

A new diminutive fossil ziphiid from the deep-sea floor off northern Chile and some remarks on the body size evolution and palaeobiogeography of the beaked whales
GIOVANNI BIANUCCI, WALTER SIELFELD, NICOLE A. OLGUIN, and GUILLERMO GUZMÁN
Bianucci, G., Sielfeld, W., Olguin, N.A., and Guzmán, G. 2023. A new diminutive fossil ziphiid from the deep-sea floor off northern Chile and some remarks on the body size evolution and palaeobiogeography of the beaked whales. Acta Palaeontologica Polonica 68 (3): 477–491.
The evolutionary history of the beaked whales (Ziphiidae), odontocetes nowadays adapted to deep diving, is well known thanks to a significant fossil record mainly from the deep ocean floors. A partial cranium of a ziphiid recovered from Plio-Pleistocene deep sea deposits (about 1000 m) off the port of Pisagua, northern Chile, during fishing activity is here described and referred to the new species Ihlengesi changoensis. Ihlengesi changoensis differs from the type species Ihlengesi saldanhae, from the sea floor off South Africa, by having a more elongated premaxillary sac fossa and consequently a more anteriorly located premaxillary foramen; dorsal margin of each premaxillary crest sloping markedly ventrolaterally and generating an acute dorsal profile of the vertex in anterior view; less anterolateral extension of the right nasal forming part of the premaxillary crest; lateral margins of the nasals not anteriorly diverging but weakly convex; nasofrontal suture anteriorly pointed. The phylogeny supports a sister-taxon relationship between I. changoensis and I. saldanhae, both members of the crown ziphiids Hyperoodontinae. Ihlengesi changoensis shares with I. saldanhae and other fossil ziphiids a small body size (estimated length 3.5 m) supporting the hypothesis that in the past small beaked whales (<4 m) were more common than today. Such recent shift of the ziphiids towards a larger size could be the result of a progressive change of diet from fish to cephalopods, to the competition with the delphinids, and the predatory impact of the white shark Carcharodon carcharias and/or of the killer whale Orcinus orca. This new Chilean ziphiid further supports the hypothesis that crown beaked whales originated and firstly dispersed in the oceanic waters of the Southern Hemisphere. Their radiation and geographical distribution could have been driven by the southern oceanic circulation and related localized concentration of trophic resources in high productivity upwelling areas.
Key words: Mammalia, Cetacea, Ziphiidae, Cenozoic, Southeastern Pacific, South America.
Giovanni Bianucci [giovanni.bianucci@unipi.it; ORCID: https://orcid.org/0000-0001-7105-0863 ], Dipartimento di Scienze della Terra, Università di Pisa, via Santa Maria 53, 56126, Pisa, Italy.
Walter Sielfeld [walter.sielfeld.kowald@gmail.com; ORCID: https://orcid.org/0000-0002-7055-5015 ], Nicole A. Olguin [nolguin@unap.cl; ORCID: https://orcid.org/0000-0002-5081-9335 ], and Guillermo Guzmán [gguzman@unap.cl; ORCID: https://orcid.org/0000-0001-8000-3815 ], Facultad de Recursos Naturales Renovables, Universidad Arturo Prat, 2120, Iquique, Chile.
Received 6 April 2023, accepted 26 May 2023, available online 28 August 2023.
Copyright © 2023 G. Bianucci et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The beaked whales (family Ziphiidae) are the most enigmatic among the cetaceans, due to the deep-sea habits and elusive behaviour of their extant species (Ellis and Mead 2017; MacLeod 2018). Their skulls are characterized by an elevated vertex, anteriorly well developed pterygoid hamuli and one or two pairs of apical or subapical mandibular teeth being more developed in the males (tusks) and generally combined with a rostral pachyostose (Moore 1968; Heyning 1989). Beaked whales also display an evolutionary tendency to reduce the number of functional teeth as an adaptation to suction feeding in deep waters (Bianucci et al. 2016b).
These medium to large-sized odontocetes comprise 24 extant species distributed in 6 genera (Berardius, Hyperoodon, Indopacetus, Mesoplodon, Tasmacetus, and Ziphius) (Mead 1989; Dalebout et al. 2002; Yamada et al. 2019) and, after Delphinidae, they represent the second richest family of cetaceans in species number.
The evolutionary history of beaked whales was almost unknown until a few decades ago, but recently many fossils have been discovered and many new genera and species have been described highlighting that the extant high diversity of this family is the consequence of a large radiation having its maximum intensity towards the end of the Miocene (Bianucci et al. 2016b).
Many fossil remains of ziphiids come from Mio-Pliocene marine deposits exposed in Europe and North America, including both old (e.g., Bianucci 1997; Lambert 2005; Lambert and Louwye 2006, 2016) and recent acquisitions (Bianucci et al. 1992, 1994, 2016a, 2019; Bianucci and Post 2005; Fuller and Godfrey 2007; Post et al. 2008; Ramassamy 2016). Outside of these two continents, the remains found in Japan (Tanaka et al. 2019; Kawatani and Kohno 2021), Argentina (Buono and Cozzuol 2013) and Peru (Bianucci et al. 2016b) are the more significant. In particular, the fossils of beaked whales from the Upper Miocene deposits of the Pisco and Sacaco basins in Peru represent the best preserved remains of this family due their completeness and quality of preservation. Many of these fossils belong to the stem beaked whales Messapicetus gregarious and Ninoziphius platyrostris, two species featured by elongated rostrum and mandibles, both still bearing a complete set of functional teeth (Muizon 1984; Bianucci et al. 2010; Lambert et al. 2010, 2015, 2013; Ramassamy et al. 2018). The discovery of the presumed last meal associated with a M. gregarius skeleton allowed the authors to hypothesize a diet based on epipelagic fish of these basal beaked whales (Lambert et al. 2015). By contrast Nazcacetus urbinai, also from Peru, already displays an incipient dental reduction and was placed within the crown ziphiids (Lambert et al. 2009; Bianucci et al. 2016b)
Additionally, abundant cranial material has been recovered from the seafloor, mainly from phosphorites beds, of most oceans off Iberian Peninsula (Bianucci et al. 2013; Miján et al. 2017), Faroe Islands (Post and Jensen 2013), Florida and California (Whitmore et al. 1986), Japan (Horikawa et al. 1987; Tazaki et al. 1987; Miyazaki and Hasegawa 1992), Peru (Eastman 1906), Brazil (Ichishima et al. 2017), South Africa (Bianucci et al. 2007, 2008), New Zealand (Fordyce and Cullen 1979), Crozed and Kergelen islands (Robineau 1973; Lambert et al. 2018), and the Antarctic (Gol’din and Vishnyakova 2013). Such material indicates that the beaked whales in the past had an incredible disparity including bizarre morphologies, most having a restricted geographical distribution. Furthermore, many species and genera are represented by a single specimen suggesting that the diversity of the ziphiids in the past is still far from being fully understood.
Despite the existence of sedimentary basins off northern Chile (Fischer and Raitt 1962; Coulbourn and Moberly 1977; Coulbourn 1981) and extensive marine Miocene–Pleistocene formations within Chile itself, some of these bearing numerous cetacean fossils (Walsh 2001; Gutstein et al. 2008, 2015; Pyenson et al. 2014), beaked whale remains are documented only on the basis of three fragmentary poorly diagnostic remains (Sielfeld 1995; Walsh 2001; Gutstein et al. 2008, 2015).
The aim of this study is to describe a fossil skull of ziphiid collected off the Chilean coast at a depth of approximately 1000 m, while fishing for Patagonian toothfish Dissostichus eleginoides. The body size evolution and palaeobiogeography of beaked whales are also discussed.
Nomenclatural acts.—This published work and the nomenclatural acts it contains have been registered in ZooBank: lsid:zoobank.org:pub:73F67704-27DA-4993-88D5-E5E1E9A2EE13.
Institutional abbreviations.—MUAP, Zoological Collection, Universidad Arturo Prat, Iquique, Chile; SAM, Iziko South African Museum, Cape Town, South Africa.
Geological setting
As for other similar finds collected on the deep seafloor, providing a high-resolution stratigraphic overview of the fossil cranium of the ziphiid here examined is not easy, since it is difficult to carry out direct observations of the strata outcropping in the discovery area. Furthermore, the cranium is not associated with any sediment whose examination could have possibly allowed the identification of micro- or macrofossils useful for biostratigraphic analyses. However, the data provided by several articles on the tectonics and stratigraphy of the find area provide us with indirect but valuable information on the age of this fossil ziphiid. Indeed, the deep seafloor where the fossil cranium has been collected is located on the oceanward forearc of the northern Chile continental margin where the Nazca Plate subducts beneath the South American Plate (Armijo et al. 2015). This oceanward forearc deepens from 400 to 1500 m and holds a string of interconnected basins, named, from north to south, Arequipa, Arica, and Iquique basins (Coulbourn and Moberly 1977; Coulbourn 1981). Considering the geographical position and the depth of the sea where it was found, the fossil skull comes from the Iquique Basin or the southern part of the Arica basin.
The analysis of seismic section profiles and a few collected samples indicate that these basins are filled by Cenozoic clays, muds, turbidites, pelagic, and hemipelagic marine sediments (Fischer and Raitt 1962; Coulbourn and Moberly 1977; Coulbourn 1981; Moberly et al. 1982; Henriquez et al. 1981; Von Huene et al. 1999; Geersen et al. 2015, 2018a, b; Ranero et al. 2006; Sallarès and Ranero 2005). More recently, Gonzáles et al. (2023), based on a seismic section profile off Pisagua, distinguished three stratigraphical units covering the seafloor, named, from bottom to top, SU1, SU2 and SU3, being SU3 the unit filling the Iquique Basin. According to the same authors SU3 has likely been deposited after most of the deformation took place in this region. Since Gonzáles et al. (2003) referred the last extensional deformations of the northern Coastal Cordillera of Chile to the Pliocene-Late Pleistocene, a Plio-Pleistocene age for the SU3 sedimentary unit containing the ziphiid cranium examined is here proposed.
Material and methods
The fossil here examined is a partial cranium recovered from the ocean floor, at a depth of approximately 1000 m, while fishing for Patagonian toothfish Dissostichus eleginoides in May 2022. The collection area is located off the port of Pisagua, Northern Chile (Fig. 1). The fossil was collected by Dany Manzo in the fishing boat ”La Huayca” and is deposited in the Zoological Collection of the Universidad Arturo Prat, Iquique, Chile. The fossil is strongly mineralized, as generally the ziphiid skull found in deep sea deposits, but it does not show phosphorite or manganese concretions adhering to the surface of bones.
