

An enigmatic tropical conifer from the Early Cretaceous of Gondwana
MARIA EDENILCE P. BATISTA, ANTÔNIO ÁLAMO F. SARAIVA, FLAVIANA J. DE LIMA, RENAN A.M. BANTIM, ALLYSSON P. PINHEIRO, DANIEL B. SILVA, and LUTZ KUNZMANN
Batista, M.E.P., Saraiva, A.Á.F. de Lima, F.J., Bantim, R.A.M., Pinheiro, A.P., Silva, D.B., and Kunzmann, L. 2024. An enigmatic tropical conifer from the Early Cretaceous of Gondwana.Acta Palaeontologica Polonica 69 (3): 375–393.
We describe a new genus of Leliacladus Batista & L.Kunzmann with the type species Leliacladus (Brachyphyllum) castilhoi (Duarte, 1985) Batista & L.Kunzmann gen. nov. et comb. nov., a rare fossil conifer that has been described from the Aptian (Lower Cretaceous) Romualdo Formation of the Araripe Basin, northeastern Brazil. We decided to leave this new fossil-genus unasigned to any existing family as it does not display sufficient number of anatomical characters. We re-studied the type material of the type species as well as we described additional new material from the Aptian Crato Formation of the same basin. The more recently found fossil materials include large portions of leafy shoots excavated from laminated lacustrine limestones. In contrast to the type material, the new material shows the replacement of organic matter by iron oxide, which is suitable for investigating anatomical features of the wood and leaves. The material allowed for a reappraisal of the systematic position of the fossil plant. Together, the morphological and anatomical characters revealed sufficient evidence to separate the conifer from Brachyphyllum and accommodate it in a new fossil-genus. Leliacladus gen. nov. is defined by the presence of comparatively thick and short claviform lateral branches, and the absence of thinner ultimate order branches with gradual tapering axes. The wood is characterized by dense tracheids with uniseriate pitting in the radial walls and cross-fields that possess one or two large pits. The rays are one to three cells high. The epidermises of the densely and helically arranged scale leaves show non-papillate cyclocytic stomatal complexes slightly sunken that are mostly scattered and randomly arranged across the abaxial surface. This combination of characters suggests the material belongs to the conifer families Podocarpaceae or Cheirolepidiaceae. The arrangement of the sparsely branched, but woody shoots of Leliacladus castilhoi gen. nov. et comb. nov. suggest a hypothetical candelabra-like growth habit of the plant, and the remarkable thickness of the axes suggests a hypothetical xeromorphic adaptation to the semiarid paleoenvironment.
Key words: Cheirolepidiaceae, Podocarpaceae, Leliacladus castilhoi, leaf epidermis, wood anatomy, Araripe Basin, Santana Group.
Maria Edenilce P. Batista [edenilce.peixoto@urca.br; ORCID: https://orcid.org/0000-0002-1239-0902 ], Departamento de Ciências Biológicas, Universidade Regional do Cariri, Crato, Ceará, Brazil.
Antônio Álamo F. Saraiva [alamocariri@yahoo.com.br; ORCID: https://orcid.org/0000-0003-0127-8912 ], Renan A. M. Bantim [renanbantimbiologo@gmail.com; ORCID: https://orcid.org/0000-0003-4576-0989 ], Laboratorio de Paleontologia, Universidade Regional do Cariri, Crato, Ceará, Brazil.
Flaviana J. De Lima [flaviana.jorge@ufpe.br; ORCID: https://orcid.org/0000-0001-8602-6508 ], Laboratorio de Paleontologia e Microestruturas, Universidade Federal do Pernambuco, Centro Acadêmico de Vitória, Vitória de Santo Antão, Pernambuco, Brazil.
Allysson P. Pinheiro [allysson.pinheiro@urca.br; ORCID: https://orcid.org/0000-0003-1565-6371 ], Museu de Paleontologia Plácido Cidade Nuvens, Santana do Cariri, Ceará, Brazil.
Daniel B. Silva [daniel.silva@ufca.edu.br; ORCID: https://orcid.org/0000-0002-3106-1557 ], Laboratório de Caracterização de Materiais, Universidade Federal do Cariri, Juazeiro do Norte-CE, Brazil.
Lutz Kunzmann [lutz.kunzmann@senckenberg.de; ORCID: https://orcid.org/0000-0001-6445-3920 ], Senckenberg Natural History Collections Dresden, Königsbrücker Landstr. 159, 01109 Dresden, Germany.
Received 1 November 2023, accepted 8 May 2024, published online 17 September 2024.
Copyright © 2024 M.E.P. Batista et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
From about 630 extant conifer species, a minority is native to tropical regions, and the concentration of species in these regions is comparatively low (e.g., Farjon 2017). New Caledonia (Melanesian Islands, Southern Pacific) is an exception, being home to 44 endemic conifer species belonging to four families (Farjon 2017). Tropical conifers often grow as single individuals or small groups among angiosperm-dominated vegetation. During the Early Cretaceous, however, tropical paleoecosystems were still dominated by gymnospermous seed plants including conifers, although nascent flowering plants (angiosperms) had already started to diversify and prosper. One of the few Early Cretaceous paleoecosystems in tropical Gondwana where the diversity and paleoecology of tropical conifers can be studied comes from the Santana Group of the Araripe Basin in northeastern Brazil. In particular, the Crato Formation flora, found in the Crato Fossil Lagerstätte, comprises 45 macrofossil taxa of seed plants, including 14 conifer taxa belonging to three families (Kunzmann et al. 2021). In addition, 37 taxa of coniferous palynomorphs from four extant families and one extinct family have also been described (Kunzmann et al. 2023). The most abundant plant macrofossils from this Lagerstätte are coniferalean leafy twigs of Brachyphyllum that belong to either very abundant or rare fossil-species (Mohr et al. 2007; Batista et al. 2017, 2020; Kunzmann et al. 2023).
