


Enamel microstructure and dental histology in a heterodontosaurid dinosaur: Heterodontosaurus tucki
CECILIA E. CALVERT, TYLER C. HUNT, NIALL S. WHALEN, JONAH N. CHOINIERE, MARK A. NORELL, and GREGORY M. ERICKSON
Calvert, C.E., Hunt, T.C., Whalen, N.S., Choiniere, J.N., Norell, M.A., and Erickson, G.M. 2023. Enamel microstructure and dental histology in a heterodontosaurid dinosaur: Heterodontosaurus tucki. Acta Palaeontologica Polonica 68 (4): 603–612.
Among non-avian dinosaurs, Heterodontosaurus tucki is unique for possessing complex dental features including both morphological and proportional heterodonty, sub-hypsodonty, tooth occlusion, and extensive low-angled wear facets—a collection of derived traits made additionally noteworthy by their appearance in one of the earliest-branching ornithischian lineages. In many taxa with similar dental characteristics, complex suites of modified dental tissues shape functional occlusal surfaces through wear. It remains unknown if H. tucki possesses similar histological complexity. Here, we investigate the histology and enamel microstructure of H. tucki maxillary cheek teeth from the Early Jurassic upper Elliot Formation of South Africa. Despite possessing a superficially complex dentition, the maxillary teeth exhibit a thin, relatively simple, three-layered enamel schmelzmuster (basal unit, columnar unit, and parallel crystallite) with enamel tubules. On the labial face, the enamel thins out drastically (<6 µm) and is discontinuous with a more simplified enamel microstructure. Surprisingly, a thick band of wear-resistant, histologically distinct dentine arises concurrent with the thinning enamel and appears to form the primary cutting crest of the functional occlusal surface, a role typically filled by enamel. This represents both the phylogenetically and chronologically earliest known acquisition of this form of modified dentine within Ornithischia.
Key words: Dinosauria, Heterodontosauridae, Heterodontosaurus tucki, enamel, microstructure, histology.
Cecilia E. Calvert [cecilia_calvert@nps.gov; ORCID: https://orcid.org/0009-0007-2745-5549 ] and Niall S. Whalen [nwhalen@bio.fsu.edu; ORCID: https://orcid.org/0000-0001-9732-1182 ], Department of Biological Science, Florida State University, 319 Stadium Dr, Tallahassee, FL 32306, USA.
Tyler C. Hunt [thunt@bio.fsu.edu; ORCID: https://orcid.org/0000-0001-6826-780X ] (corresponding author) and Gregory M. Erickson [gerickson@bio.fsu.edu; ORCID: https://orcid.org/0009-0003-4881-4900 ], Department of Biological Science, Florida State University, Tallahassee, FL 32306, USA; Mechanical and Physical Properties Laboratory National High Magnetic Field Laboratory-Florida State University, 1800 E Paul Dirac Dr, Tallahassee, FL 32310, USA.
Jonah N. Choiniere [Jonah.choiniere@wits.ac.za; ORCID: https://orcid.org/0000-0002-1008-0687 ], Evolutionary Studies Institute, University of Witwatersrand, 1 Jan Smuts Avenue, Johannesburg, South Africa.
Mark A. Norell [mark.norell@icloud.com; ORCID: https://orcid.org/0000-0002-1084-5555 ], Division of Vertebrate Paleontology, American Museum of Natural History, 200 Central Park West, New York, NY, USA.
Received 11 February 2023, accepted 20 September 2023, available online 1 December 2023.
Copyright © 2023 C.E. Calvert et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
In contrast to the simple, non-occluding teeth of most reptiles, some lineages of ornithischian dinosaurs evolved dental occlusion (Lambe 1902; Edmund 1960; Erickson et al. 2012, 2015). These derived dental features generally did not arise until late in the Mesozoic; Late Cretaceous hadrosaurs and ceratopsians exhibited complex multi-tooth grinding and high-angle slicing dentitions. These groups maintained their derived functional occlusal topography through the differential wear of a suite of exposed, modified dental tissues including enamel, mantle dentine, orthodentine, secondary dentine, vasodentine, and giant tubules (Erickson et al. 2012, 2015). Conversely, most early dinosaur dentitions retain the morphologically and histologically plesiomorphic reptilian condition; homodont crowns composed solely of orthodentine and a relatively thin enamel shell. In a notable exception to this trend, the earliest diverging ornithischian clade, Heterodontosauridae, exhibit a remarkably derived dentition featuring heterodonty, occlusion, derived episodic tooth replacement, and complex wear facets, a condition maximally developed in Heterodontosaurus tucki (Crompton and Charig 1962; Norman et al. 2011; Sereno 2012; Becerra et al. 2020).
Late Triassic to Early Cretaceous heterodontosaurids are broadly characterized by a differentiated dental arcade composed of: (i) incisor-like premaxillary teeth; (ii) laterally compressed premaxillary and dentary caniniform teeth; and (iii) a post-caniniform tooth row of worn, leaf-shaped (foliodont) teeth (Crompton and Charig 1962; Sereno 2012; Becerra et al. 2014). In derived heterodontosaurids, these post-caniniform teeth likely performed the bulk of the oral processing, as indicated by their substantial wear facets (Sereno 2012). Thought to be flanked by fleshy cheeks, these distal teeth are uniquely modified in Heterodontosaurus tucki (Sereno 2012). In contrast to their chisel-like appearance in most heterodontosaurids, H. tucki post-caniniform cheek teeth are labio-lingually expanded with a sub-rectangular outline (Sereno 2012; Norman et al. 2011). Their apices are completely effaced through use, resulting in a broad, flat, and block-like functional morphology in the distal-most teeth. Maxillary teeth are angled lingually while the dentary teeth are canted slightly labially relative to the orientation of their characteristic bluntly-rounded roots (Sereno 2012).