The anatomical terms follow Mead and Fordyce (2009). The measurements mainly follow Ross (1984) and Lambert (2005).
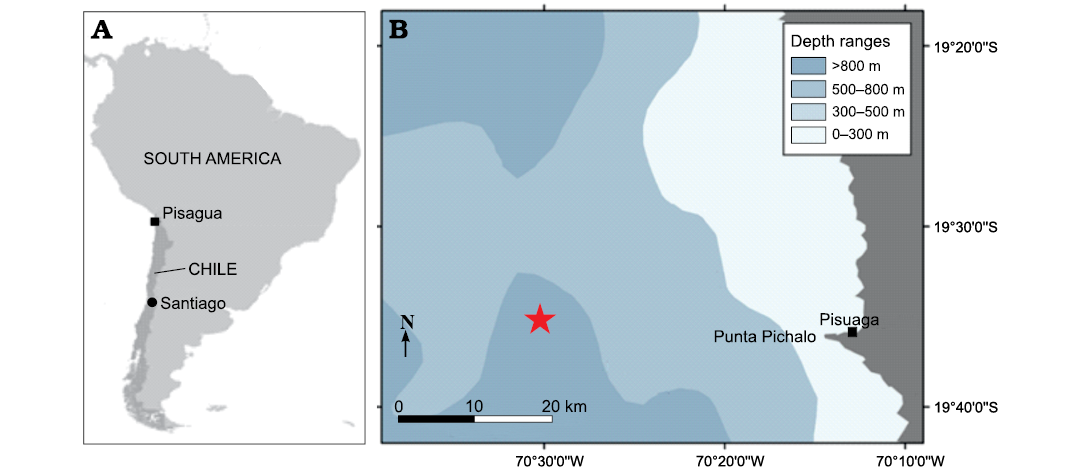
Fig. 1. A. Schematic map providing the position of Pisagua in South America. B. Schematic map of Northern Chile coast around Pisagua and sea floor bathymetry showing approximative discovery locality of the holotype MUAP(MM)-068 cranium of the beaked whale Ihlengesi changoensis sp. nov. at a depth of 1000 m (star).
Phylogenetic analysis.—The phylogenetic relationships of Ihlengesi changoensis with the other Ziphiidae are investigated here using the same methods and the same matrix as in Bianucci et al. (2016b), just adding, beside the new diagnosed I. changoensis, the two known species of Khoikhoicetus (K. agulhasis and K. kergueleni), both sharing several cranial similarities with Ihlengesi (see Appendix 1). The modified matrix includes 37 taxa (31 belonging to the family Ziphiidae), coded for 51 morphological characters (28 binary, 17 multistate and ordered, and six multistate and unordered) (see SOM, Supplementary Online Material available at http://app.pan.pl/SOM/app68-Bianucci_etal_SOM.pdf). The analysis was executed with the software PAUP (v. 4.0, Swofford 2002), using the branch-and-bound algorithm. The characters were analyzed under both equal and implied weight. In the phylogenetic analysis here proposed we have not modified the state of the characters of the genera Beneziphius and Berardius to take into account the species Beneziphius cetariensis, Beradius kobayashii, and Berardius minimus recently described respectively by Miján et al. (2017), Kawatani and Kohno (2021), and Yamada et al. (2019). Indeed, these taxa are phylogenetically distant from I. changoensis and reviewing the entire phylogeny of the beaked whales is outside the aim of this work.
Body size.—The body size of the new ziphiid here described and of the other fossil beaked whale examined in the discussion paragraph are estimated using the following equation proposed by Bianucci et al. (2008: som: fig. b; see also Lambert et al. 2013): y = 9.464x + 1137, where y is the body length and x the width of the cranium (postorbital width), both in millimetres.
Systematic palaeontology
Cetacea Brisson, 1762
Odontoceti Flower, 1867
Ziphiidae Gray, 1850
Hyperoodontinae Gray, 1866
(sensu Bianucci et al. 2007)
Genus Ihlengesi Bianucci, Lambert, and Post, 2007
Type species: Ihlengesi saldanhae Bianucci, Lambert, and Post, 2007, by original designation; from the sea floor off Saldanha Bay, South Africa; age unknown.
Species included: Type species and Ihlengesi changoensis sp. nov.
Emended diagnosis.—Ihlengesi differs from all other Hyperoodontinae for the following unique combination of characters: narrow rostrum in dorsal view; presence of a distinct maxillary tubercle and a wide and shallow prominental notch anteromedial to the antorbital notch; dorsal infraorbital foramen close to the prominental notch at the rostrum base; premaxillary foramen distinctly posterior to the antorbital notch; roughly flat dorsal surface of the preorbital area without distinct maxillary crest; moderate elevation of the vertex (less than in the other hyperoodontines but Khoikhoicetus); ascending process of the premaxilla in lateral view concave with posterodorsal portion partly overhanging the bony nares; transverse distance between the lateral margins of the premaxillary crests less than in the other hyperoodontines; wide space between the premaxillary crests (greater than in Mesoplodon and Hyperoodon); inclusion of the nasal into the premaxillary crest with the at least the left nasal covering about half the length of the median margin of the crest; deep anteromedian excavation of the nasals.
Ihlengesi changoensis sp. nov.
Figs. 2–5, Table 1.
Zoobank LSID: urn:lsid:zoobank.org:act:503E74DA-918C-4125-B419-C4A084E9F317
Etymology: From “Changos”, the indigenous people and fishermen, who originally inhabited the coast of Northern Chile.
Holotype: MUAP(MM)-068, a partial cranium including the complete rostrum, the medial facial area (premaxillary sac fossae and vertex) and part of both supraorbital regions.
Type locality: Off the port of Pisagua (19°35’50”S; 70°12’48’’W), Northern Chile, at a depth of 1000 m (Fig. 1), collected by Dany Manzo in May 2022.
Type horizon: Iquique Basin, Plio-Pleistocene (Gonzáles et al. 2023).
Diagnosis.—Ihlengesi changoensis sp. nov. differs from Ihlengesi saldanhae in the following characters: more elongated premaxillary sac fossa and consequently premaxillary foramen more anteriorly located (ratio between the longitudinal distance between the right premaxillary foramen and the rostrum base and the width of rostrum base equals 0.10 contra 0.32 in I. saldanhae); dorsal margin of each premaxillary crest sloping markedly ventrolaterally and generating an acute dorsal profile of the vertex in anterior view (rounded profile in I. saldanhae); less anterolateral extension of the right nasal forming part of the premaxillary crest; lateral margins of the nasals not anteriorly diverging but weakly convex; nasofrontal suture not convex posteriorly but anteriorly pointed.
Description.—Judging from the size of the cranium (Table 1), Ihlengesi changoensis was a small ziphiid which, like Ihlengesi saldanhae, did not reach 4 m in length. Indeed, by roughly estimating the postorbital width of the cranium to 250 mm and applying the aforementioned equation proposed by Bianucci et al. (2008), the body length results to be 3.5 m, a value close to that estimated for I. saldanhae (3.1 m). Therefore, based on these estimations, species of Ihlengesi were smaller than all extant ziphiids with the exception of the pygmy beaked whale Mesoplodon peruvianus (maximum body length 3.72 m; Reyes et al. 1991).
Table 1. Measurements (in mm) of the crania of Ihlengesi changoensis sp. nov. and Ihlengesi saldanhae (measurements from Bianucci et al. 2007). Abbreviations: e, estimate; –, no data.
| |
Ihlengesi changoensis sp. nov. |
Ihlengesi saldanhae Bianucci, Lambert, and Post, 2007 |
|
|
MUAP(MM)-068 |
SAM PQ 2792 |
SAM PQ 69673 |
|
|
Length of rostrum |
540 |
– |
– |
|
Width of rostrum at mid-length |
38 |
– |
– |
|
Width of rostrum base at prominental notch |
104 |
108 |
104 |
|
Width of rostrum base at antorbital notch |
180 |
e143 |
e142 |
|
Width of premaxillae at rostrum base (anterior notch) |
55 |
47 |
– |
|
Distance rostrum base—anterior apex of palatine |
120 |
– |
– |
|
Preorbital width of skull |
e230 |
– |
– |
|
Longitudinal distance right
premaxillary foramen |
47 |
31 |
– |
|
Width of premaxillary sac fossae |
130 |
105 |
101 |
|
Width of right premaxillary sac fossa |
69 |
51 |
e52 |
|
Width of left premaxillary sac fossa |
55 |
46 |
44 |
|
Width of bony nares |
45 |
49 |
– |
|
Minimum width of right ascending process of premaxilla |
32 |
25 |
– |
|
Width of premaxillary crests |
110 |
103 |
– |
|
Width of right premaxillary crest |
59 |
47 |
– |
|
Width of left premaxillary crest |
42 |
38 |
– |
|
Minimum distance between premaxillary crests |
34 |
34 |
– |
|
Maximum width of nasals |
41 |
52 |
– |
|
Length of medial suture of nasals on vertex |
45 |
e45 |
– |
|
Minimum posterior distance between maxillae |
37 |
32 |
– |
The complete rostrum is moderately elongated and, similarly to the paratype of Ihlengesi saldanhae (SAM PQ 69673), shows a strong lateral compression generating a narrow dorsal profile and a transverse section with a height significantly greater than its width (Figs. 2, 5). Due to its narrow dorsal shape, the rostrum of Ihlengesi changoensis clearly differs from Khoikhoicetus spp. Indeed, the rostrum of this latter genus is significantly transversely wider than in I. changoensis, a feature particularly marked in Khoikhoicetus kergueleni having a triangular outline of the rostrum in dorsal view (Lambert et al. 2018).
The medial margins of the premaxillae on the rostrum never contact medially and the mesorostral groove is filled by the pachyosteoscleorotic vomer along its whole rostral portion (Fig. 2A1), a feature that, at least in the extant ziphiids, is only observed in adult males (Bianucci et al. 2016b). The vomer greatly inflates towards the posterior portion of the rostrum as in the holotype of Ihlengesi saldanhae (SAM PQ 2792). A median longitudinal suture between the lateral walls of the vomer in the rostrum base area is visible as in all hyperoodontines.
In ventral view the rostrum does not show a distinct alveolar groove whereas a clear maxilla-palatine suture extends about 12 cm anterior to the antorbital notch (Fig. 2A3).