The fossil-genus Brachyphyllum was defined by Brongniart (1828), based on its distinct external morphology of coniferous foliage shoots, including spirally arranged scale leaves. The type species B. mamillare was later validly established by Lindley and Hutton (1836). According to the taxonomic concepts and nomenclatural rules prevailing at the time, Brachyphyllum was later emended by Harris (1979) to a form-genus. In contrast to Kendall (1947), who proposed including characters of the leaf cuticles in the generic concept, Harris (1979) omitted the anatomical characters from his diagnosis, making Brachyphyllum an artificial morphotype. The scale leaves of Brachyphyllum consist of basal cushions and small apical free parts, but the total height of the cushion and free leaf part does not exceed the width of the cushion (Harris 1979). This definition unambiguously separates Brachyphyllum from other coniferous leaf morphotypes, such as Pagiophyllum (Kendall 1947; Harris 1979). However, it can be difficult to assign larger, more complex foliage shoots to a specific genus because the ultimate and penultimate shoot portions can reveal leaves of the Brachyphyllum morphotype, while the leaves of thicker shoot portions often show characters of the Pagiophyllum morphotype (Batista et al. 2020; Noll and Kunzmann 2020). Additionally, the helical phyllotaxis can also vary, changing to decussate within the same shoot, as observed in Brachyphyllum sattlerae (Batista et al. 2020). Commonly, Brachyphyllum is considered a morphological entity that cannot be related to any particular fossil or extant conifer family (Seward 1895; Kendall 1947; Harris 1979; Kunzmann et al. 2004; Batista et al. 2020). Where additional anatomical characters, such as the epidermal cell structures of the leaves and wood anatomy of the axes, can be observed in Brachyphyllum, the individual species may indeed have been attributed to different conifer families (Harris 1979). Consequently, various species of Brachyphyllum have been placed in the Araucariaceae, Cheirolepidiaceae, and Cupressaceae, as well as the Taxodiaceae and Podocarpaceae (Harris 1979; Alvin 1982; Stockey 1982, 1994; Van der Ham et al. 2003; Kunzmann et al. 2004; Passalia 2009; Du et al. 2013; Batista et al. 2017, 2020, 2021). Although the form-genus concept was abandoned in paleobotanical nomenclature in 2012 (“Melbourne Code”), the generic concept of Brachyphyllum is still widely accepted and commonly used for the sterile foliage of Mesozoic conifers.
So far, three Brachyphyllum species have been reported from the Araripe Basin (Duarte 1985; Kunzmann et al. 2004; Batista et al. 2017, 2020). Most of the fossil materials are well preserved, revealing information on the epidermal cell structure of the leaves and/or the wood anatomy of the axes, making it possible to assign these fossil-species to a conifer family, i.e., Brachyphyllum obesum (Kunzmann et al. 2004; Batista et al. 2017) and Brachyphyllum sattlerae (Batista et al. 2020) from the Crato Formation to the Araucariaceae. However, Brachyphyllum castilhoi, first described from the Romualdo Formation (Duarte 1985) and later also reported from the older Crato Formation (Martill et al. 2007), has not yet been assigned to a higher taxonomic level due to its lack of distinct anatomical characters. Therefore, this fossil-species has remained enigmatic, and even its taxonomic relationship with conifers has been called into question. The attribution of this fossil plant to Brachyphyllum was based on the helical arrangement of its scale leaves (Duarte 1985), although it differs from other conifers in this fossil-genus, including other species from the Araripe Basin, due to its characteristic claviform lateral shoots, which are comparatively thick and not branching (Duarte 1985; Kunzmann et al. 2004; Batista et al. 2017, 2020). More slender and thinner ultimate shoot portions are unknown from this rare fossil-species.
Considering the need for more detailed studies on B. castilhoi, we revisited the original material from the Romualdo Formation and have described new specimens from the Romualdo and Crato formations, herein presenting novel anatomical characteristics of the stem and leaf epidermis. Based on these new data, we have proposed separating this fossil-species into a new fossil-genus Leliacladus gen. nov., and have reconsidered the family assigment of Leliacladus castilhoi comb. nov.
Institutional abbreviations.—DBAV-UERJ, Departmento de Biologia Animal e Vegetal, University of the State of Rio de Janeiro, Rio de Janeiro, Brazil; MCNHBJ, Museu de Ciências Naturais e de História Barra do Jardim, Jardim, Ceará, Brazil; MMG PB, Paleobotanical collection of Museum of Mineralogy and Geology, Senckenberg Natural History Collections Dresden, Germany; MPPCN, Museu de Paleontologia Plácido Cidade Nuvens; Santana do Cariri, Ceará, Brazil.
Nomenclatorial acts.—The nomenclatural acts included in this work has been registered in Plant Fossil Names Registry (PFNR).
Geological setting
The Araripe Basin is an intracontinental basin in northeastern Brazil. It bears two of the most important fossil localities in the Mesozoic of equatorial Gondwana, namely the terrestrial Crato Fossil Lagerstätte and the marine to terrestrial Romualdo Fossil Lagerstätte (Fig. 1) (Martill 2007; Vila Nova et al. 2011; Nascimento et al. 2023). Both formations belong to the Lower Cretaceous Santana Group. Due to their extraordinary wealth of exceptionally well-preserved plant and animal fossils, the two localities are considered Konservat Lagerstätten (Martill 2007), protected by the establishment of the Araripe UNESCO Global Geopark. The Mesozoic Araripe Basin developed on the Neoproterozoic terrain of the Borborema Province and occupies an area of ~8.000 km² in the states of Ceará (South), Pernambuco (Northeast), and Piauí (East corner) (Fig. 1; Assine 2007; Fürsich et al. 2019). The origin and sedimentary history of the basin are closely related to the breakup of western Gondwana and the opening of the South Atlantic during the Jurassic and Cretaceous.
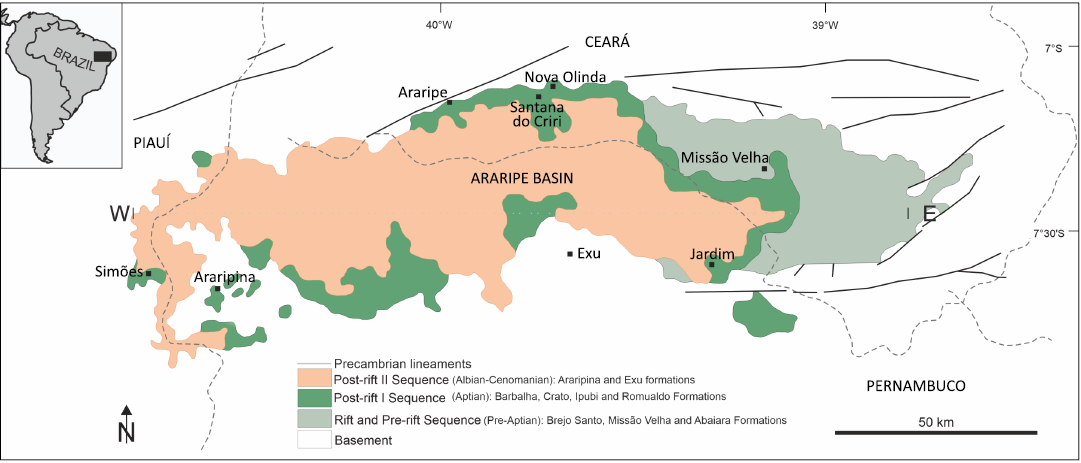
Fig. 1. Geographical location of the Araripe Basin and its main geomorphological features (modified from Barreto et al. 2020).