Specific to H. tucki, the post-caniniform teeth are uniquely arranged such that adjacent teeth are buttressed directly against one another. The wear facets formed by the occlusion of the 11 maxillary teeth with the 10 dentary teeth therefore result in near-continuous, planar functional surfaces across the tooth row (Sereno 2012). This composite surface formed by adjacent/successive wear facets forms a broad interface for contact with ingested food stuffs (Norman et al. 2011; Sereno 2012). While tooth morphology remains fixed throughout ontogeny, the angle of the wear surface varies both through ontogeny and with position along the tooth row. Additionally, the number of teeth making up this surface increases with the eruption of additional maxillary teeth in older specimens (Compton and Charig 1962; Butler et al. 2008). The wear surface is generally more planar in the adult dentition, where apical wear facets form nearly perpendicular to the apico-basal axis of the tooth, resulting in nearly flat, transversely-oriented surfaces (Sereno 2012). Wear surfaces are more steeply inclined in juveniles and subadults (Butler et al. 2008; Sereno 2012). However, across all age groups the surface becomes increasingly horizontal distally along the tooth row (Crompton and Charig, 1962; Norman et al. 2011). The pulp cavities are notably enlarged, to such an extent that they become exposed at the tooth surface during later stages of wear (Sereno 2012). Heterodontosaurids also possessed a derived mode of staggered, active tooth replacement that allowed for the continuity of a consistent wear surface despite different degrees of crown eruption across the tooth row (Norman et al. 2011; Sereno 2012).
Such a complex dentition and associated dental occlusion would imply substantial underlying complexity in enamel microstructure, a frequently observed phenomenon in both dinosaurs and mammals (Sander 1999; Hwang 2011; Becerra and Pol 2020; Wilmers and Bargmann 2020). The dental microstructure of only one heterodontosaurid genus has been previously investigated: Manidens condorensis from the Lower Jurassic of Argentina (Becerra and Pol 2020). Manidens condorensis exhibits relatively primitive dental characteristics relative to H. tucki. The small, high angle wear facets suggest a shearing mode of mastication and limited dental occlusion. Additionally, M. condorensis retains a post-diastema tooth morphology more reminiscent of the ancestral ornithischian condition (Becerra et al. 2014; Sereno 2012). Manidens condorensis is similarly simple in terms of enamel microstructure, composed primarily of parallel and divergent crystallites, with many of the latter organized into incipient columnar units (Becerra and Pol 2020). Incremental lines occur in conjunction with parallel crystallites, and numerous tubules are randomly distributed throughout the enamel thickness of the maxillary teeth, while the dentary teeth possess no tubules. Unlike all other known ornithischians, M. condorensis does not possess a basal unit layer (BUL); a thin (5–15 µm), distinct polygonal unit layer adjacent to the enamel dentine junction (EDJ), that is present even in the most basal reptilian taxa sampled to date (e.g., Captorhinus and Procolophon) (Sander 1999; Becerra and Pol 2020). It remains a mystery if a more complex enamel microstructure than that of the closely related M. condorensis accompanies the higher dental complexity of H. tucki.
In this study, we examine the microstructural features of H. tucki dental tissues in the maxillary teeth of an individual from the Early Jurassic upper Elliot Formation of South Africa (Fig. 1). Through histological and SEM analyses of H. tucki we describe the earliest known acquisition of wear-resistant and histologically distinct dentine in ornithischian dinosaurs and describe the enamel microstructure.
Institutional abbreviations.—BP, Evolutionary Studies Institute, University of the Witwatersrand, Braamfontein, Johannesburg, South Africa (formerly Bernard Price Institute).
Other abbreviations.—BUL, basal unit layer; DI, de-ionized water; EDJ, enamel dentine junction; OES, outer enamel surface; SEM, scanning electron microscope.
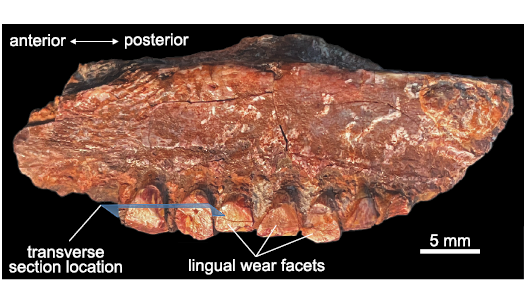
Fig. 1. Heterodontosaurid dinosaur Heterodontosaurus tucki Crompton and Charig, 1962 (BP/1/9007), from Lower Jurassic upper Elliot Massospondylus Assemblage Zone of South Africa. Left partial maxillary tooth row in lingual view; preserves partial crowns with wear facets. Blue plane indicates location of histological transverse sections from the middle of the tooth row.
Material and methods
Specimen acquisition.—BP/1/9007 is a partial sub-adult to adult Heterdontosaurus tucki maxilla from the Lower Jurassic upper Elliot Massospondylus Assemblage Zone of South Africa (Bordy et al. 2020; Viglietti et al. 2020). It preserves seven partial tooth crowns from the middle–posterior region of the maxillary tooth row. Attribution to H. tucki was based on the subrectangular cross-sectional morphology of the maxillary teeth, the presence of mesial and distal labial fossae separated by a primary ridge, and the lack of a cingulum (Sereno 2012).
Specimen preparation.—The unprocessed jaw was photographed using an Olympus SZX12 dissecting microscope (Olympus Corp., Tokyo, Japan) and imaging software Infinity Analyze (Lumenera, Ottawa, ON, CA). Prior to sectioning and preparation for scanning electron microscopy (SEM), the tooth crowns were molded using Silputty (Douglas & Sturgess, San Francisco, CA) to document the three-dimensional crown structure. The jaw was frontally sectioned and then embedded along with its resident teeth in epoxy resin pucks (Epoxyset, Allied High Tech Products, Inc., Rancho Dominguez, CA, USA). This section of the jaw was selected because the teeth it contained exhibited the best-preserved crowns of the tooth row. The embedded specimen was cut longitudinally and transversely using a slow-speed diamond saw (Buehler, Isomet 1000, Lake Bluff, IL, USA). The section was then polished on an automatic polisher (Struers Rotopol, Struers Inc., Cleveland, OH, USA) using increasingly finer grit silicon carbide paper grading 400–1200 grit with de-ionized (DI) water as a lubricant. The section was polished down to a thickness of 200 µm. Finer scale polishing was achieved using a microfiber polishing pad, 0.3 µm alumina powder, and DI water as a lubricant. The specimen was then cleansed of potential debris in an ultrasonic bath (Branson 200 Ultrasonic Cleaner, Emerson Electric, St. Louis, MO, USA) for one minute and dried with pressurized air. The section was mounted on a petrographic slide and then viewed and described using an Olympus BX60 compound microscope (Olympus Corp., Tokyo, Japan).
Etching was performed on the petrographic slides using 0.1M HCl followed by a rinse in DI water. The highly permineralized nature of the specimen necessitated lengthy etching times: 95 seconds for transverse sections and 90 seconds for longitudinal sections. Sections were then sputter-coated with 4 nm of iridium and fixed with copper tape to minimize charging during SEM analysis. The enamel and dentine were then analyzed with a scanning electron microscope (FEI Nova 400 Nano SEM, Hillsboro, OR, USA) at both 5kV and 10kV. Only transverse and longitudinal sections were analyzed as the thin and heavily permineralized nature of the enamel made tangential sectioning infeasible.