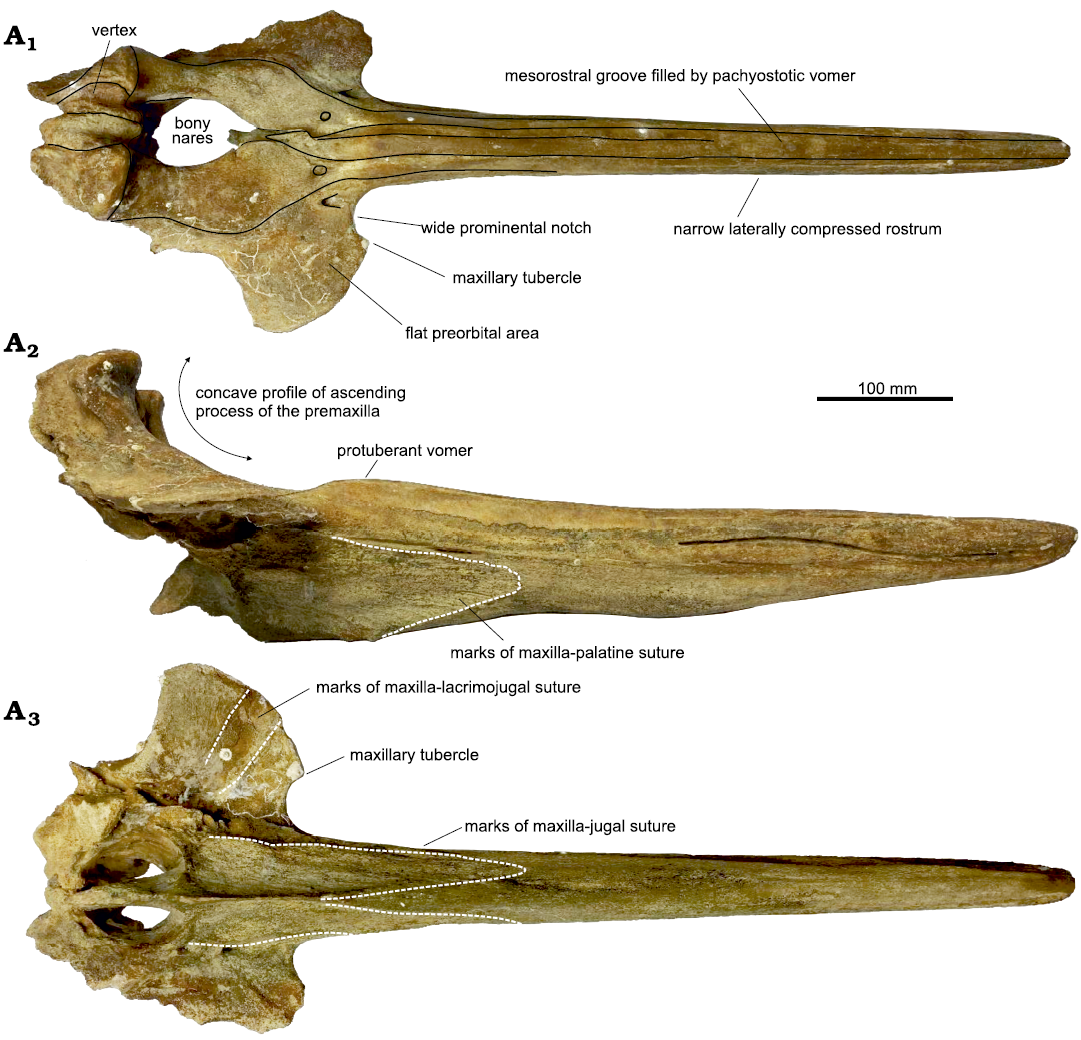
Fig. 2. Holotype of the beaked whale Ihlengesi changoensis sp. nov. (MUAP(MM)-068) from the sea floor off Pisagua, Northern Chile; Plio-Pleistocene. Cranium in dorsal (A1), right lateral (A2), and ventral (A3) views.
Medial to the narrow antorbital notch, a distinct maxillary tubercle is visible. The position the suture between the maxilla and the missing lacrimojugal complex, well visible in the ventral surface of the left side of the neurocranium, supports that this tip, apparently laterally located to the rostrum base, is actually the maxillary tubercle. Indeed, this suture is located well lateral to the lateral margin of the rostrum, leaving exposed ventrally a wide portion of the maxilla that anteriorly bears what we interpret as maxillary tubercle. The presence of a well-defined maxillary tubercle separated from the lateral margin of the rostrum by a distinct prominental notch is a feature shared by most hyperoodontines including Ihlengesi saldanhae but absent in Khoikhoicetus spp.
The prominental notch of the Ihlengesi changoensis holotype is transversely wide, U-shaped and shallow as in the paratype of I. saldanhae, whereas in the I. saldanhae holotype this notch is V-shaped and transversely shorter, suggesting that this character is subject to intraspecific variation (Fig. 3).
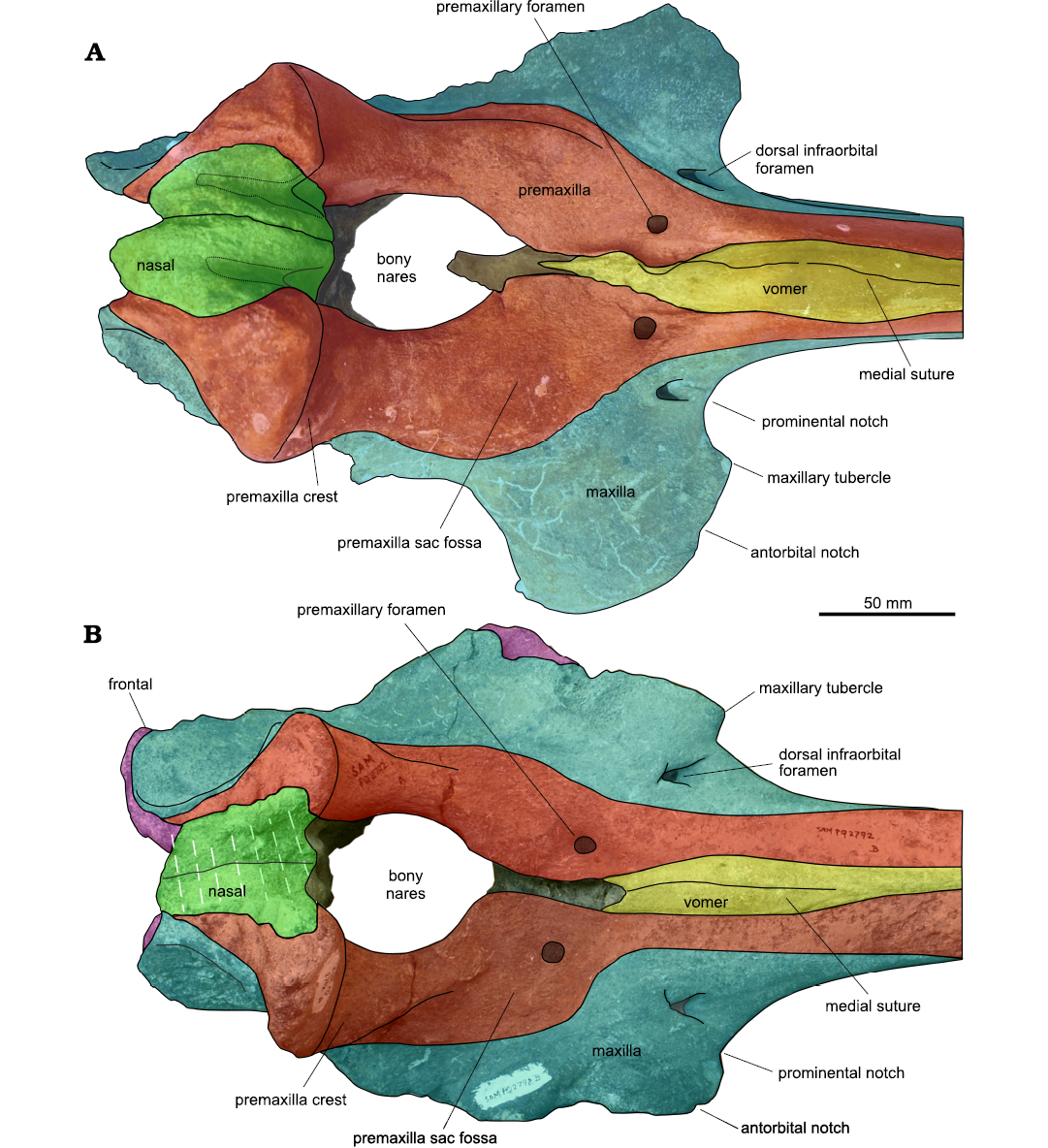
Fig. 3. Comparison of the neurocrania and the posterior portion of the rostra in dorsal view in two beaked whales. A. The holotype of Ihlengesi changoensis sp. nov. (MUAP(MM)-068) from the sea floor off Pisagua, Northern Chile; Plio-Pleistocene. B. The holotype of Ihlengesi saldanhae (SAM PQ 2792) from the sea floor off Saldanha Bay, South Africa; age unknown.
The dorsal surface of the preorbital area is roughly flat without a distinctive maxillary crest, as in I. saldanhae, while in most of the other crown Ziphiidae, including Khoikhoicetus, the maxillary crest is present and generally elevated to form a peculiar dome.
Both the right and the left maxilla are pierced by a single dorsal infraorbital foramen near the prominental notch, 13- and 7-mm posterior to the rostrum base, respectively. Similar dorsal infraorbital foramina are observed in Ihlengesi saldanhae but more posteriorly located in I. saldanhae paratype.
The right and the left premaxillary foramina are distinctly posterior to the rostrum base (5 mm) but less than in Ihlengesi saldanhae as well quantified by the ratio between the longitudinal distance between the right premaxillary foramen and the rostrum base and the width of rostrum base, being this ratio equal to 0.10 in I. changoensis against 0.34–0.32 in I. saldanhae. This significant difference in the position of the premaxillary foramina is related to the greater anteroposterior extension of the premaxillary sac fossae in I. changoensis compared to I. saldanhae. Indeed, the premaxillary sac fossae of I. saldanhae are unusually short so that this feature was previously considered an apomorphy of the Ihlengesi (Bianucci et al. 2007). Interestingly, in I. saldanhae the premaxillary foramina are distinctly posterior to the dorsal infraorbital foramina, an unusual condition in beaked whales. The premaxillary foramina of I. changoensis are posteriorly located to the dorsal infraorbital foramina but to a lesser degree than I. saldanhae. Regardless the position of the foramina with respect to the rostrum base, in all hyperoodontines, except some species of Mesoplodon (e.g., Mesoplodon grayi von Haast, 1876), the premaxillary foramina are always roughly at the same level or weakly anterior to the maxillary foramina.