The sediments of the Santana Group represent the post-rift 1 tectonosedimentary sequence (Neumann and Assine 2015). The group is divided into four formations, (from oldest to youngest) the Barbalha, Crato, Ipubi and Romualdo (Corecco et al. 2022). During the late Barremian to Aptian, the Araripe Basin experienced a transition from an entirely terrestrial to a predominantly marginal marine paleoenvironment proximal to the gradually widening South Atlantic basin. The topographic depression was initially filled with terrestrial sediments deposited on alluvial plains and in freshwater lakes and ephemeral deltas (Nascimento et al. 2023). The first marine ingressions from the early South Atlantic occurred during the deposition of the Batateira Beds of the Barbalha Formation, which underlies the Crato Formation (Fauth et al. 2023). The latter formation represents mainly continental lacustrine deposits. The so-called Crato lake is currently understood to have been a freshwater lake that experienced partial and local saline conditions caused by the equatorial hot and semiarid paleoclimate (e.g., Nascimento et al. 2023 and references therein). The semiarid and arid paleoclimate led to the development of evaporites in the subsequent Ipubi Formation, which represents a transitional marine-terrestrial paleoenvironment (e.g., Lúcio et al. 2020). Eventually, during the deposition of the Romualdo Formation, the Araripe Basin became part of an epicontinental shallow marine corridor under predominantly tropical and semiarid conditions. For more detailed geological and lithostratigraphic information on the Santana Group and the entire basin see, e.g., Coimbra and Freire (2021).
The Crato Formation consists mainly of lacustrine deposits, comprising gray and dark brown to black shales and light gray to light brown laminated limestones. The laminated limestones of this formation, which contain a diverse and abundant fauna and flora, have been divided into six units (C1–C6), which refer to successive phases of lake development (e.g., Nascimento et al. 2023). The Romualdo Formation consists of marine shales, marls, and limestones that contain abundant carbonate concretions that formed around various vertebrate and plant fossils (e.g., Fara et al. 2005; Vila Nova et al. 2011). The stratigraphic ages of the Santana Group formations are under reconsideretion due to recent new results (Lucio et al. 2020; Melo et al. 2020; Fauth et al. 2023). The age of the Crato Formation was considered to be late Aptian to early Albian based on its palynomorph assemblages (e.g., Batten 2007). Based on the presence of the planktonic foraminiferal genus Leupoldina, Fauth et al. (2023) have indicated an early Aptian age for the Barbalha Formation. Lucio et al. (2020) published an early Aptian age for the black shale of the Ipubi Formation, and Melo et al. (2020) have demonstrated a late Aptian age for the Romualdo Formation, based on new foraminiferal, ostracod and microfacies data. Thus, it can be preliminarily assumed that the Crato Formation most likely represents a relatively short time span in the early Aptian (for more detailed discussion, see Gobo et al. 2023).
Material and methods
The type material of Leliacladus castilhoi (Duarte, 1985) gen. nov. et comb. nov. from the Romualdo Formation was re-studied, and new material from the Romualdo and Crato formations has been additionally described.
The exact provenance of the holotype of L. castilhoi (Duarte, 1985) gen. nov. et comb. nov. (DBAV-UERJ 93 Pb) remains unconfirmed. It was donated by the geologists Moacyr Castilho and Benedito Alves without any information on the sampling locality. Unfortunately, both passed away before the fossil-species was described (Duarte 1985). However, proof of it coming from the Romualdo Formation has been confirmed by the carbonate matrix adhering to the specimen. Two further specimens, collected in 2001 by staff of the MCNHBJ in the Jardim municipality, from the same formation (MCNHBJ 171 and MCNHBJ 176) have recently been identified and confirm the lithostratigraphic position of the holotype. All the Romualdo Formation specimens were three-dimensionally preserved in carbonate nodules (concretions) typical of this formation (Martill 2007).
Two specimens are available from the Crato Formation. MPPCN PL 6752 was collected from Unit C5 of the lacustrine limestones (sensu Neumann and Cabrera 1999) from an outcrop of the Crato Formation at the Antônio Finelon Mine (S 07°07′22.5″ W 39°42′01″) in Nova Olinda municipality, Ceará State. Specifically, it came from 5.74 m from the top of the carbonate section in the outcrop. The plant fossil was associated with specimens of the common fish taxon Dastilbe sp. MMG PB SAK 59 has previously been figured in Martill et al. (2007: fig. 19.5A), where it was noted as a rare conifer of unknown affinity, with no description given. It came from an exposure in one of the commercial limestone quarries near Nova Olinda, but the exact quarry the sample came from is unknown. The lithology of the sample is similar to that of the C5 limestone unit. Both of the Crato Formation specimens are sterile leafy branches. They are almost three-dimensionally preserved and embedded in a matrix of yellowish finely laminated limestone. The original plant tissues underwent iron oxide replacement during fossilization, which retained their anatomical details (Nascimento et al. 2023: fig. 7).
MPPCN PL 6752 was mechanically prepared in the Laboratory of Paleontology at the Universidade Regional do Cariri (LPU-URCA) to remove the sediment that covered parts of the fossil. MMG PB SAK 59 also underwent mechanical preparation, but it is unknown when this was done or by whom. For the Romualdo Formation specimens, there is no information on any mechanical preparation.
All the specimens were photographed using a Canon EOS Rebel T6 camera. The DBAV-UERJ 93 Pb was photographed under an Ez4w Leica stereomicroscope in the Semiarid Crustacean Laboratory (LACRUSE-URCA). Details of MMG PB SAK 59 were photographed using a Keyence VHX 7000 digital microscope using a stacked image routine.
Large portions the brittle iron oxide material could not be removed from the sediment for scanning electron microscope (SEM) studies of the Crato Formation specimens (MPPCN PL 6752, MMG PB SAK 59), unfortunately. These specimens were also too large for direct investigation in the SEM chamber under low vacuum mode, as used by Kunzmann et al. (2011).