The enamel microstructure and dental histology terminology used in this study follow Avery (1991), Sander (1999), Hals (1983), Schmidt and Keil (1971), Hillson (1986). Note: LeBlanc and colleagues (2016, 2017) utilize alternative terminological characterizations for some dinosaurian tissues recognized here, or do not treat them as distinct tissues.
Results
Enamel description.—A notable asymmetry in enamel thickness is present in the worn maxillary teeth of Heterodontosaurus tucki. The enamel is thickest (~30 µm) on the mesial and distal edges of the teeth. In all teeth sampled, the enamel is extremely thin (~6 µm) and discontinuous on the labial edge, while it is completely removed by wear on the lingual sides, if originally present at all (Fig. 2A). The mesio-distal enamel begins thinning on the labial edges of the mesial and distal marginal ridges (Fig. 2A4). This thinning is likely a true reduction rather than a result of wear, as evidenced by the concurrent simplification in microstructure, future studies investigating the histology of unworn teeth could be performed to definitively confirm this interpretation. The labial edge is composed exclusively of simple parallel crystallite enamel (~6 µm) with intermittent incremental lines (Fig. 3A1). In contrast, the mesial and distal tooth margins preserve a more complex enamel schmelzmuster (the three-dimensional arrangement of enamel types; Sander 1999) comprised of a basal unit layer (BUL) (~6.9 µm, ~24% total thickness), followed by columnar unit enamel (~18.21 µm, 64% total thickness), and a thin outer layer of parallel crystallites (~3.37 µm, 12% total thickness) that are best visualized in longitudinal view (Fig. 3A3). Crystallite bundles composing the columnar divergence units increase in size towards the outer enamel surface (OES). Individual columnar units are separated by prominent planes of convergence that occasionally coincide with sinuous, radially emanating enamel tubules (Fig. 3A3). These tubules originate within the BUL and do not appear to continue past the columnar units. Interestingly, a marked difference was observed in the microstructural complexity between transverse and longitudinal views (Fig. 3A2, A3). While longitudinal views exhibit a well-defined, tripartite, BUL-columnar-parallel enamel schmelzmuster, transverse views present a schmelzmuster composed solely of parallel crystallite enamel. This discrepancy is likely a result of the three-dimensional orientation of the diverging crystallites (Fig. 3B). Crystallites diverge in apico-basal directions along an axis in the transverse plane; transverse sections therefore exhibit only the apical or basal half of the diverging crystallite pairs, and so appear parallel (Sander 1999). The tubules that coincide with zones of convergence are also oriented and undulate along primarily transverse planes. Therefore, tubules are visible in transverse view only when the tooth is sectioned directly along a convergence zone, resulting in their higher abundance in longitudinal view where multiple layers of convergence are captured in a single cut (Fig. 3B).
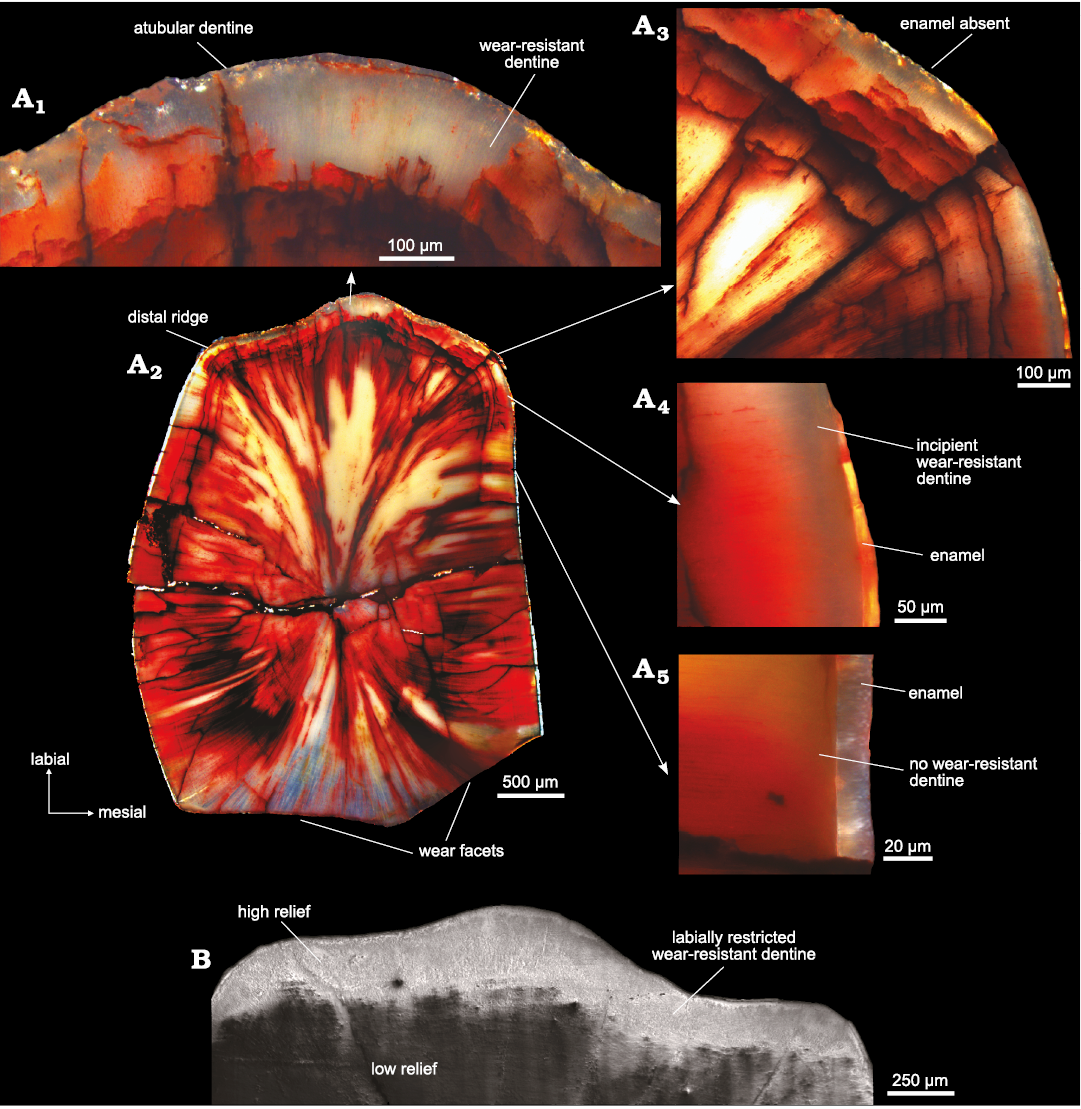
Fig. 2. Maxillary tooth histology of heterodontosaurid dinosaur Heterodontosaurus tucki Crompton and Charig, 1962 (BP/1/9007), from Lower Jurassic upper Elliot Massospondylus Assemblage Zone of South Africa. A. Histological sections: A1, primary ridge: thick band of translucent dentine lines the labial edge of the tooth, no enamel is present; A2, whole tooth: transversely sectioned at the base of the wear facet; A3, mesial ridge: histologically distinct dentine thickens and enamel is absent; A4, labial edge: on the mesial distal edges the enamel is extremely thin, resulting from wear and likely a natural thinning of the enamel, histologically distinct dentine is present, but thin; A5, lateral edge: the enamel is thickest with no distinct dentine present. B. Scanning electron micrograph of labial edge of tooth showing crest formation from differential wear from the last stage of polishing.
