amino acid. Proceedings of the Royal Society of London (B) 185: 51–72. Crossref
Sire, J.Y., Donoghue, P.C., and Vickaryous, M.K. 2009. Origin and evolution of the integumentary skeleton in non-tetrapod vertebrates. Journal of Anatomy 214: 409–440. Crossref
Thangadurai, S., Brumfeld, V., Milgram, J., Li, L., and Shahar, R. 2022. Osteodentin in the Atlantic wolffish (Anarhichas lupus): Dentin or bone? Journal of Morphology 283: 219–235. Crossref
Tyborowski, D. 2017. Large predatory actinopterygian fishes from the Late Jurassic of Poland studied with X-ray microtomography. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen 283: 161–172. Crossref
Underwood, C.J., Mitchell, S.F., and Veltkamp, C.J. 1999. Microborings in mid-Cretaceous fish teeth. Proceedings of the Yorkshire Geological Society 52: 269–274. Crossref
Wagner, J.A. 1851. Beiträge zur kenntniss der iden lithographischen schiefern abgelagerten urweltlichen fische. 96 pp. Abhandlungen der Mathematisch-Physikalischen Klasse der Königlich Bayerischen Akademie der Wissenschaften, München.
Wierzbowski, H., Błażejowski, B., and Tyborowski, D. 2019. Oxygen isotope profiles of uppermost Jurassic vertebrate teeth and oyster shells: a record of paleoenvironmental changes and animal habitats. Palaios 34: 585–599. Crossref
Wierzbowski, H., Dubicka, Z., Rychliński, T., Durska, E., Olempska, E., and Błażejowski, B. 2016. Depositional environment of the Owadów-Brzezinki conservation Lagerstätte (uppermost Jurassic, central Poland): evidence from microfacies analysis, microfossils and geochemical proxies. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen 282: 81–108. Crossref
Weitzel, K. 1930. Drei Riesenfische aus den Solnhofener Schiefern von Langenaltheim. Abhandlungen der Senckenbergischen Naturforschenden Gesellschaft 42: 85–113.
Wenz, S., Bernier, P., Barale, G., Bourseau, J.-P., Buffetaut, E., Gaillard, C., and Gall, J.-C. 1994. L’ichthyofaune des calcaires lithographiques du Kimm´eridgien sup´erieur deCerin (Ain, France). Geobios 27 (Supplement 1): 61–70. Crossref
Woodward, A.S. 1893. On some British Upper–Jurassic fish-remains, of the genera Caturus, Gyrodus, and Notidanus. Journal of Natural History 12: 398–402. Crossref
Woodward, A.S. 1895. Catalogue of the Fossil Fishes in the British Museum of Natural History. 544 pp. British Museum of Natural History, London. Crossref
Woodward, A.S. 2014, The Fossil Fishes of the English Wealden and Purbeck Formations: Cambridge Library Collection, Monographs of the Palaeontographical Society. 149 pp. Cambridge University Press, Cambridge. Crossref
Acta Palaeontol. Pol. 68 (3): 493–512, 2023
https://doi.org/10.4202/app.01058.2023