For the SEM analysis, small fragments of MPPCN PL 6752 and DBAV-UERJ 93 Pb were removed and observed directly using a HITACHI SU3500 SEM in the URCA Electronic Microscopy Laboratory. Small fragments of MPPCN PL 6752, MCNHBJ 171, and MCNHBJ 176 were sputter coated with gold and observed under a TESCAN VEGA3 SEM in the Laboratório de Caracterização de Materiais (Universidade Federal do Cariri). Small fragments of scale leaves were removed from MMG PB SAK 59 at the Senckenberg Natural History Collections, Dresden, Germany. Sample pieces were mounted on stubs and sputtered with Au/Pd using a Polaron SC7640 sputter coater. These were studied and photographed using a Zeiss Evo 50 SEM under high-vacuum mode (20 kV).
Systematic palaeontology
Class Spermatophyta [no authorities specified according to APG IV]
Order Cupressales Link, 1829
Family unknown
Genus Leliacladus Batista & L.Kunzmann nov.
Figs. 2–9.
Plant Fossil Registry Number: PFN003297.
Etymology: In honour of Lelia Duarte (1930–2013), a pioneering Brazilian paleontologist and paleobotanist, author of Brachyphyllum castilhoi redescribed in this work.
Type species: Brachyphyllum castilhoi Duarte, 1985 ≡ Leliacladus castilhoi (Duarte, 1985) Batista & L.Kunzmann gen. nov. et comb. nov. (see below).
Diagnosis.—Foliated shoots, conspicuously thick, isotomous trifurcate and bifurcate branching mode. Leading branches cylindrical, frequently with cup-shaped apices, lateral branches claviform. Branches bear uniform Brachyphyllum-type adpressed leaves. Leaf epidermises smooth, with cyclocytic stomatal complexes abaxially, mostly arranged in loose and ill-defined rows. Xylem tracheid walls with abietinean radial pitting, one or two large pits per cross-field.
Leliacladus castilhoi (Duarte, 1985) Batista & L.Kunzmann gen. nov. et comb. nov.
Plant Fossil Registry Number: PFN003298.
Basionym: Brachyphyllum castilhoi Duarte, 1985: pl. 1: 1–3.
Epitype: MPPCN PL 6752, selected herein, Figs. 2, 4–7.
Plant Fossil Registry Number: PFN003299
Type locality: Antônio Finelon Mine (S 07°07′22.5″ and W 39°42′01″), Nova Olinda municipality, Ceará, Brazil.
Type horizon: C5 limestone unit, Crato Formation, Santana Group, Araripe Basin; lower Aptian, Lower Cretaceous (Assine et al. 2014; Nascimento et al. 2023; Gobo et al. 2023).
Emended diagnosis.—Foliated sparsely branched shoots, conspicuously thick; leading branches cylindrical and straight, lateral branches claviform, arranged in two ranks; isotomous trifurcate and bifurcate branching modes. Main axes either terminate into slightly tapering rounded apices or into widened shallow cup-shaped apices. Branches completely and densely covered by uniform Brachyphyllum-type leaves, helical phyllotaxis. Nine and 10 contact parastichies clockwise and counterclockwise on the leading branches, and seven contact parastichies clockwise and counterclockwise on the claviform branches. Free apical leaf parts very small and adpressed, margin frilled. Leaf epidermises smooth, cyclocytic stomatal complexes on abaxial side, randomly arranged in apical and central parts, in loose and ill-defined rows at the base. Xylem tracheid walls with areolate pits, one or two large pits per cross-field. Uniseriate rays, one to three cells high.
Our emendation of the specific diagnosis encompasses characters that are not observable in the holotype. To demonstrate these diagnostic characters, MPPCN PL 6752 is proposed as the epitype (Turland et al. 2018).
Description.—In order to demonstrate that all the specimens from the two formations belong to a single fossil-species, the materials from the formations are described separately before being compared.
Crato Formation material: MPPCN PL 6752 and MMG PB SAK 59 represent portions of larger shoot systems and consist of differently shaped sterile foliated axes that exhibit sparse branching (Figs. 2, 3). These branches are composed of (i) main cylindrical long axes of two orders with conspicuous widths, and (ii) shorter lateral cup-shaped (claviform) branchlets with remarkable widths that do not branch out. All axes are three-dimensionally preserved, but markedly flattened. Thinner and slender (pen-)ultimate-order axes or their putative scars are not observed. In MPPCN PL 6752, one main axis shows isotomous trifurcate branching, while another shows isotomous bifurcate branching and a third branching axis is probably not preserved or hidden in the host sediment (Fig. 2). MMG PB SAK 59 also exhibits seemingly trifurcate branching in one case, but this is less visible (Fig. 3).
The main cylindrical axes terminate into two types of apices: a slightly tapering rounded apex and a widened, shallow cup-shaped (caliculatous) apex (Fig. 2). The latter seems to be a supporting unit for larger organs, although these were not preserved. The claviform branchlets are borne in two ranks on the main axes (opposite phyllotaxis); the two remaining sides of the main axes show no scars of putatively removed or detached branchlets. The branchlets spread at acute angles and are often rather adpressed to the main axes. The claviform branchlets have their widest diameter approximately in the middlle or after two-thirds of their entire length, while they markedly taper towards their bases and apices. The latter are obtuse-rounded in shape.

Fig. 2. Conifer Leliacladus castilhoi (Duarte, 1985) Batista & L.Kunzmann gen. nov. et comb. nov., from Aptian, Lower Cretaceous, Crato Formation, Araripe Basin, Ceará State, NE Brazil. A. MPPCN PL 6752 showing the general morphology of the part (A1) and counterpart (A2). Detail of the leaves, possible base of a reproductive structure and the small branch emerging (arrow) (A3). Detail of the leaves and possible base of a reproductive structure (A4). Top view of possible base of a reproductive structure (A5).
MPPCN PL 6752 presents four larger leading axes, ranging 14–14.9 mm in width. The largest preserved cylindrical axis fragment is 215 mm long, but may have originally been longer, since the specimen is broken. The secondary (terminal) cylindrical branches are 85–109 mm in length and 11–12 mm in width, mostly 11.5 mm. Ten of the terminal branches carry a widened, shallow cup-shaped (caliculatous) apex. These structures show modified and condensed scale leaves. The cup-shaped structures are 16–21 mm wide markedly wider than their supporting axes. Among the claviform branchlets are obviously younger and ontogenetically older elements. Overall these measure 7–38 mm in length and 3–90 mm in width (Fig. 2). In MMG PB SAK 59, which is a 490 mm long shoot portion, the cup-shaped apices of the main axes are not present. Instead, one of the main secondary cylindrical branches exhibits a slightly tapering obtuse-acute apex. The eight claviform branchlets are 17–50 mm in length and 9–16 mm in width.