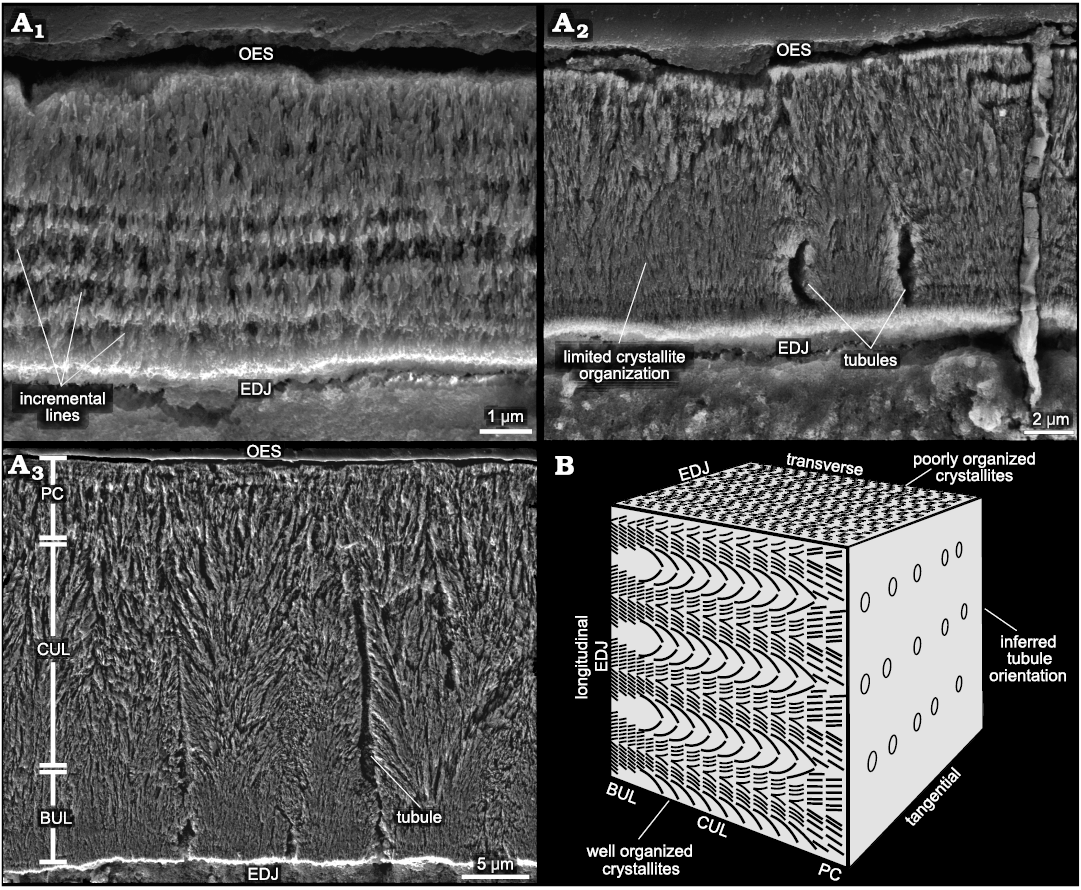
Fig. 3. Enamel microstructure of heterodontosaurid dinosaur Heterodontosaurus tucki Crompton and Charig, 1962 (BP/1/9007), from Lower Jurassic upper Elliot Massospondylus Assemblage Zone of South Africa. A. Scanning electron micrographs: A1, simplified, thin, labial edge enamel with incremental lines in transverse view; A2, thick, mesio-distal edge enamel exhibiting poorly organized crystallites and extremely infrequent enamel tubules in transverse view; A3, mesio-distal edge enamel exhibiting three enamel layers in longitudinal view. Long, sinuous, and continuous enamel tubules are frequently observed in this sectioning plane. B. Schematic enamel block depicting how enamel crystallite complexity might differ in different planes of sectioning. Abbreviations: BUL, basal unit layer; CUL, columnar unit layer; EDJ, enamel dentine junction; OES, outer enamel surface; PC, parallel crystallite.