The weakly concave premaxillary sac fossae of I. changoensis are distinctly asymmetrical as in I. saldanhae, being the right fossa transversally wider than the left fossa (ratio between the widths of the left and right premaxillary fossae = 0.78).
The ascending process of the premaxilla rises towards the vertex with its posterodorsal portion partly overhanging the bony nares (Fig. 2A2). Therefore, with the cranium in lateral view, the anterior profile of this portion of the ascending process of the premaxilla is clearly concave in Ihlengesi changoensis as in I. saldanhae, not straight and vertical as in Khoikhoicetus spp.
The vertex is moderately elevated as in Ihlengesi saldanhae and Khoikhoicetus spp. (ratio between the vertical distance between the dorsal margin of the maxilla at the rostrum base and the top of the vertex and the width of the premaxillary sac fossae between 0.70 and 1.0). All other hyperoodontines have a higher vertex (ratio > 1.0).
The ascending process of the premaxilla displays a strong constriction between premaxillary sac fossa and premaxillary crest (ratio between the minimal width of ascending process of the right premaxilla and the width of right premaxillary crest = 0.56).
As in Ihlengesi saldanhae and Khoikhoicetus agulhasis, the posterolaterally directed premaxillary crests are transversally narrower than in the other hyperoodontines (ratio between the transverse width of the premaxillary crests and the width of premaxillary sac fossae = ca. 1.0), whereas the distance between the premaxillary crests is greater than in Mesoplodon and Hyperoodon (ratio between the minimum distance between the right and left premaxillary crests and the width of the premaxillary sac fossae = 0.3).
The dorsal margin of each premaxillary crest slopes markedly ventrolaterally generating an acute dorsal profile of the vertex in anterior view, similarly to species of Mesoplodon, Hyperoodon, Khoikhoicetus but not Ihlengesi saldanhae, this latter having premaxillary crests with a rounded dorsolateral profile (Fig. 4).
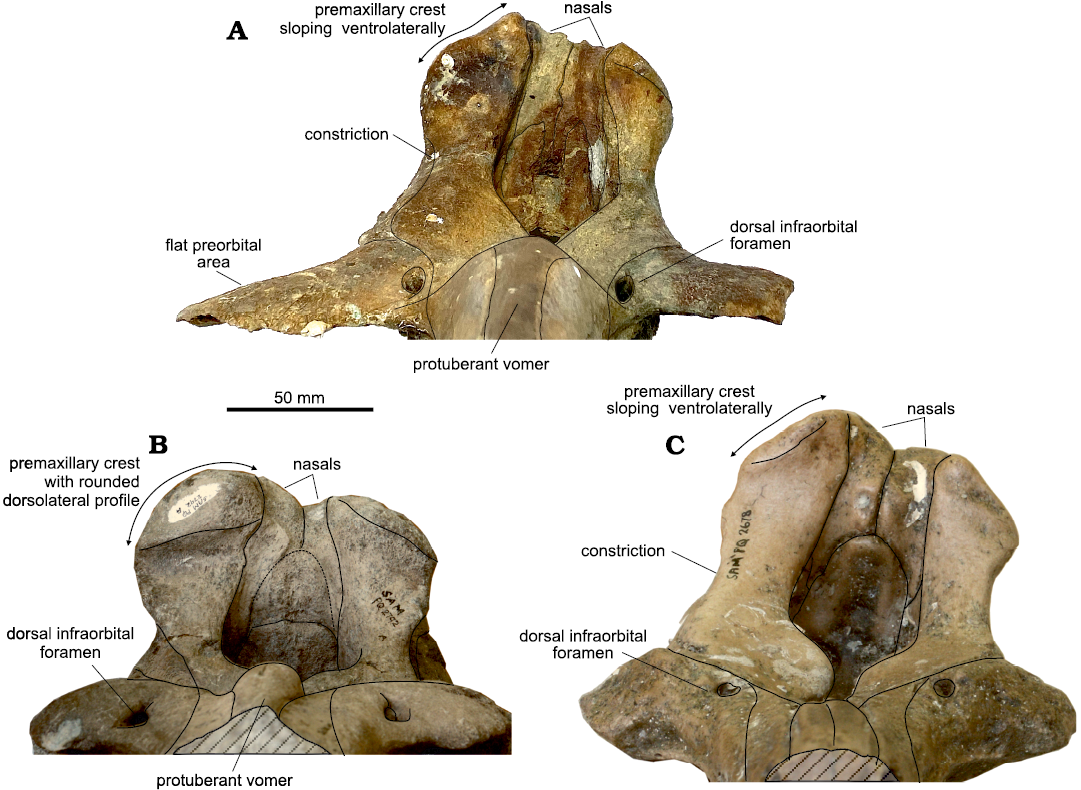
Fig. 4. Comparison of the crania in anterior view of the holotype of three beaked whales. A. Ihlengesi changoensis sp. nov. (MUAP(MM)-068) from the sea floor off Pisagua, Northern Chile; Plio-Pleistocene. B. The holotype of Ihlengesi saldanhae (SAM PQ 2792) from the sea floor off Saldanha Bay, South Africa; age unknown. C. The holotype Khoikhoicetus agulhasis (SAM PQ 2678) from the sea floor off Cape Agulhas, South Africa; age unknown. Diagonal lines represent broken surfaces.
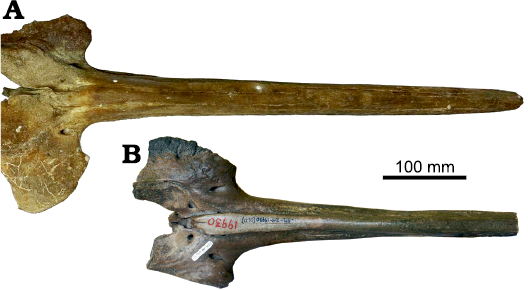
Fig. 5. Comparison of the rostra and posterior portion of the neurocrania in dorsal view of two beaked whales. A. The holotype of Ihlengesi changoensis sp. nov. (MUAP(MM)-068) from the sea floor off Pisagua, Northern Chile; Plio-Pleistocene. B. The paratype of Ihlengesi saldanhae (SAM PQ 69673) from the sea floor off Saldanha Bay, South Africa; age unknown.
The elongated nasals (ratio between the length of medial suture of nasals on vertex and the maximum width of nasals = 1.1) show a deep anteromedial excavation on their joined dorsal surface as in the other hyperoodontines but Indopacetus pacificus (Longman, 1926). Due to a significant anterolateral expansion of the nasals forming part of the premaxillary crests, a deep notch is visible on the dorsal margin of each nasal, a feature not discernible in Ihlengesi saldanhae holotype due to the bad preservation of the vertex. The inclusion of the nasals in the premaxillary crests is significant, although less for the right than for the left nasal, a condition shared with Khoikhoicetus spp. rather than with I. saldanhae having a greater anterolateral expansion of the right nasal.
The lateral margins of the nasals are weakly convex as in Khoikhoicetus spp., not anteriorly diverging as in Ihlengesi saldanhae. The nasofrontal suture is anteriorly pointed as in Khoikhoicetus spp., not convex posteriorly as in I. saldanhae.
Remarks.—All characters listed in the diagnosis separating Ihlengesi changoensis from Ihlengesi saldanhae are shared with Khoikhoicetus spp. Indeed Khoikhoicetus is a hyperoodontine with a cranium roughly similar to Ihlengesi (i.e., for the size and for the moderate elevation of the vertex). Nevertheless Khoikhoicetus does not show many of the character above listed of the genus Ihlengesi (i.e., rostrum narrow in dorsal view, presence of a distinct maxillary tubercle and a wide and shallow prominental notch anteromedial to the antorbital notch; premaxillary foramen distinctly posterior to the antorbital notch, roughly flat dorsal surface of the preorbital area without distinct maxillary crest; ascending process of the premaxilla in lateral view concave with posterodorsal portion partly overhanging the bony nares) and therefore we consider reliable the appurtenance of the new Chilean species to Ihlengesi rather than Khoikhoicetus, being also supported by the phylogenetic analysis.
Stratigraphic and geographic range.—Type locality and horizon only.
Phylogeny
As for the phylogeny in Bianucci et al. (2016b), the best result was obtained using implied weight analysis with constant K = 3 (Goloboff 1993). This analysis generated a single most parsimonious tree, with tree length = 172, Goloboff fit = -37.65, CI = 0.48, and RI = 0.77 (Fig. 6). The addition of Ihlengesi changoensis, Khoikhoicetus agulhasis, and Khoikhoicetus kergueleni did not change the topology of the tree compared to the tree published in Bianucci et al. (2016b). Indeed, I. changoensis results to be sister taxon of I. saldanhae and Ihlengesi to be sister taxon of Hyperoodon, both in derived position within the Hyperoodontinae. Khoikhoicetus kergueleni is confirmed to be congeneric with K. agulhasis, both forming a clade basal to Mesoplodon. Khoikhoicetus was not included in the analysis by Bianucci et al. (2016b), whereas in the consensus tree of five most parsimonious cladograms published in Bianucci et al. (2007) it shows the same position than the
Khoikhoicetus agulhasis + Khoikhoicetus kergueleni clade of the analysis here proposed.
The two following reversals, that define the Ihlengesi clade, also support the close relationship of Ihlengesi changoensis with I. saldanhae:

Fig. 6. Single most parsimonious tree of the heuristic search with downweighted homoplastic characters (K = 3) showing the relationships of Ihlengesi changoensis sp. nov. (in bold) with the other ziphiids. Numbers associated with branches are bootstrap values. (E) genera with extant species. See text, Appendix 1, and Bianucci et al. (2016b) for data matrix and description of characters.
Discussion
Body size evolution.—The estimated body size of Ihlengesi changoensis is compared with those of the other ziphiids and the evolution of body size amongst ziphiids is analysed putting this character in the most parsimonious cladogram produced by the phylogenetic analysis. This approach is similar to that in Lambert et al. (2013: fig. 16) but the selected body size categories are different: small size (3–4 m); medium size (4–5 m); medium-large size (5–7 m); and large size (6–11 m).
The obtained graph (Fig. 7) evidences a low disparity of body size within the stem ziphiids being all medium-sized apart from the small-sized Chimuziphius and the medium-large-sized Imocetus and Tusciziphius. The size disparity in the crown beaked whales appears greater being this wide clade represented by all four body size categories from the small size (e.g., Ihlengesi), to the large size of Hyperoodon and Berardius. These different sizes were independently achieved by parallel evolution several times during the evolution of the crown beaked whales.