All the branches are densely covered by scale leaves in a helical phyllotaxis. The dense arrangement does not vary between the older cylindrical and younger claviform axes (Figs. 2A2–A4, 3A2, A3). The leaves are exclusively of the Brachyphyllum-type, and do not transition to the Pagiophyllum type on the main axes, i.e., to leaves that are much longer than wide. The leaves are differently preserved; complete leaves are rare while abraded leaf cushions or even cushion marks are more commonly present. There are no leaf scars pointing to leaf abscission. In the secondary cylindrical branches with cup-shaped apices, there are nine and 10 contact parastichies arranged in a clockwise direction and the same numbers arranged in a counterclockwise direction. On the claviform branches, there are seven clockwise and seven counterclockwise contact parastichies (Figs. 2A4, 3A2). The leaves comprise a large cushion and a very small triangular free apical part; the entire abaxial surface is rhombic in outline. The leaf bases are acute and the apices are acute-obtuse, but both are almost symmetrical in shape (Figs. 2A4, 3A3). The apices only slightly overlap the neighboring leaves (Fig. 3A3). The free leaf parts are 0.2–0.3 mm long. The leaf blades are markedly thick (coriaceous), tapering to a thin margin consisting of a rim of elongated epidermal cells oriented almost at right angles to the margin. The rim is ~0.05 mm wide. The cushions and cushion marks present on the main branches measure 2.5–3.0 mm in width and 2.5–3.1 mm in length. However, they are mostly 2.6 mm wide and 2.6 mm long. On the slightly tapering obtuse-acute apices of the main axes, the sizes of the leaves gradually decrease. The leaves of the cup-shaped apices are larger than the leaves of the branches, measuring, on average, 3.1 mm in width and up to 3.4 mm in length (Fig. 2A3).

Fig. 3. Conifer Leliacladus castilhoi (Duarte, 1985) Batista & L.Kunzmann gen. nov. et comb. nov., from Aptian, Lower Cretaceous, Crato Formation, Araripe Basin, Ceará State, NE Brazil. A. MMG PB SAK 59 showing the general morphology (A1), claviform branches (arrows) and fish skeleton partially superimposed on one of the branches (A2), detail of the leaves (arrow pointing to the leaf apex) (A3) , frilled leaf margin (arrow) (A4), epidermis with stomata (arrow) (A5) and detail of stomata (arrow) (A6).
Leaf epidermis: The epidermal cell layer was preserved three-dimentionally and the cell luminae are filled with iron oxide. The cuticles are absent, and thus there is no potential microrelief of the outer cuticle surfaces visible. It was only possible to study the abaxial leaf surface; the remains of the adaxial surface could not be obtained.
The abaxial surface is characterized by a special distribution pattern of stomatal complexes. In the lower and central parts of the leaf, the stomata are arranged in loose longitudinal rows. In the central and apical leaf parts, the rows become ill-defined and the stomata are distributed more randomly and in a scattered fashion. No stoma directly adjoins another. There are always several ordinary epidermal cells between neighboring stomata of the same row and between the stomata of adjacent rows, as well as between the more scattered stomata in the apical parts. The stomatal complexes of the lower and central parts are longitudinally oriented, whereas the stomatal complexes of the apical part are more perpendicularly oriented in relation to the long leaf axis. The stomatal complex is generally cyclocytic with a ring of 5–6 subsidiary cells that seem to be very slightly sunken in a few cases (Fig. 3A5, A6). The subsidiary cells are isodiametric to short rectagnular (less than twice as long as wide) in shape, with straight anticlinal walls. The two guard cells are only faintly visible, but they are sunken. The ordinary epidermal cells are arranged in relatively well-defined longitudinal rows in the lower and central parts of the leaf, and more irregularly arranged in the apical part. In the former parts, the epidermal cells are elongated and subrectangular in shape, while in the latter part, they are shorter and often isodiametric in shape (4- to 6-sided). All the anticlinal walls are straight. Towards the leaf margin, the ordinary epidermal cells became more elongated again and are often very slightly curved in their longitudinal axes, diverging towards a rectangular orientation to the leaf margin. This arrangement of columnar marginal cells at a scarious leaf margin is known as “marginal frill” (Fig. 3A4). No trichomes, papillae or glands are observed on the visible leaf surfaces.
Stem: The xylem is homoxylic composed of tracheids with little to no intercellular spaces. In cross-section, the tracheids are rounded, square or pentagonal in shape, and arranged radially, without growth-rings. However, there are marks that look like growth rings. These are actually artifacts caused by cutting the sample with a knife to prepare to the SEM (Fig. 4A1, A2). The lumens are about 7.8 µm in diameter and the walls are about 5.3 µm thick. In longitudinal view, the tracheids are narrow and long, organized in an intrusive way, and exceed 900 µm in length (Fig. 4A3). The radial tracheid walls show well-separated uniseriate areolated pits (abietinean radial pitting). The pit opening diameter dimensions is ~2 µm (Fig. 4A4–A6). In some tracheids, the pits appear simple, but this may have resulted from taphonomic processes (Fig. 4A7).