Tooth histology.—In most high-wear, masticating dentitions, enamel is the primary crest-forming material due to its greater relative hardness and remarkable damage tolerance (Erickson et al. 2012; Weng et al. 2016; Wilmers and Bargmann 2020). The extremely thin and discontinuous enamel present on the labial crests of H. tucki maxillary teeth is therefore highly unexpected. However, associated with the onset of enamel thinning on the labial margins of the mesial and distal ridges is a gradually thickening band of translucent, presumably hypermineralized (see discussion), and histologically distinct dentine (Fig. 2A). This band lies immediately adjacent to the enamel-dentine junction, and at its inception on the mesial and distal edges of the tooth is ~50 µm thick (Fig. 2A4). It rapidly thickens along the curvature of the mesial and distal marginal ridges to ~100 µm and continues thickening to a maximum of ~180 µm at the primary ridge (Fig. 2A1–A4). In etched SEM samples, the semi-transparent, unstained dentine can be split into three histologically distinct regions along the labial edge: (i) an inner region (~70 µm thick) with radially oriented dentinal tubules (Fig. 4A1, A4), (ii) a middle region (~90 µm thick) with apico-basally oriented tubules displaying circular cross-sections (Fig. 4A1, A3), and (iii) an outer region (~20 µm thick) that is atubular and composed solely of intertubular dentine (Fig. 4A1, A2). In transmitted light, the outer exceptionally translucent dentine is likely caused by the passing of light parallel to the long axis of the tubules which would obscure and decrease the visual contrast of the tubules with the surrounding tissue, causing a larger portion of the dentine to appear optically “atubular” when only the outer ~20 µm is truly atubular (Figs. 2A1, A2, 4A1, A2). The shifting dentinal tubule orientation explains why the outer ~110 µm is translucent, however, it does not explain why the region with radially oriented tubules is similarly translucent. In the last stage of polishing, a compliant pad and alumina powder permit differential wear to occur (i.e., the preferential removal of material from softer regions of the tooth leaving the harder regions as zones of higher relief). In the SEM, we observed a raised region coinciding with the outer dentine (Fig. 2B). This suggests that in addition to being histologically distinct this region is also relatively harder than the inner orthodentine. While not a direct measure of this tissue’s mineralization state, the high relief in conjunction with the decrease in the density of the tubules (decreasing the porosity) in the outermost dentine suggests that this region may be more mineralized than the inner unmodified orthodentine. The absence of iron staining and permineralization in this region further supports decreased porosity and thus increased mineralization relative to the adjacent orthodentine.
Hadrosaurids and ceratopsids bear a similar type of modified dentine immediately adjacent to the EDJ (termed mantle dentine sensu Erickson et al. 2012 following Avery 1991). These tissues exhibit a hardness approaching that of enamel, allowing for its role as a secondary crest-forming tissue in hadrosaurs (Erickson et al. 2012). In H. tucki, this modified dentine forms the primary cutting crest on the functional occlusal surface, as the thin and microstructurally simple enamel in this region is readily effaced by wear. Similar to the tissues in the more derived ornithischians, the material properties observed here suggest that over the functional life of the tooth its histological components will self-wear to the observed functional morphology.
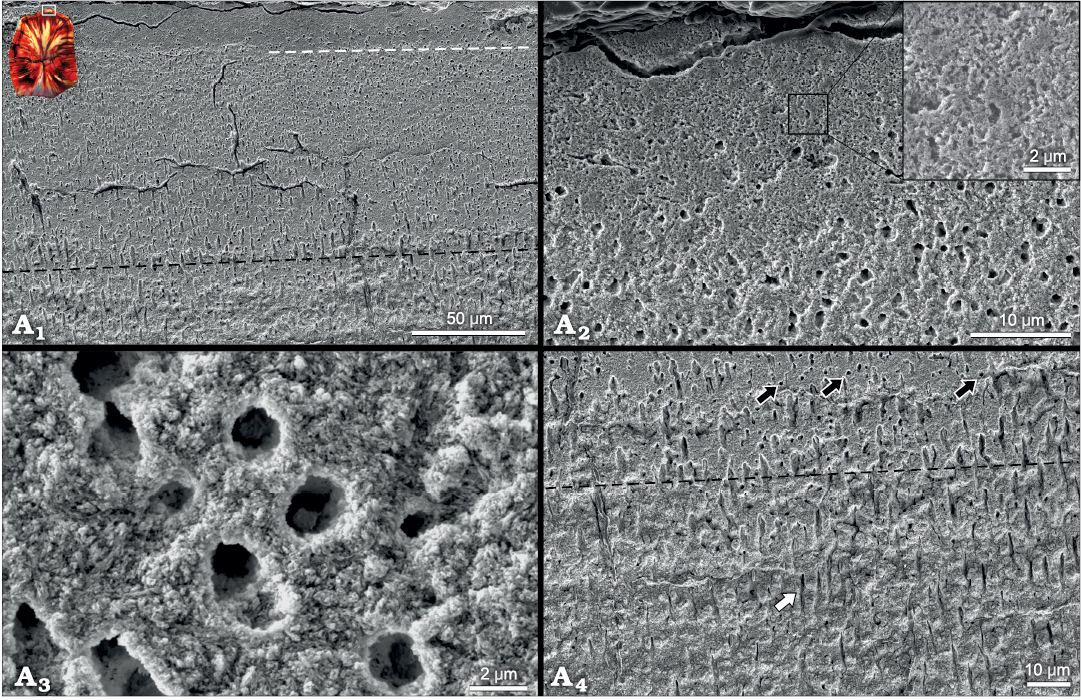
Fig. 4. Scanning electron micrographs depicting histologically distinct dentine on the outer labial edge of maxillary teeth of heterodontosaurid dinosaur Heterodontosaurus tucki Crompton and Charig, 1962 (BP/1/9007), from Lower Jurassic upper Elliot Massospondylus Assemblage Zone of South Africa. A1, shows the change in dentinal tubule orientation from radially emanating (below black dashed line) to longitudinally emanating (above black dashed line, circular cross-sections) to the atubular dentine present on the outermost edge (above white dashed line). Inset shows the position of the micrograph on the tooth cross section. A2, depicts the marked decrease in dentinal tubules in the outer ~20 µm of dentine. Inset shows that only intertubular dentine is present and the absence of occluded tubules which would be expected if this was derived from sclerotization of the dentine. A3, high magnification image of open dentinal tubules in the histologically distinct enamel (above black dashed line in A1). A4, close up showing the transition zone of tubule orientation. White arrow shows tubule cut along its long axis; black arrows show orthogonally sectioned tubules (circular). More circular tubules are present above the black dashed line than below indicating the zone of tubule orientation shift.
Discussion
Heterodontosaurus tucki represents one of the chronologically earliest heterodontosaurids yet exhibits one of the most derived dentitions for the entire family (Sereno 2012). H. tucki tooth macrostructure varies significantly from related heterodontosaurids, including Manidens condorensis, the sole other microstructurally analyzed heterodontosaurid (Becerra and Pol 2020). While the post-diastema teeth of most heterodontosaurids exhibit a high-angled, chisel-like, worn morphology throughout their entire ontogeny, the teeth of adult H. tucki wear to a flattened block-like morphology forming a near-continuous functional surface composed of adjacent teeth (Becerra et al. 2020; Sereno 2012). These macro-scale differences are of potential significance to the enamel microstructure in the two species, as microstructural complexity tends to be correlated with dental complexity as a whole (Hwang 2005, 2011).