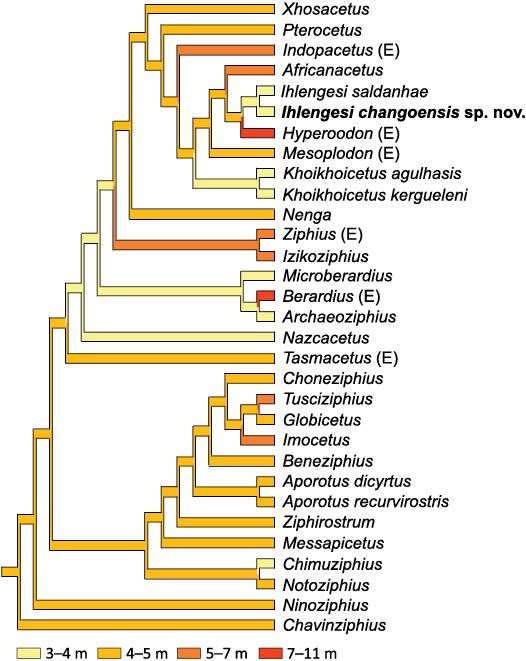
Fig. 7. Body size evolution amongst ziphiids. The tree is the single most parsimonious as presented in Fig. 6. (E) genera with extant species. See text and Lambert et al. (2013: fig. 16) for details.
Such changes in body size among extant and fossil ziphiids have been related, by Bianucci et al. (2008), to a dietary niche partitioning (different prey size) of species living in the same area (MacLeod et al. 2003) and to a wider exploration of the water column for foraging. The development of a larger body, in fact, improves the dive capacity of airbreathing vertebrates (Schreer and Kovacs 1997). Moreover, the progressive global climatic cooling culminating in the Quaternary glaciations could have contributed to the origin of the extant large beaked whale (i.e., Berardius and Hyperoodon) confined to the cold waters at high latitudes (Lambert et al. 2013; Bianucci et al. 2016b).
Interestingly, a small body size characterizes not only Ihlengesi changoensis and I. saldanhae, but also other four fossil species of crown ziphiids: Archaeoziphius microglenoideus, Khoikhoicetus agulhasis, K. kergueleni, Microberardius africanus, and Nazcacetus urbinai. Instead among the extant ziphiids only the pygmy beaked whale Mesoplodon peruvianus does not reach 4 m in length (Reyes et al. 1991). Based on few stomach contents analysed, M. peruvianus feeds only on fish (Reyes et al. 1991) while a diet preferentially based on cephalopods was observed in larger extant ziphiids (MacLeod et al. 2003). These data seem to suggest that Ihlengesi changoensis and other small fossil beaked whales had a predominantly piscivorous diet and that the drastic reduction in number of small species and concomitant appearance of large species in the extant ziphiid could at least in part be related to a shift towards a diet based mainly on octopuses and squids. In turn, this partial change of diet in the extant ziphiids could be due to feeding competition with the mainly fisheater delphinids which have a recent explosive radiation. A similar but older competition had already been claimed, when observing that the extinction of the epipelagic feeder stem ziphiids coincides with the radiation of the delphinids (Lambert et al. 2015). Nevertheless, the case here reported concerns a more recent competition between bottom feeder crown ziphiids and ecologically related delphinids.
Predatory pressure may have also contributed to the extinction of small-sized beaked whales. Analysing the documented cases of active predation on cetaceans by the white shark Carcharodon carcharias, Long and Jones (1996) noted that the biggest reported preys are two juvenile Mesoplodon stejnegeri and Ziphius cavirostris, about 3 m long. According to the same authors this datum implies an upper limit for the prey of white sharks due to their maximum body size that generally does not exceed 6 m. The shift of the minimum size towards 4 m in the extant beaked whales could therefore be interpreted as a consequence of the predatory impact of white shark whose appearance is reported toward the end of Messinian (Ehret et al. 2012). The weakness of this hypothesis is that it is not possible to well correlate the change in size of the beaked whales with the evolution of C. carcharias and other large predatory sharks due to the poor constrain of the ages of Ihlengesi changoensis and of most of the other small fossil beaked whales. It should be also considered that other macropredatory sharks lived before and some (e.g., the giant Carcharocles megalodon) also together with C. carcharias, becoming extinct well after the appearance of the white shark (Pimiento and Clements 2014; Boessenecker et al. 2019). The selective pressure on beaked whales by these large sharks may have been very strong, shifting the lower limit of body size of the beaked whale even higher than today. Therefore, the impact of the shark predation on the evolution of the size of the beaked whales is still far from being clarified.
Another macropredator that could have driven the recent evolution of beaked whales is the killer whale Orcinus orca (Aguilar de Soto et al. 2020). Indeed, Bianucci et al. (2022) suggested that the ability to prey on tetrapods, including other cetaceans, has been achieved quite recently (possibly during the Pleistocene) in the O. orca lineage. However, even if there are some documented evidences of predation by killer whales on beaked whales (Jefferson et al. 1991; Wellard et al. 2016), it is unclear how much this predatory pressure may have directly influenced the reduction in the number of species of small ziphiids. In fact, unlike sharks, killer whales can attack prey much larger than themselves by hunting in groups (Ford 2018). However, the predation by Orcinus orca could have indirectly favoured the increase in body size of beaked whales by progressively pushing them towards deeper waters. Indeed, as mentioned above, the diving capacity is greater in larger cetaceans and therefore beaked whales might have suffered an indirect selective pressure towards their present large size.
Palaeobiogeography.—The attribution of the Chilean fossil skull to a hyperoodontine further supports the hypothesis that the crown beaked whales originated and radiated in the oceanic waters of the southern hemisphere. In fact, all the beaked-whale fossil assemblages found on the sea floor of the southern oceans (Fig. 8) are characterized by the absence of stem ziphiids (Whitmore et al. 1986; Bianucci et al. 2007; Gol’din and Vishnyakova 2013; Ichishima et al. 2017; Lambert et al. 2018), while stem ziphiids are abundant and diversified in the deep sea assemblages of North Atlantic (Bianucci et al. 2013; Miján et al. 2017). This geographic difference has been related to a phenomenon of convergent evolution in disjunct antitropical areas that would have led to similar adaptations to deep-sea feeding (Bianucci et al. 2007, 2016b; Lambert et al. 2018). Interestingly, all the ziphiid remains found on the sea floor of the Southern Hemisphere, including the cranium here described, were collected in an area presently characterized by high productivity due to oceanic circulation and upwelling (Bianucci et al. 2008). These areas, where even today beaked whales are abundant and quite diversified (Ross 1984; Mead 1989), could have contributed to past radiation of crown beaked whales in the Southern Hemisphere oceanic environments. In particular, the cold Humboldt Current, which originated during the Eocene or Oligocene and intensified between 15 to 10 Ma, generates coastal upwelling activity along the Peruvian and Chilean margins which still today represents one of the most productive marine areas (Karstensen and Ulloa 2009; Armijo et al. 2015; Collareta et al. 2021). Therefore it is not surprising that, even in the eastern South Pacific off the coast of Chile, beaked whales show a high diversity today, although, as usual for beaked whales, their record is rather scarce and fragmentary. In particular, information on present species in this area has been summarized by Aguayo-Lobo et al. (1998) who reported Berardius arnouxii, Hyperoodon planifrons, Tasmacetus shepherdii, Ziphius cavirostris, Mesoplodon densirostis, M. grayi, M. hectori, M. layardii, M. peruvianus, and M. traversii. The occurrence of B. arnouxii is based on direct observations in Antarctic waters (Aguayo-Lobo 1994) and skeletal remains stranded in the eastern Strait of Magellan (Goodall 1978; Sielfeld 1983). Hyperoodon planifrons presents sightings off 33°S (Aguayo 1966) and Antarctic waters (Mörch 1911). Ziphius cavirostris has been sighted between Valparaiso and Easter Island (Aguayo-Lobo et al. 1998) and off central Chile (Hucke-Gaete 1998; Findley et al. 1998). Strandings of Z. cavirostris have been reported for the coast of Concepción (Oliver-Schneider 1946), Robinson Crusoe Island (Cárdenas and Yáñez 1988) and the eastern outlet of the Strait of Magellan: Cabo Espiritu Santo (Venegas and Sielfeld 1978; Sielfeld 1983). Tasmacetus shepherdii has only been reported from carcasses stranded on Gable Island: Beagle Channel (Goodall 1978) and Alejandro Selkirk Island (Brownell et al. 1976). Mesoplodon densirostris has been reported based specimens stranded in Puerto Montt: Bahía Pargua (Pastene et al. 1990) and Isla de Pascua (Aguayo-Lobo et al. 1998). The presence of M. grayi has been recorded on the basis of bone remains collected in the Strait of Magellan: Punta Wreck and Punta Catalina: Bahía Munición (Sielfeld 1979, 1983). The inclusion of M. hectori in the ziphiid list of the Chilean waters is supported by a skull stranded on the Atlantic coast of Tierra del Fuego (Goodall 1978) and Bahía Windhond: Isla Navarino (Sielfeld 1979). M. layardii has a stranding on the north coast of the Strait of Magellan: Rio Seco (Venegas and Sielfeld 1978), Tierra del Fuego: Cabo Espiritu Santo (Goodall 1978), and Isla Navarino: Bahía Windhond (Venegas and Sielfeld 1978; Sielfeld 1979). M. peruvianus, firstly described only for the coast of Peru (14°S) (Reyes et al. 1991; Reyes and Van Waerebeek 2018), afterwards was sighted south of Iquique (Tarapacá) and off Punta Choros (Atacama) and also reported through a skull stranded on Playa Choros (Sanino et al. 2007). M. traversii (= M. bahamondi) was reported on the basis of a skull found on Robinson Crusoe Island (Reyes et al. 1995). Most of these species of beaked whales found off the Chilean coasts have also been reported off South Africa, Australia, and New Zealand (Table 2), evidencing a circumpolar distribution (McLeod et al. 2006). A similar distribution can be hypothesized for the fossil genus Ihlengesi which, in addition to being reported in Chile, was previously known from Cape Columbine, west coast of South Africa, in the Atlantic Ocean (Bianucci et al. 2007). But unfortunately, the Chilean beaked whale fossil record is too scarce to analyse its biogeographic relationships and more generally to reconstruct the mode and tempo of the evolution of beaked whales in this part of the eastern south Pacific Ocean. Indeed, besides the described cranium, the Chilean fossil record of ziphiids is represented only by three fragmentary remains from the Upper Miocene–lower Pliocene Bahía Inglesa Formation (Caldera, Atacama Region). These fossils consist of an indeterminate rostrum (Sielfeld 1995), an incomplete tympanic bulla attributed to Ninoziphius aff. N. platyrostris by Walsh (2001) but that does not actually retain sufficient characters for a generic attribution (GB personal observation), and an unfigured tooth referred to Ziphiidae indet. (Gutstein et al. 2008, 2015).