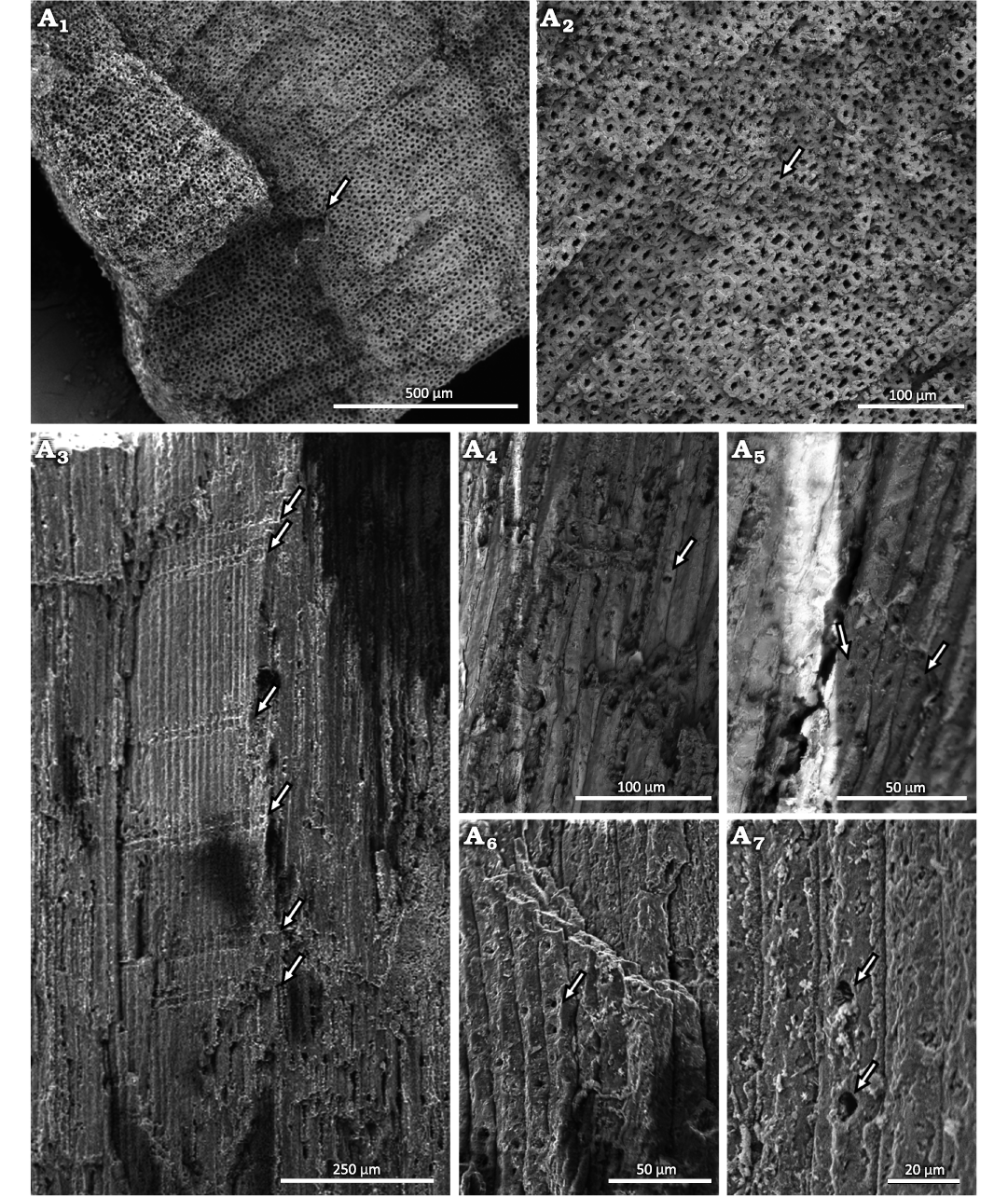
Fig. 4. Conifer Leliacladus castilhoi (Duarte, 1985) Batista & L.Kunzmann gen. nov. et comb. nov., from Aptian, Lower Cretaceous, Crato Formation, Araripe Basin, Ceará State, NE Brazil. Xylem of MPPCN PL 6752 in transverse section (A1, A2), showing radially organized tracheids with no intercellular spaces (arrow); the marks that look like growth rings in A1 are artifacts caused by cutting the sample with a knife to prepare the SEM. Longitudinal view of the tracheids showing abundant rays (arrows) composed of one and two cells in height (A3), tracheid with a simple-looking joint (arrow) (A4), bordered pits (arrows) (A5, A6) and tracheids with simple-looking pits (arrows) (A7).
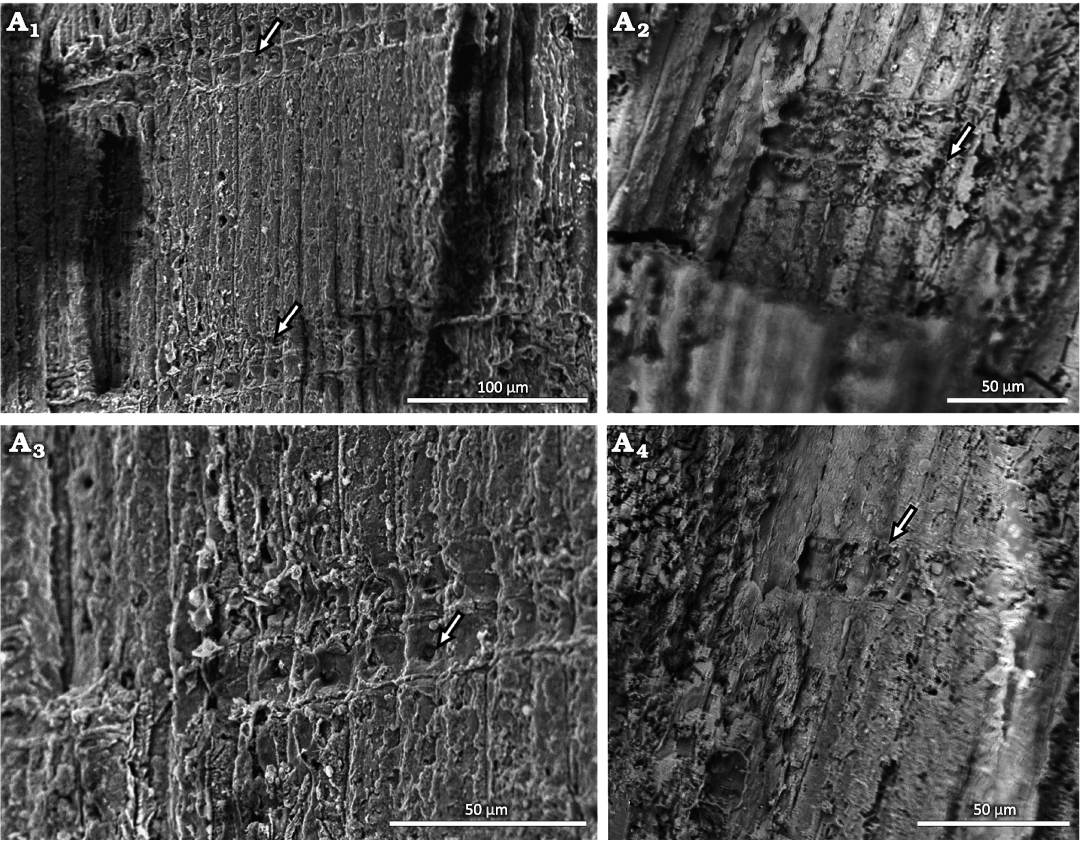
Fig. 5. Conifer Leliacladus castilhoi (Duarte, 1985) Batista & L.Kunzmann gen. nov. et comb. nov., from Aptian, Lower Cretaceous, Crato Formation, Araripe Basin, Ceará State, NE Brazil. Radial longitudinal SEM view of MPPCN PL 6752, showing details of two cross-fields, each with one large pit (arrows) (A1), cross-field with three cells high (arrow) (A2), one pit per cross-field (arrow) (A3), cross-field with one cell high and two pits (arrow) (A4).