Although the enamel microstructures of Heterodontosaurus tucki and its closely related taxon Manidens condorensis are broadly similar, both being composed primarily of divergent crystallite enamel with a thin parallel crystallite layer adjacent to the OES, the microstructures of the two taxa are distinguished by substantial differences in organization. Most notable is the presence of a well-defined BUL in H. tucki, a level of organization lacking in M. condorensis. The BUL is thought to represent the initial phase of mineralization, with each polygonal basal unit representing an ameloblast (Sander 1999). Heterdontosaurus tucki shares this trait with thyreophorans, marginocephalians, and ornithopods (Fig. 5) (Hwang 2005, 2011; Chen et al. 2018; Becerra and Pol 2020). The lack of this distinctive microstructural trait in M. condorensis was previously interpreted as implying its absence in the ancestral ornithischian form. Its presence in H. tucki adds a new degree of ambiguity to the ancestral ornithischian condition and the evolutionary history of the acquisition and loss of the BUL.
Heterodontosaurus tucki enamel also differs from Manidens condorensis in the organization of the middle, divergent crystallite layer. While the incipient divergent columnar units of M. condorensis are poorly defined and frequently discontinuous, the divergent crystallites of H. tucki enamel form well-defined columnar units that persist, albeit sinuously, from the EDJ to near the OES, where they grade into parallel crystallite enamel. The convergence zones that define the edges of these columnar units frequently coincide with tubules in H. tucki. Similar tubules are present within the maxillary teeth of M. condorensis, although they appear randomly arranged and do not occur within convergence zones, unlike those in H. tucki (Becerra and Pol 2020). The dentary teeth of M. condorensis lack enamel tubules; the presence or absence of tubules within the enamel of H. tucki dentary teeth could not be determined in this study due to the lack of material available for sectioning.
Despite possessing characteristics typically associated with derived enamel schmelzmusters (heterodonty, tooth occlusion, episodic tooth replacement) H. tucki exhibits an unremarkable enamel microstructure similar to many other ornithischian lineages (Fig. 5). Virtually all major ornithischian radiations display some combination of a BUL, divergent crystallite columnar unit layer, and parallel crystallite enamel types. While major ornithischian lineages typically exhibit characteristic schmelzmusters, the enamel types composing these schmelzmusters are largely shared between groups (Fig. 5). Ceratopsians and ornithopods mark an important exception to this observation, possessing derived tubule-riddled and wavy enamel fabrics, respectively (Sander 1999; Hwang 2005). Despite possessing a degree of macro-scale dental complexity comparable to ceratopsians and derived ornithopods, H. tucki lacks derived enamel fabrics.
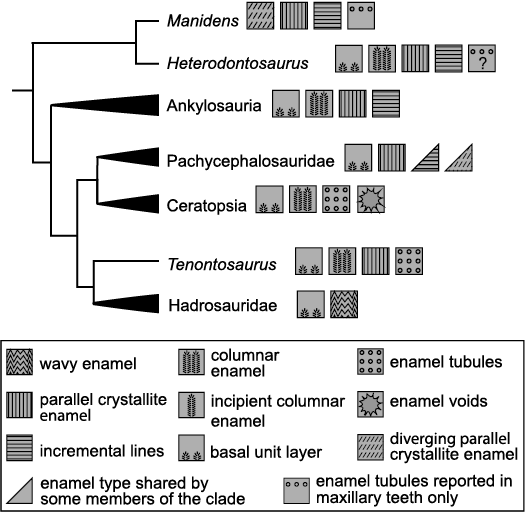
Fig. 5. Simplified ornithischian phylogeny depicting characteristic schmelzmusters and enamel types for studied clades. Note: Presence of enamel tubules in Heterodontosaurus tucki dentary teeth remains unknown. Modified from Hwang (2005).
Like ceratopsians and ornithopods, H. tucki possesses an exceptionally wear-resistant dentine layer, a secondarily derived histological tissue known, amongst ornithischians, only from these three lineages (Erickson et al. 2012, 2015). This layer has previously been observed in computed-tomographic (CT) scans of H. tucki maxillary material, where it was interpreted as a thickened edge of enamel on the labial side of the tooth due to its higher comparative density to the adjacent unmodified dentine (Sereno 2012). Increased density under CT analysis is considered one of the primary means of identifying hypermineralized dentine, its higher mineral to collagen ratio lends it a high-density signature that has allowed for its identification in the functional crests of fossil xenarthran teeth that are entirely composed of dentine (Kalthoff 2020). Through histological analysis, we here clarify its identity as a distinct band of dentine of a particularly high density that is presumably related to its mineralization state. This high density signature relative to unmodified dentine likely led to its misidentification as enamel, leading to the proposal of asymmetric enamel as an autapomorphy defining H. tucki (Sereno 2012). While the condition of the dentary teeth remains unknown, we posit that this unique trait is an asymmetric mineralization of the dentine; with the labial side of the maxillary teeth bearing a thick, histologically distinct, wear-resistant band of dentine. Further evidence that this tissue may be hypermineralized is demonstrated in how diagenetic processes have stained the underlying orthodentine with ferric red hues (Fig. 2A2), while the outer region appears less affected, retaining a clear translucency in transmitted light (Fig. 2A1). The ferric red appears within the histologically distinct region only where cracks have penetrated it (Fig. 2A1), such cross-cutting attests to the apparently native origin of the translucent band. This tissue likely survived fossilization due to its presumably hypermineralized nature; an increase in the density of hydroxyapatite within this tissue would decrease the porosity, thereby decreasing interaction with pore fluids and minimizing subsequent permineralization.
The wear-resistant dentine leading to crest-formation in H. tucki is likely related both to the mineralization state and the structural shift in tubule direction within this tissue. While the higher mineral content would presumably lend the tissue elevated mechanical hardness and increased wear resistance, the shifting tubule direction changes the surface roughness of the exposed dentine and therefore changes how that dentine interacts with abrasive particles traversing the occlusal surface (Fig. 4A1, A4). As the inner orthodentine wears down and interacts with abrasive particles, the tubule tracts present a source of surface roughness and thus elevated wear throughout the functional lifespan of the tooth. As an abrasive particle slides over this tissue, it will dip down into the tubule tract and collide with the opposing edge of the tubule creating extremely high localized pressures that will drastically increase the removal of material (Fig. 4A4). In the dentine with apico-basally oriented tubules a similar interaction would occur, however the reduced cross-sectional area of the tubules would diminish the removal of material in comparison to the inner orthodentine leaving the histologically distinct dentine elevated (Fig. 4A1). In the outer-most atubular dentine no such interaction would occur; therefore, this tissue would be expected to have the highest wear-resistance and the highest relief (Fig. 4A2). In Triceratops horridus similar histological control of wear has been demonstrated in the dentine; the ridge-forming orthodentine and the fuller-forming vasodentine possessed hardness values that were statistically indistinguishable, yet the extremely porous vasodentine had a wear rate ~1.6 times that of orthodentine (Erickson et al. 2015).