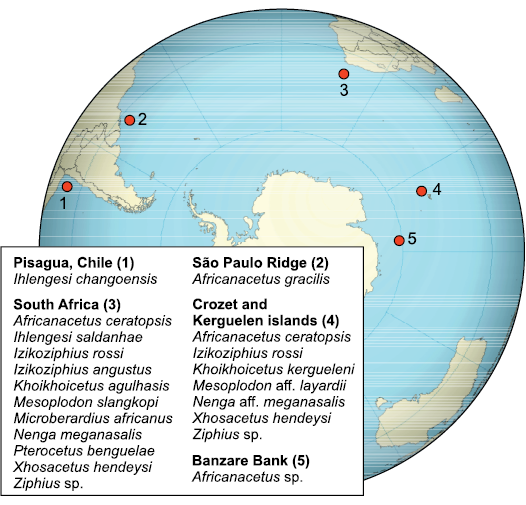
Fig. 8. Geographic distribution of the main fossils of ziphiids recovered from the seafloor of the Southern Hemisphere. Data from: 1, this study; 2, Ichishima et al. (2017); 3, Bianucci et al. (2006, 2007); 4, Lambert et al. (2018); 5, Gol’din and Vishnyakova (2013).
Table 2. Extant species of beaked whales recorded along the coast of Chile and their geographical distribution in the other main circumantarctic areas. For detailed references of the Chilean record see the text whereas the other data are from MacLeod et al. (2006).
| |
Chile |
South Africa |
Antarctic |
Australia |
New Zealand |
|
Berardius arnouxii |
× |
× |
× |
× |
× |
|
Hyperoodon planifrons |
× |
× |
× |
× |
× |
|
Mesoplodon densirostis |
× |
× |
|
× |
× |
|
Mesoplodon grayi |
× |
× |
× |
× |
× |
|
Mesoplodon hectori |
× |
× |
|
× |
× |
|
Mesoplodon layardii |
× |
× |
× |
× |
× |
|
Mesoplodon peruvianus |
× |
|
|
|
|
|
Mesoplodon traversii |
× |
|
|
|
× |
|
Tasmacetus shepherdii |
× |
|
|
× |
× |
|
Ziphius cavirostris |
× |
× |
× |
× |
× |
Conclusions
Morphological characters and phylogeny support affinities, but also distinctive differences, of the Chilean fossil cranium described in this study with the fossil hyperoodontine Ihlengesi saldanhae. Therefore this cranium is here described as holotype of the new specie I. changoensis.
The small body size of Ihlengesi changoensis (estimated length 3.5 m) supports the hypothesis that in the past small beaked whales (body length under 4 m) were more common than at present. The following reasons for the recent shift towards larger body sizes in the ziphiids have been hypothesized: (i) a progressive change of diet with a greater component of cephalopods rather than fish partially related to the competition with delphinids; and (ii) the predatory impact of white sharks and killer whales.
Given that the new Chilean fossil ziphiid is an hyperoodontine, the previously formulated hypothesis that the crown beaked whales originated and radiated in the oceanic waters of the Southern Hemisphere is further supported. In particular, the fossil and extant record of beaked whales of the whole Southern Hemisphere suggests that the radiation and the geographical distribution of crown beaked whales may have been driven by the oceanic circulation and related localized concentration of trophic resources in high productivity upwelling areas. Even if the high diversity observed in the extant beaked whales off the coast of Chile and the fossil specimen here described seem to support this evolutionary and paleogeographic pattern, new fossil remains from the ocean floor as well as from inland outcrops (e.g., the fossil-rich Bahia Inglesa Formation) will better clarify the rise of the southeastern Pacific current beaked whale assemblage.
Acknowledgements
The authors wish to thank the captain Dany Manzo and his crew of the MV “La Huayca”, for collecting from the sea floor the here described fossil cranium. We are also grateful to Giancarlo Molli (Dipartimento di Scienze della Terra, Università di Pisa, Italy) for fruitful discussions about the geology and tectonic evolution of the northern Chile continental margin; to Graham Avery and Margaret Avery for the access to the SAM collection; and to Anelio Aguayo (Chilean Antarctic Institute, Punta Arenas, Chile) for his critical comments and observations on extant ziphiids off the coast of Chile. Aldo Benites-Palomino (Department of Paleontology, University of Zurich, Switzerland) and Cheng-Hsiu Tsai (Department of Life Science, National Taiwan University, Taipei, Taiwan) contributed with important improvements during the review process.
References
Aguayo, A.1966. Observaciones de cetáceos frente a la costa de Chile, durante el año 1966. Informe al Ministerio de Agricultura. Informe Técnico. 11 pp. Departamento de Pesca y Casa, Montemar.
Aguayo-Lobo, A. 1994. Registro de mamíferos y aves marinas en la Antártica, durante los inviernos 1993 y 1994. Boletin Antárico Chileno 13 (2): 13–14.
Aguayo-Lobo, A., Torres, D., and Acevedo, J. 1998. Los mamíferos marinos de Chile: I. Cetacea. Serie Científica, Instituto Nacional Antártico de Chile (INACH) 48: 19–159.
Aguilar de Soto, N., Visser, F., Tyack, P.L., Alcazar, J., Ruxton, G., Arranz, P., Madsen, P.Y., and Johnson, M. 2020. Fear of killer whales drives extreme synchrony in deep diving beaked whales. Scientific Reports 10: 13. Crossref
Armijo, R., Lacassin, R., Coudurier-Curveur, A., and Carrizo, D. 2015. Coupled tectonic evolution of Andean orogeny and global climate. Earth-Science Reviews 143: 1–35. Crossref
Bianucci G. 1997. The Odontoceti (Mammalia: Cetacea) from Italian Pliocene. The Ziphiidae. Palaeontologia italica 84: 163–192.
Bianucci, G. and Post, K. 2005. Caviziphius altirostris, a new beaked whale from the Miocene southern North Sea basin. Deinsea 11: 1–6.
Bianucci, G., Collareta, A., Post, K., Varola, A., and Lambert, O. 2016a. A new record of Messapicetus from the Pietra Leccese (Late Miocene, Southern Italy): Antitropical distribution in a fossil beaked whale (Cetacea, Ziphiidae). Rivista Italiana di Paleontologia e Stratigrafia 122: 63–74.
Bianucci, G., Di Celma, C., Urbina, M., and Lambert, O. 2016b. New beaked whales from the late Miocene of Peru and evidence for convergent evolution in stem and crown Ziphiidae (Cetacea, Odontoceti). PeerJ 4: e2479. Crossref
Bianucci, G., Geisler, J.H., Citron, S., and Collareta, A. 2022. The origins of the killer whale ecomorph. Current Biology 32: 1843–1851. Crossref
Bianucci G., Lambert, O., and Post, K. 2007. A high diversity in fossil beaked whales (Mammalia, Odontoceti, Ziphiidae) recovered by trawling from the sea floor off South Africa. Geodiversitas 29: 561–618.
Bianucci, G., Lambert, O., and Post, K. 2010. High concentration of long-snouted beaked whales (genus Messapicetus) from the Miocene of Peru. Palaeontology 53: 1077–1098. Crossref
Bianucci, G., Landini, W., and Varola, A. 1992. Messapicetus longirostris, a new genus and species of Ziphiidae (Cetacea) from the late Miocene of “Pietra Leccese” (Apulia, Italy). Bolletino della Società Paleontologica Italiana 31: 261–264.
Bianucci, G., Landini, W., and Varola, A. 1994. Relationships of Messapicetus longirostris (Cetacea, Ziphiidae) from the Miocene of South Italy. Bollettino della Società Paleontologica Italiana 33: 231–241.
Bianucci, G., Llàcer, S., Cardona, J.Q., Collareta, A., and Florit, A.R. 2019. A new beaked whale record from the upper Miocene of Menorca, Balearic Islands, based on CT-scan analysis of limestone slabs. Acta Palaeontologica Polonica 64: 291–302. Crossref
Bianucci, G., Miján, I., Lambert., O., Post, K., and O., Mateus. 2013. Bizarre fossil beaked whales (Odontoceti, Ziphiidae) fished from the Atlantic Ocean floor off the Iberian Peninsula. Geodiversitas 35: 105–153. Crossref
Bianucci G., Post, K., and Lambert, O. 2008. Beaked whale mysteries revealed by seafloor fossils trawled off South Africa. South African Journal of Science 104: 140–142.
Boessenecker, R.W., Ehret, D.J., Long, D.J., Churchill, M., Martin, E., and Boessenecker, S.J. 2019. The Early Pliocene extinction of the mega-toothed shark Otodus megalodon: a view from the eastern North Pacific. PeerJ 7: e6088. Crossref
Brisson, M.-J. 1762. Regnum Animale in classes IX distributum, sine synopsis methodica. 296 pp. Theodorum Haak, Paris.
Brownell, R.L., Aguayo, A., and Torres, D. 1976. A Shepherd’s beaked whale, Tasmacetus shepherdii, from the Eastern South Pacific. Scientific Report of the Whales Research Institute, Tokyo 28: 127–128.
Buono, M.R., and Cozzuol, M.A. 2013. A new beaked whale (Cetacea, Odontoceti) from the late Miocene of Patagonia, Argentina. Journal of Vertebrate Paleontology 33: 986–997. Crossref
Cárdenas, J.C. and Yáñez, J. 1988. Importancia del desarrollo de un programa de investigaciones cetológicas en las islas oceánicas chilenas. In: Primer Taller sobre Conservación y Manejo de Mamíferos Marinos Chilenos, Valdivia, 19–20 August 1988, extended Abstracts, 1–13. Valdivia.
Collareta, A., Lambert, O., Marx, F.G., de Muizon, C., Varas-Malca, R., Landini, W., Bosio, G., Malinverno, E., Gariboldi, K., Gioncada, A., Urbina M., and Bianucci, G. 2021.Vertebrate palaeoecology of the Pisco Formation (Miocene, Peru): glimpses into the ancient Humboldt Current ecosystem. Journal of Marine Science Engineering 1188: 1–25. Crossref
Coulbourne, W.T. 1981. Tectonics of the Nazca Plate and the continental margin of Western South America 18–25°S. Geological Society of America Memoirs 154: 587–681. Crossref
Coulbourne, W.T. and Moberly, R. 1977. Structural evidence of the evolution of fore-arc basins off South America. Canadian Journal of Earth Sciences 14: 102–116. Crossref
Dalebout, M.L., Mead, J.G., Baker, C.S., Baker, A.N., and Van Helden, A.L. 2002. A new species of beaked whale Mesoplodon perrini sp. n. (Cetacea: Ziphiidae) discovered through phylogenetic analyses of mitochondrial DNA sequences. Marine Mammal Science 18: 577–608. Crossref
Eastman, C.R. 1906. Report of the scientific results of the expedition to the eastern tropical Pacific, in charge of Alexander Agassiz, by the U.S. Fish Commission Steamer: “Albatros”, from October, 1904, to March, 1905, lieut. commander L.M. Garrett U.S.N., commanding. VII. Shark’s teeth and cetacean bones. Bulletin of the Museum of Comparative Zoology at Harvard College 5 (4): 75–100.