Uniseriate rays are visible in radial and tangential sections (Figs. 4A3, 5, 6A1–A3). These vary from one to three cells in height, with two cells being the most frequent. The rays are ~6 µm in width and 15–18 µm in height. Rays with thicker walls are visible (Fig. 6A3). The cross-field pitting is similar to the podocarpoid type, where usually only one pit in the cross-field. In our material, there are usually one or two simple and large pits per cross-field, measuring 3.7 µm in average diameter (Figs. 5A1, A3, A4). In one case, a branch attachment scar can be seen. The scar is ~227 µm high and 119 µm wide (Fig. 6A4). The tracheids in this area have a lumen diameter of ~4 µm and walls ~3 µm thick.
Shallow cup-shaped structures: The anatomical details of these structures show little vascularization and the presence of a large number of parenchyma cells (Fig. 7A1–A4). In the vascular strands, the xylem is well organized (Fig. 7A5). In longitudinal view, the tracheids show scalariform thickenings in the inner part of an isolated cell next to the parenchyma cells (Fig 7A6). The parenchyma cells are irregular in shape, ranging from rounded to rectangular. The more rounded ones have diameters of 20–50 µm, while the rectangular ones average 75 µm in length and are 20–35 µm wide.
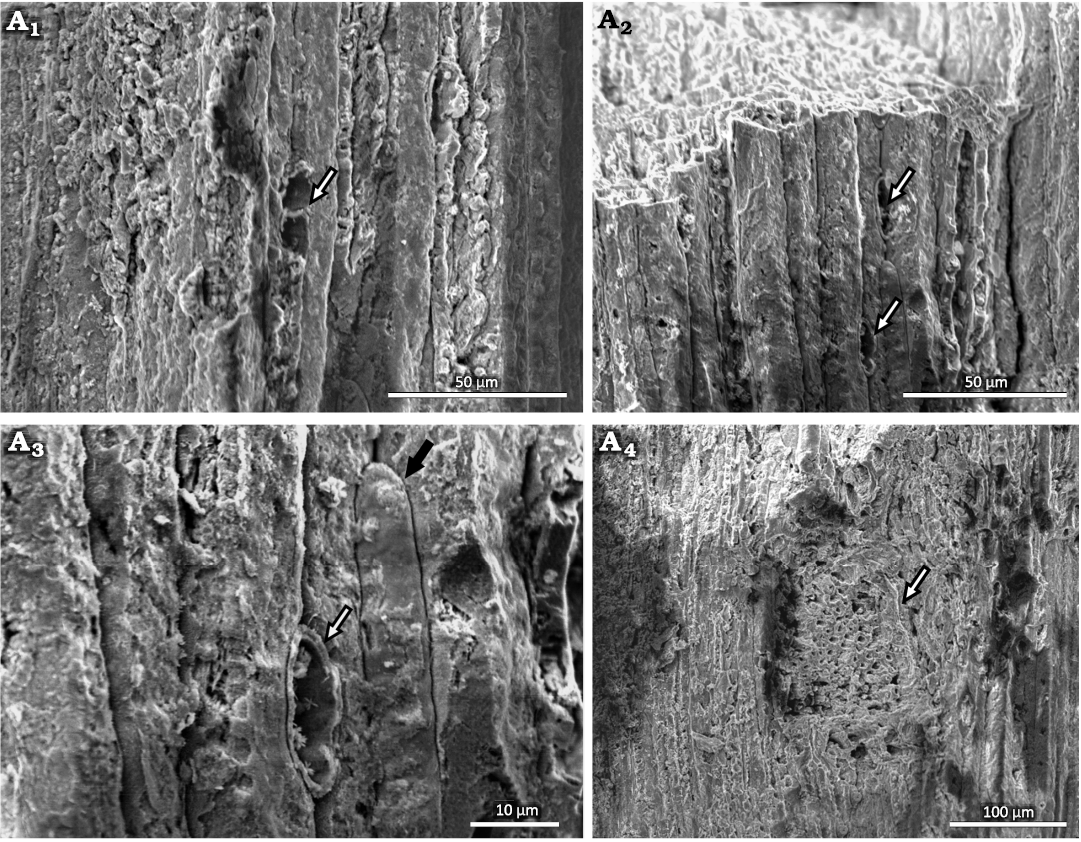
Fig. 6. Conifer Leliacladus castilhoi (Duarte, 1985) Batista & L.Kunzmann gen. nov. et comb. nov., from Aptian, Lower Cretaceous, Crato Formation, Araripe Basin, Ceará State, NE Brazil. Longitudinal tangential SEM view of MPPCN PL 6752, showing a ray with two cells high (arrow) (A1), two rays with one cell high (arrows) (A2), ray with thickened walls (white arrow) and a tracheid with an intrusive socket (black arrow) (A3), branch base (arrow) (A4).