Together the hypermineralization and the shift in tubule direction likely promoted the outer dentine layer to form a crest relative to the more internal orthodentine during polishing (Fig. 2B). This effect was observed in multiple teeth in multiple sections and occurred despite the heavy permineralization of the topographically recessed orthodentine. Permineralization is generally expected to increase the hardness of dental tissues (Erickson et al. 2012; Kundanati et al. 2019). The ability of the unaltered, translucent, and wear-resistant dentine to form substantial crests above the diagenetically altered orthodentine with its presumably increased hardness attests to the significant in vivo crest-forming properties of this histologically distinct dentine. In vivo the non-altered orthodentine would have likely been even less wear-resistant and formed even deeper basins than observed here. Similar to xenarthrans and other mammalian herbivores, the presence of a more resistant dental tissue on the occlusal surface of H. tucki teeth would have resulted in the development of triturating crests of modified dentine, enhancing the complexity of the occlusal surface and aiding in mastication (Erickson et al. 2012; Kalthoff 2020).
Modified dentinal tissues have been reported in a handful of other dinosaurian taxa. In theropods, modified dentine is found within the tooth reinforcing the spaces between serrate denticles (Brink et al. 2015). In ornithischians, hard wear-resistant dentine plays a fundamentally different role, directly participating in oral processing at the occlusal surface. Within hadrosaurids, wear-resistant dentine is found buttressing the enamel, as well as forming isolated, secondary shearing crests across the chewing pavements (Erickson et al. 2012). In ceratopsians like Leptoceratops gracilis and Protoceratops andrewsi wear-resistant dentine again buttresses the enamel, and in some taxa like T. horridus, even directly composes the shearing crest on the non-enameled mesial and distal edges (Erickson et al. 2015; Varriale 2016). Heterodontosaurus tucki takes this trend to the extreme, in that rather than limiting the wear-resistant dentine to secondary internal crests or using it to reinforce the enamel edge, the entire primary, labial, maxillary, shearing crest solely consists of wear-resistant dentine. Further work should be done to investigate how and by what mechanisms these functionally, and in H. tucki histologically distinct, dentines have elevated wear resistance.
This level of histological complexity is unexpected for an Early Jurassic ornithischian, as the derived hadrosaurs and ceratopsians that would eventually incorporate secondary dental tissues into their occlusal surface did not arise until the middle Cretaceous (Butler et al. 2009). The evolutionary anachronism of modified dentine in a mid-Mesozoic, early diverging ornithischian could imply the tissue is in fact more prevalent than previously thought, and further histological examination of other taxa for elaborated dentinal tissues is warranted. However, when considered in the light of the overall highly derived dentition of Heterodontosaurus, the presence of this dental tissue likely represents a derivation exclusive to this early diverging taxon. The high degree of specialization in the dentition of H. tucki should call for caution in its use as a model for the plesiomorphic ornithischian condition, at least until similar information for other basal lineage ornithischians or earlier heterodontosaurids such as Echinodon, Fruitadens, or Tianyulong are available. This would permit polarization of the evolutionary change of enamel in Heterodontosauridae and provide a better understanding of early ornithischian dental characteristics.
Conclusions
Microstructural and histological analysis of Heterodontosarus tucki maxillary teeth indicates that tooth crown complexity does not always beget enamel microstructural complexity. Despite its highly derived dental morphology, H. tucki possesses a relatively typical ornithischian enamel schmelzmuster on the mesial and distal edges of the maxillary teeth. The maxillary labial edge, representing the first point of occlusal contact, exhibits exceedingly thin enamel and a highly simplified microstructure, even less remarkable than that of the mesio-distal edges. This reduced and simplified enamel presumably renders the enamel largely non-functional as a shearing crest, a role that has likely been assumed by the crest-forming layer of modified wear-resistant dentine underlying the enamel of the labial edge. This histological structure represents the chronologically and phylogenetically earliest known acquisition of wear-resistant modified dentine in ornithischians and the earliest occurrence of its presence on the occlusal surface in dinosaurs. The discovery of this structural tissue emphasizes the unique and derived nature of the complex heterodontosaurid dentition, suggesting that despite its early-diverging phylogenetic position, caution must be exercised in treating dental characteristics in members of the heterodontosaurid clade as representative of the basal ornithischian condition.
Acknowledgements
The reviews and comments of Marcos G. Becerra (Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Museo Paleontológico Egidio Feruglio, Trelew, Argentina), Aaron LeBlanc (Centre for Oral, Clinical & Translational Sciences, King’s College London, London, UK), and an anonymous reviewer greatly improved this manuscript. This research was funded by National Science Foundation Grant CMMI BMMB EAGER 1937050 (G.M.E.) and through the generous support of the Mario and Vanessa Fernandez Graduate Research Fund. J.N.C. was supported by GENUS: the DSI-NRF Centre of Excellence in Palaeosciences and NRF African Origins Platform grants #98800 and 136516.
References
Avery, J.K. 1991. Dentine. In: S.N. Bhaskar (ed.), Orban’s Oral Histology and Embryology. 11th ed., 106–138. Mosby-Year Book, St. Louis, Missouri.