Ehret, D.J., MacFadden, B.J., Jones, D.S., DeVries, T.J., Foster, D.A., and Salas Gismondi, R. 2012. Origin of the white shark Carcharodon (Lamniformes: Lamnidae) based on recalibration of the upper Neogene Pisco Formation of Peru. Palaeontology 55: 1139–1153 Crossref
Ellis, R. and Mead, J.G. 2017. Beaked Whales: A Complete Guide to Their Biology and Conservation. 194 pp. Johns Hopkins University Press, Baltimore.
Findlay, K., Pitman, R., Tsurui, T., Sakai, K., Ensor, P., Iwakami, H., Ljungblad, D., Shimada, D., Thiele, D., Van Waerebeek, K., Hucke-Gaete, R., and Sanino, G.P. 1998. 1997/1998 IWC-Southern Whale and Ecosystem Research (IWC/SOWER) Blue Whale Cruise, Chile. 39 pp. International Whaling Commission Scientific Committee Muskat, Omán.
Fisher, R.L. and Raitt, R.L. 1962. Topography and structure of the Perú-Chile Trench. Deep Sea Research 5: 423–443. Crossref
Flower, W.H. 1867. Description of the skeleton of Inia geoffrensis and the skull of Pontoporia blainvillii, with remarks on the systematic position of these animals in the Order Cetacea. Transactions of the Zoological Society of London 6: 87–116. Crossref
Ford, J.K.B. 2018. Killer whale: Orcinus orca. In: B. Würsig, J.G.M. Thewissen, and K.M. Kovacs (eds.), Encyclopedia of Marine Mammals, Third Edition, 531–537. Academic Press, London. Crossref
Fordyce, R.E. and Cullen, D.J. 1979. A Miocene ziphiid whale (Odontoceti: Cetacea) from Central Chatham Rise, East of New Zealand. New Zealand Oceanographic Institute Records 4: 45–53.
Fuller, A.J. and Godfrey, S.J. 2007. A late Miocene ziphiid (Messapicetus sp.: Odontoceti: Cetacea) from the St. Marys Formation of Calvert Cliffs, Maryland. Journal of Vertebrate Paleontology 27: 535–554. Crossref
Geersen, J., Ranero, C.R., Barckhausen, U., and Reichert, C. 2015. Subducting seamounts control interplate coupling and seismic rupture in the 2014 Iquique earthquake area. Nature Communications 6 (1): 8267. Crossref
Geersen, J., Ranero, C.R., Klaucke, I., Behrmann, J.H., Kopp, H., Tréhu, A.M., Contreras-Reyes, E., Barckhausen, U., and Reichert, C. 2018a. Active Tectonics of the North Chilean Marine Forearc and Adjacent Oceanic Nazca Plate. Tectonics 37: 4194–4211. Crossref
Geersen, J., Ranero, C.R., Kopp, H., Behrmann, J.H., Lange, D., Klaucke, I., Barrientos, S., Diaz-Naveas, J., Barckhausen, U., and Reichert, C. 2018b. Does permanent extensional deformation in lower forearc slopes indicate shallow plate-boundary rupture? Earth and Planetary Science Letters 489: 17–27. Crossref
Gonzáles, G., Bello-Gonzáles, J.P., Contreras-Reyes, E., Tréhu, A.M., and Geersen, J. 2023. Shallow structure of the Northern Chilean marine forearc between 19°S–21°S using multichannel seismic reflection and refraction data. Journal of South American Earth Science 123 (2023): 104243. Crossref
Gonzáles, G., Cembrano, J., Carrizo, D., Macci, A., and Schneider, H., 2003. The link between forearc tectonics and Pliocene–Quaternary deformation of the Coastal Cordillera, northern Chile. Journal of South American Earth Science 16: 321–342. Crossref
Gol’din, P.E. and Vishnyakova, K.A. 2013. Africanacetus from the sub−Antarctic region: the southernmost record of fossil beaked whales. Acta Palaeontologica Polonica 58: 445–452.
Goloboff, P.A. 1993. Estimating character weights during tree search. Cladistics 9: 83–91 Crossref
Goodall, R.N.P. 1978. Report on the small cetaceans stranded on the coasts of Tierra del Fuego. Scientific Reports of the Whales Research Institute 30: 197–230.
Gray, J.E. 1850. Catalogue of the Specimens of Mammalia in the Collections of the British Museum. Part I Cetacea. 153 pp. Richard and John E. Taylor, London.
Gray, J.E. 1866. Catalogue of Seals and Whales in the British Museum. 402 pp. Trustees of the British Museum, London.
Gutstein, C.S., Horwitz, F.E., Valenzuela-Toro, A.M., and Figueroa-Bravo, C.P. 2015. Cetáceos fósiles de Chile: Contexto evolutivo y paleobiogeográfico. Publicación Ocasional del Museo Nacional de Historia Natural, Chile 63: 339–383.
Gutstein, C.S., Yury, R.Y., Soto, S., Suárez, M.E., and Rubilar-Rogers, D.E. 2008. La fauna de vertebrados fósiles del “bonebed” de la Formación Bahía Inglesa y aspectos taxonómicos. Actas del I Simposio Paleontología en Chile, Santiago 1: 102–108.
Haast, J. von. 1876. On a new ziphioid whale. Proceedings of the Zoological Society, London 1876: 7–13. Crossref
Henriquez, G., Rodriguez, L., and Kong, I. 1981. Exploración y prospección de recursos pesqueros del talúd continental. Instituto de Fomento Pesquero, CORFO, Chile, Informe Técnico 8: 1–15.
Heyning, J.E. 1989. Comparative facial anatomy of beaked whales (Ziphiidae) and a systematic revision among the families of extant Odontoceti. Contributions in Science, Natural History Museum of Los Angeles County 405:1–64. Crossref
Horikawa, H., Tazaki, K., and Kanno, T. 1987. Fossil Ziphiidae from Koshiji-Syo off Sado Island, central Japan. Publication of Sado Museum 9: 225–230.
Hucke-Gaete, R. 1998. Crucero de Investigación sobre la ballena azúl en aguas chilenas IWC/SOWER 1997/98. Informe de terreno. Observador científico embarcado en el Shonan-Maru. 41 pp. Subsecretaría de Pesca, Ministerio de Economía, Fomento y Reconstrucción, República de Chile, Santiago de Chile.
Ichishima, H., Augustin, A.H., Toyofuku, T., and Kitazato, H. 2017. A new species of Africanacetus (Odontoceti: Ziphiidae) found on the deep ocean floor off the coast of Brazil. Deep Sea Research Part II: Topical Studies in Oceanography 146: 68–81. Crossref
Jefferson, T.A., Stacey, P.J., and Baird, R.W. 1991. A review of killer whale interactions with other marine mammals: predation to coexistence. Mammal Review 21: 151–180. Crossref
Karstensen, J. and Ulloa, O. 2009. The Peru-Chile Current System. In: J.H. Steele, K.K. Turekian, and S.A. Thorpe (eds.), Encyclopedia of Ocean Sciences, 2nd Edition, 385–392. Elsevier, Amsterdam. Crossref
Kawatani, A. and Kohno, N. 2021. The oldest fossil record of the extant genus Berardius (Odontoceti, Ziphiidae) from the Middle to Late Miocene boundary of the western North Pacific. Royal Society Open Science 8: 201152. Crossref
Lambert, O. 2005. Systematics and phylogeny of the fossil beaked whales Ziphirostrum du Bus, 1868 and Choneziphius Duvernoy, 1851 (Mammalia, Cetacea, Odontoceti), from the Neogene of Antwerp (North of Belgium). Geodiversitas 27: 443–497.
Lambert, O. and Louwye, S. 2006. Archaeoziphius microglenoideus, a new primitive beaked whale (Mammalia, Cetacea, Odontoceti) from the Middle Miocene of Belgium. Journal of Vertebrate Paleontology 26: 182–191. Crossref
Lambert, O. and Louwye, S. 2016. A new early Pliocene species of Mesoplodon: a calibration mark for the radiation of this species-rich beaked whale genus. Journal of Vertebrate Paleontology 36: 1–10. Crossref
Lambert, O., Bianucci, G., and Post, K. 2009. A new beaked whale (Odontoceti, Ziphiidae) from the middle Miocene of Peru. Journal of Vertebrate Paleontology 29: 911–922. Crossref
Lambert, O., Bianucci, G., and Post K. 2010. Tusk-bearing beaked whales from the Miocene of Peru: sexual dimorphism in fossil ziphiids? Journal of Mammalogy 91: 19–26. Crossref
Lambert, O., Collareta, A., Landini, W., Post, K., Ramassamy, B., Di Celma, C., Urbina, M., and Bianucci, G. 2015. No deep diving: evidence of predation on epipelagic fish for a stem beaked whale from the Late Miocene of Peru. Proceedings of the Royal Society B 282: 20151530. Crossref
Lambert, O., Muizon, C. de, and Bianucci, G. 2013. The most basal beaked whale Ninoziphius platyrostris Muizon, 1983: clues on the evolutionary history of the family Ziphiidae (Cetacea: Odontoceti). Zoological Journal of the Linnean Society 167: 569–598. Crossref
Lambert, O., Muizon, C. de, Duhamel, G., and Van der Plicht, J. 2018. Neogene and Quaternary fossil remains of beaked whales (Cetacea, Odontoceti, Ziphiidae) from deep-sea deposits off Crozet and Kerguelen islands, Southern Ocean. Geodiversitas 40: 135–160. Crossref
Long, D.J. and Jones, R.E. 1996. White shark predation and scavenging on cetaceans in the eastern North Pacific Ocean. In: P.A. Klimley and D.G. Ainley (eds.), Great White Sharks: The Biology of Carcharodon carcharias, 293–307. Academic Press, San Diego. Crossref
Longman, H.A. 1926. New records of Cetacea, with a list of Queensland species. Memoirs of the Queensland Museum 8: 266–278.