Becerra, M.G. and Pol, D. 2020. The enamel microstructure of Manidens condorensis: New hypotheses on the ancestral state and evolution of enamel in Ornithischia. Acta Palaeontologica Polonica 65: 59–70. Crossref
Becerra, M.G., Pol, D., Marsicano, C.A., and Rauhut, O.W.M. 2014. The dentition of Manidens condorensis (Ornithischia; Heterodontosauridae) from the Jurassic Cañadón Asfalto Formation of Patagonia: morphology, heterodonty and the use of statistical methods for identifying isolated teeth. Historical Biology 26: 480–492. Crossref
Becerra, M.G., Pol, D., Whitlock, J., and Porro, L. 2020. Tooth replacement in Manidens condorensis: baseline study to address the replacement pattern in dentitions of early ornithischians. Wiley Online Library 7 (2): 1167–1193. Crossref
Bordy, E.M., Abrahams, M., Sharman, G.R., Viglietti, P.A., Benson, R.B.J., McPhee, B.W., Barrett, P.M., Sciscio, L., Condon, D., Mundil, R., Rademan, Z., Jinnah, Z., Clark, J.M., Suarez, C.A., Chapelle, K.E.J., and Choiniere, J.N. 2020. A chronostratigraphic framework for the upper Stormberg Group: Implications for the Triassic–Jurassic boundary in southern Africa. Earth-Science Reviews 203: 103120. Crossref
Brink, K.S., Reisz, R.R., Leblanc, A.R.H., Chang, R.S., Lee, Y.C., Chiang, C.C., Huang, T., and Evans, D.C. 2015. Developmental and evolutionary novelty in the serrated teeth of theropod dinosaurs. Scientific Reports 5: 12338. Crossref
Butler, R.J., Porro, L.B., and Norman, D.B. 2008. A juvenile skill of the primitive ornithischian dinosaur Heterodontosaurus tucki from the “Stormberg” of Southern Africa. Journal of Vertebrate Paleontology 28: 702–711. Crossref
Butler, R.J., Barrett, P.M., Kenrick, P., and Penn, M.G. 2009. Diversity patterns amongst herbivorous dinosaurs and plants during the Cretaceous: implications for hypotheses of dinosaur/angiosperm co-evolution. Journal of Evolutionary Biology 22: 446–459. Crossref
Chen, J., Leblanc, A.R.H., Jin, L., Huang, T., and Reisz, R.R. 2018. Tooth development, histology, and enamel microstructure in Changchunsaurus parvus: Implications for dental evolution in ornithopod dinosaurs. PLoS ONE 13 (11): e0205206. Crossref
Crompton, A.W. and Charig, A.J. 1962. A new ornithiscian from the upper Triassic of South Africa. Nature 196: 1074–1077. Crossref
Edmund, A.G. 1960. Tooth replacement phenomena in lower vertebrates. Contributions to the Life Sciences Division of the Royal Ontario Museum 52: 1–190. Crossref
Erickson, G., Krick, B., Hamilton, M., Bourne, G., Norell, M., Lilleodden, E., and Sawyer, W. 2012. Complex dental structure and wear biomechanics in hadrosaurid dinosaurs. Science 338: 98–101. Crossref
Erickson, G.M., Sidebottom, M.A., Kay, D.I., Turner, K.T., Ip, N., Norell, M.A., Sawyer, W.G., and Krick, B.A. 2015. Wear biomechanics in the slicing dentition of the giant horned dinosaur Triceratops. Science Advances 1 (5): e1500055. Crossref
Hals, E. 1983. Polarized light study of giant tubules in human and red deer coronal dentin. European Journal of Oral Sciences 91: 105–111. Crossref
Hillson, S. 1986. Teeth. xix + 376 pp. Cambridge University Press, Cambridge.
Hwang, S.H. 2005. Phylogenetic patterns of enamel microstructure in dinosaur teeth. Journal of Morphology 266: 208–240. Crossref
Hwang, S.H. 2011. The evolution of dinosaur tooth enamel microstructure. Biological Reviews 86: 183–216. Crossref
Kalthoff, D.C. 2020. Short review of dental microstructure and dental microwear in xenarthran teeth. In: W. von Koenigswald and T. Martin (eds.), Mammalian Teeth—Form and Function, 231–241. Verlag Dr. Friedrich Pfeil, München.
Kundanati, L., D’Incau, M., Bernardi, M., Scardi, P., and Pugno, N.M. 2019. A comparative study of the mechanical properties of a dinosaur and crocodile fossil teeth. Journal of the Mechanical Behavior of Biomedical Materials 97: 365–374. Crossref
Lambe, L.M. 1902. New genera and species from the Belly River Series (mid-Cretaceous). Contributions to Canadian Palaeontology 3: 69–70.
LeBlanc, A.R.H., Reisz, R.R., Evans, D.C., and Bailleul, A.M. 2016. Ontogeny reveals function and evolution of the hadrosaurid dinosaur dental battery. BMC Evolutionary Biology 16 (1): 1–13. Crossref
LeBlanc, A.R.H., Brink, K.S., Cullen, T.M., and Reisz, R.R. 2017. Evolutionary implications of tooth attachment versus tooth implantation: A case study using dinosaur, crocodilian, and mammal teeth. Journal of Vertebrate Paleontology 37 (5): e1354006. Crossref
Norman, D., Crompton, A., and Butler, R. 2011. The Lower Jurassic ornithischian dinosaur Heterodontosaurus tucki Crompton & Charig, 1962: cranial anatomy, functional morphology, taxonomy, and relationships. Zoological Journal of The Linnaean Society 163: 182–276. Crossref
Sander, P.M. 1999. The microstructure of reptilian tooth enamel: Terminology, function, and phylogeny. Münchner geowissenschaftliche Abhandlungen, Reihe A, Geologie und Palaontologie 38: 1–103.
Schmidt, W.J. and Keil, A. 1971. Polarizing Microscopy of Dental Tissues. 584 pp. Pergamon Press, Oxford.
Sereno, P. 2012. Taxonomy, morphology, masticatory function and phylogeny of heterodontosaurid dinosaurs. ZooKeys 226: 1–225. Crossref
Varriale, F.J. 2016. Dental microwear reveals mammal-like chewing in the neoceratopsian dinosaur Leptoceratops gracilis. PeerJ 4: e2132. Crossref
Viglietti, P.A., McPhee, B.W., Bordy, E.M., Sciscio, L., Barrett, P.M., Benson, R.B.J., Wills, S., Chapelle, K.E.J., Dollman, K.N., Mdekazi, C., and Choiniere, J.N. 2020. Biostratigraphy of the Massospondylus Assemblage Zone (Stormberg Group, Karoo Supergroup), South Africa. South African Journal of Geology 123: 249–262. Crossref
Weng, Z.Y., Liu, Z.Q., Ritchie, R.O., Jiao, D., Li, D.S., Wu, H.L., Deng, L.H., and Zhang, Z.F. 2016. Giant panda׳s tooth enamel: Structure, mechanical behavior and toughening mechanisms under indentation. Journal of the Mechanical Behavior of Biomedical Materials 64: 125–138. Crossref
Wilmers, J. and Bargmann, S. 2020. Nature’s design solutions in dental enamel: Uniting high strength and extreme damage resistance. Acta Biomaterialia 107: 1–24. Crossref
Acta Palaeontol. Pol. 68 (4): 603–612, 2023
https://doi.org/10.4202/app.01060.2023