MacLeod, C.D. 2018. Beaked whales overview. In: B. Würsig, J.G.M. Thewissen, and K.M. Kovacs (eds.), Encyclopedia of Marine Mammals, Third Edition, 80–83. Academic Press, London. Crossref
MacLeod, C.D., Perrin, W.F., Pitman, R., Barlow, J., Balance, L., D’Amico, A., Gerrodette, T., Joyce, J., Mullin, K.D., Palka, D.L., and Waring, G.T. 2006. Known and inferred distributions of beaked whale species (Cetacea: Ziphiidae). Journal of Cetacean Research and Management 7: 271–286. Crossref
MacLeod, C.D., Santos, M.B., and Pierce, G.J. 2003. Review of data on diets of beaked whales: evidence of niche separation and geographic segregation. Journal of the Marine Biological Association of the United Kingdom 83: 651–665. Crossref
Mead, J.G. 1989. Beaked whales of the genus Mesoplodon. In: S.H. Ridgway and R. Harrison (eds.), Handbook of Marine Mammals, Vol. 4, River Dolphins and the Larger Toothed Whales, 349–430. Academic Press, London.
Mead, J.G. and Fordyce, R.E. 2009. The therian skull: a lexicon with emphasis on the Odontocetes. Smithsonian Contributions to Zoology 627: 1–249. Crossref
Miján, I., Louwye, S., and Lambert, O. 2017. A new Beneziphius beaked whale from the ocean floor off Galicia, Spainand biostratigraphic reassessment of the type species. Acta Palaeontologica Polonica 62: 211–220. Crossref
Miyazaki, N. and Hasegawa Y. 1992. A new species of fossil beaked whale, Mesoplodon tumidirostris sp. nov. (Cetacea, Ziphiidae) from the Central North Pacific. Bulletin of the National Science Museum Tokyo, Serie A 18: 167–174.
Moberly, R., Shepherd, G.L., and Coulbourn, W.T. 1982. Forearc and other basins, continental margin of northern and southern Peru and adjacent Ecuador and Chile. Geological Society, London, Special Publications 10: 171–189. Crossref
Moore, J.C. 1968. Relationships among the living genera of beaked whales with classifications, diagnoses and keys. Fieldiana, Zoology 53: 209–289.
Mörch, J.A. 1911. On the Natural History of Whalebone Whales. Proceeding of the Zoological Society London 1911: 661–670. Crossref
Muizon, C. de 1984. Les Vertébrés de la Formation Pisco (Pérou). Deuxième partie: les Odontocètes (Cetacea, Mammalia) du Pliocène inférieur du Sud-Sacaco. Travaux de l’Institut français d’Études andines 27: 1–188.
Oliver-Schneider, C. 1946. Catálogo de los mamíferos de Concepción. Boletin de la Sociedasd de Biología de Concepción (Chile) 21: 67–83.
Pastene, L.A., Numachi, K., Jofre, M., Acevedo, M., and Joyce, G. 1990. First record of the Blainville´s beaked whale, Mesoplodon densirostris Blainville, 1817 (Cetacea: Ziphidae) in the eastern South Pacific. Marine Mammal Science 6: 82–84. Crossref
Pimiento, C. and Clements, C.F. 2014. When did Carcharocles megalodon become extinct? A new analysis of the fossil record. Plos One 9 (10): e11086. Crossref
Post, K. and Jensen, J.K. 2013. On diamonds, a mammal fossil from the Faroe Islands, and the northern most occurrence of fossil beaked whales. Cranium 30: 19–21.
Post, K., Lambert, O., and Bianucci, G. 2008. First record of Tusciziphius crispus (Cetacea, Ziphiidae) from the Neogene of the US east coast. Deinsea 12: 1–10.
Pyenson, N.D., Gutstein, C.S., Parham, J.F., LeRoux, J.P., Carreño Chavarría, C., Little, H., Metallo, A., Rossi, V., Valenzuela-Toro, A.M., Velez-Juarbe, J., Santelli, C.M., Rubilar Rogers, D., Cozzuol, M.A., and Suárez, M.E. 2014. Repeated mass strandings of Miocene marine mammals from Atacama Region of Chile point to sudden death at sea. Proceeding of the Royal Society B 281: 20133316. Crossref
Ramassamy, B. 2016. Description of a new long-snouted beaked whale from the Late Miocene of Denmark: evolution of suction feeding and sexual dimorphism in the Ziphiidae (Cetacea: Odontoceti). Zoological Journal of the Linnean Society 178: 381–409. Crossref
Ramassamy, B., Lambert, O., Collareta, A., Urbina, M., and Bianucci, G. 2018. Description of the skeleton of the fossil beaked whale Messapicetus gregarius: searching potential proxies for deep-diving abilities. Fossil Record 21: 11–32. Crossref
Ranero, C. R., Von Huene, R., Weinrebe, W., and Reichert, C. 2006. Tectonic processes along the Chile convergent margin. In: O. Oncken, G. Chong, G. Franz, P. Giese, H.-J. Götze, V.A. Ramos, M.R. Strecker, and P. Wigger (eds.), The Andes. Active Subduction Orogeny, 91–121. Springer, Berlin. Crossref
Reyes, J.C. and Van Waerebeek, K. 2018. The lesser beaked whale Mesoplodon peruvianus Reyes, Mead and Van Waerebeek, 1991 revisited, with biological observations on new specimens from Peru. Journal of Marine Biology and Oceanography 7(4): 1–10. Crossref
Reyes, J.C., Mead, J.G., and Van Waerebeek, K. 1991. A new species of beaked whale Mesoplodon peruvianus sp. n. (Cetacea Ziphiidae) from Peru. Marine Mammal Science 7: 1–24. Crossref
Reyes, J.C., Van Waerebeek, K., Cárdenas, J.C., and Yáñez, J.L. 1995. Mesoplodon bahamondei sp. n. (Cetacea, Ziphiidae) a new living beaked whale from the Juan Fernández Archipelago, Chile. Boletin Museo Nacional de Historia Natural, Chile 45: 31–44. Crossref
Robineau, D. 1973. Sur deux rostres de Mesoplodon (Cetacea, Hyperodontinae). Mammalia 37: 504–513. Crossref
Ross, G.J.B. 1984. The smaller cetaceans of the south east coast of southern Africa. Annales of Cape Provincial Museum (Natural History) 15: 173–410.
Sallarès, V. and Ranero, C.R. 2005. Structure and tectonics of the erosional convergent margin off Antofagasta, North Chile (23°300’ S). Journal of Geophysical Research 110: B06101. Crossref
Sanino, G.P., Yáñez, J., and Van Waerebeek, K. 2007. A first confirmed specimen record in Chile and sightings attributed to the lesser beaked whale Mesoplodon peruvianus Reyes, Mead and Van Waerebeek, 1991. Boletín del Museo Nacional de Historia Natural, Chile 56: 89–96. Crossref
Schreer, J.F. and Kovacs, K.M. 1997. Allometry of diving capacity in airbreathing vertebrates. Canadian Journal of Zoology 75: 339–358. Crossref
Sielfeld, W. 1983. Mamíferos Marinos de Chile. 199 pp. Ediciones de la Universidad de Chile, Santiago.
Sielfeld, W. 1979. Consideraciones acerca de tres especies de Mesoplodon Gervais (Cetacea: Ziphidae) presentes en aguas chilenas. Anales del Instituto de la Patagonia, Punta Arenas (Chile) 11: 273–280.
Sielfeld, W. 1995. El Plioceno/Pleistoceno de Caldera (III Región, Chile) y su importancia en el estudio de los Mamíferos Marinos de Chile. In: Resúmenes 1° Congreso de la Sociedad Latinoamericana de Especialistas en Mamíferos Marinos (SOLAMAC), 22–25 October, 1995, 25. Viña del Mar.
Swofford, D.L. 2002. PAUP*. Phylogenetic Analysis Using Parsimony (*and other methods). Version 4. 142 pp. Sinauer Associates, Sunderland.
Tanaka, Y., Watanabe, M., and Kimura, M. 2019. Crown beaked whale fossils from the Chepotsunai Formation (latest Miocene) of Tomamae Town, Hokkaido, Japan. Palaeontologia Electronica 22.2.31A. Crossref
Tazaki, K., Horikawa, H., and Miyazaki, S. 1987. Fossil Ziphiidae from Hyotan-Guri off Sado Island, central Japan. Publication of Sado Museum 9: 219–223.
Venegas, C. and Sielfeld, W. 1978. Registro de Mesoplodon layardii y otros cetáceos en Magallanes. Anales del Instituto de la Patagonia, Punta Arenas (Chile) 9: 171–177.
Von Huene, R., Weinrebe, W., and Heeren, F. 1999. Subduction erosion along the North Chile margin. Journal of Geodynamics 27: 345–358. Crossref
Walsh, A.A. 2001. The Bahía Inglesa Formation Bonebed: Genesis and Palaeontology of a Neogene Konzentrat Lagerstätte from North-Central Chile. 443 pp. Ph.D. Thesis, School of Earth and Environmental Sciences, University of Portsmouth, Portsmouth.
Wellard, R., Lightbody, K., Fouda, L., Blewitt M., Riggs, D., and Erbe, C. 2016. Killer whale (Orcinus orca) predation on beaked whales (Mesoplodon spp.) in the Bremer Sub-basin, Western Australia. PLoS One 11: e0166670. Crossref
Whitmore, F.C., Morejohn, G.V., and Mullins, H.T. 1986. Fossil beaked whales—Mesoplodon longirostris dredged from the ocean bottom. National Geographic Research 2: 47–56.
Yamada, T.K., Kitamura, S., Abe, S., Tajima, Y., Matsuda, A., Mead, J.G., and Matsuishi, T.F. 2019. Description of a new species of beaked whale (Berardius) found in the North Pacific. Scientific Reports 9: 1–14. Crossref
Acta Palaeontol. Pol. 68 (3): 477–491, 2023
https://doi.org/10.4202/app.01076.2023
Coding for Ihlengesi changoensis sp. nov., Khoikhoicetus agulhasis, and Khoikhoicetus kergueleni in the morphological matrix by Bianucci et al. (2016). Abbreviations: a, variable between 1 and 2; ?, missing character.
|
Ihlengesi changoensis |
22000 |
03212 |
0013a |
200?? |
????? |
?1??0 |
01021 |
????? |
????? |
??100 |
? |
|
Khoikhoicetus agulhasis |
?2000 |
0221a |
00a31 |
200?? |
????? |
?1??0 |
01011 |
????? |
????? |
??100 |
? |
|
Khoikhoicetus kergueleni |
22000 |
02212 |
20a31 |
200?? |
????? |
?1??0 |
01011 |
????? |
????? |
??100 |
? |