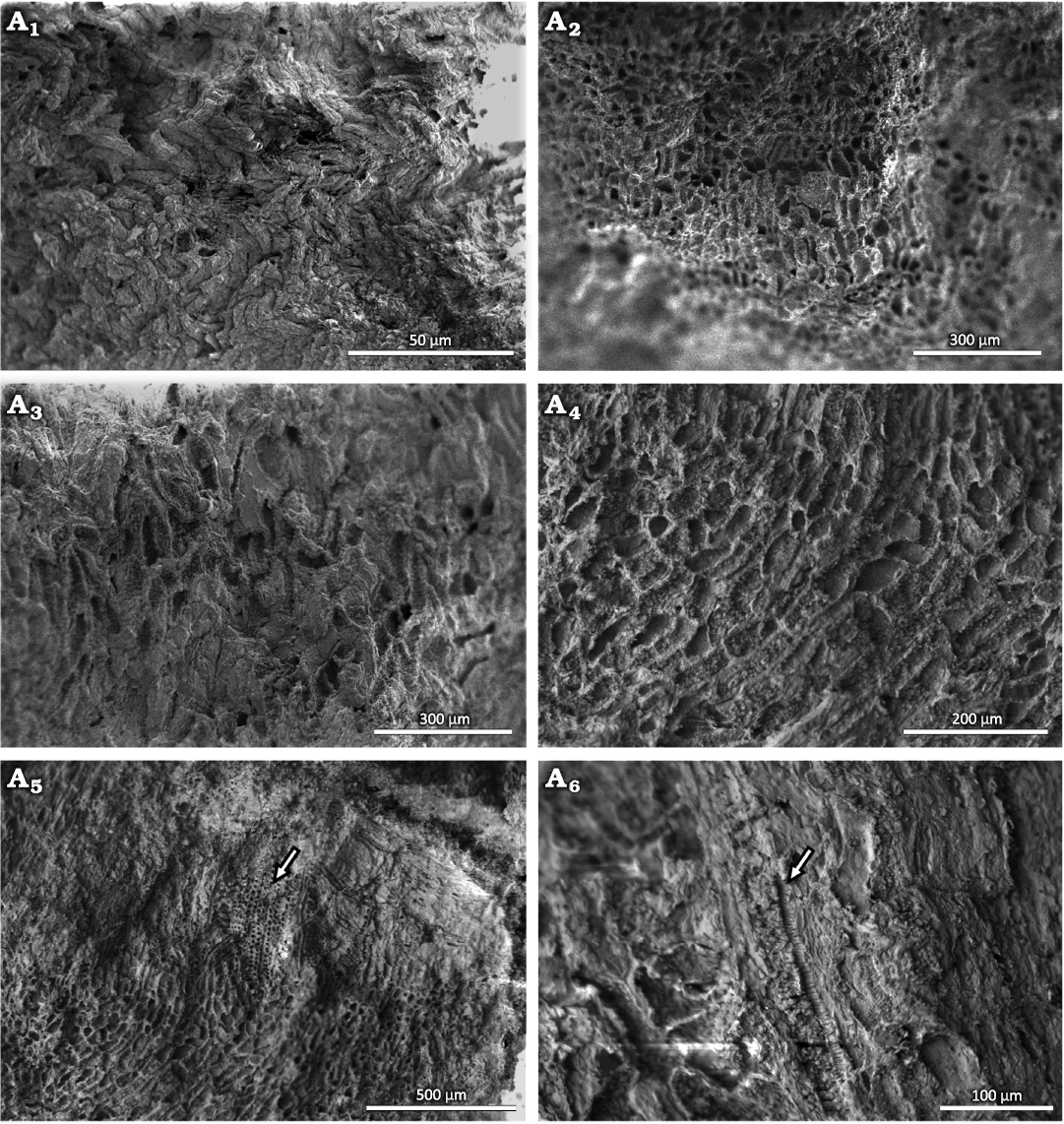
Fig. 7. Conifer Leliacladus castilhoi (Duarte 1985) Batista & L.Kunzmann gen. nov. et comb. nov., from Aptian, Lower Cretaceous, Crato Formation, Araripe Basin, Ceará State, NE Brazil. Possible base of reproductive structures in MPPCN PL 6752 viewed with the SEM showing general appearance of irregularly shaped parenchyma cells seen from the outside (A1), general appearance of parenchyma cells seen from the inside (A2–A4), xylem next to parenchyma cells (arrow) (A5), and tracheid with scalariform thickenings (arrow) (A6).
Romualdo Formation material: DBAV-UERJ93Pb (holotype), MCNHBJ 171, and MCNHBJ 176 represent portions of larger branches that exclusively consist of differently shaped foliated axes (Fig. 8). These fragments are composed of two orders of branching. The branches are remarkably wide and three-dimensionally preserved, although somewhat flattened. Thinner and slender ultimate or penultimate order axes, or their putative scars, are not visible in the MCNHBJ specimens, although the diagnostic ultimate-order claviform axes are present in the holotype DBAV-UERJ 93 Pb (Fig. 8A1).
DBAV-UERJ 93 Pb is a simple, non-furcating portion of a cylindrical main branch that is 190 mm long and 20 mm wide (Fig. 8A1). Seven distichously arranged pairs of claviform lateral branchlets are preserved and attached at acute angles to the main axis. The remaining surface of the main axis shows no scars from additional, putatively detached claviform branchlets.
MCNHBJ 171 shows trifurcated branching, although the lateral branches are not preserved, with only the bases at the main branch being present (Fig. 8B). From the main branch, another branch emerges above the trifurcation node. The main axis fragment is 169 mm long, but may have been longer in life, since the specimen is broken. It is 180 mm wide at the widest point and 130 mm wide at the branch base. This specimen is partially flattened, especially in the outermost area, where the leaves are preserved. The secondary and ultimate claviform branch is 100 mm long, 10 mm wide at the widest point and 8 mm wide at the base.
MCNHBJ 176 shows trifurcated branching, with two larger branches visible and a third branch being partially covered by sediment, but with its base exposed (Fig. 8C). There is a fourth branch, but its base is not preserved. Of the three branches that emerge from the same point, only one is fully exposed, and this is 145 mm long, 13 mm wide at its widest point, and 9 mm wide at its base. The other two branches are embedded in the sediment, so it is not possible to measure their lengths. Their widths at the base are 7 mm.
All branches of the three specimens are densely covered by scale leaves in a helical phyllotaxis. In DBAV-UERJ 93 Pb~10 contact parastichies, arranged in clockwise and anticlockwise directions, are visible. In MCNHBJ 171 and MCNHBJ 176, only the leaf parastichies of the main branches can be counted. These are arranged in 7–9 contact parastichies in clockwise and anticlockwise directions.
The dense arrangement of scale leaves does not vary between axes. The leaves are almost exclusively of the Brachyphyllum-type, although some leaves of the Pagiophyllum type occur on the main axes. The leaves are rhombic in abaxial outline, consisting of a large cushion and a tiny free apical part adpressed to the axis (Fig. 8A2, A3). The bases are acute and the apices acute-obtuse, but both are almost symmetrical in shape. They have relatively smooth, although somehow shriveled, surfaces, a few cases showing a narrow longitudinal keel on the abaxial side (Fig. 8A3). The free triangular apical leaf part only slightly overlaps or does not overlap the neighboring leaf. The leaves are generally isodimensional in length and width (up to 3 mm), although a few leaves are slightly longer than they are wide, being 4 by 3 mm, without a marked change in their shapes compared to the majority of the isodimensional leaves. The leaves that are longer than the width of the cushion fit the diagnostic characters of the Pagiophyllum type leaf. The free leaf parts are 1.5–2 mm long. The leaf blades are markedly thick (coriaceous).

Fig. 8. Conifer Leliacladus castilhoi (Duarte, 1985) Batista & L.Kunzmann gen. nov. et comb. nov., from Aptian, Lower Cretaceous, Romualdo Formation, Araripe Basin, Ceará State, NE Brazil. A. Holotype DBAV-UERJ 93 Pb, unbranched main axis with 7 pairs of lateral claviform branchlets (A1), detail of the leaves (A2, A3). B. MCNHBJ 171, part (B1) and counterpart (B2) of a smaller trifurcately branching shoot fragment. C. MCNHBJ 176, trifurcately (?) branched shoot fragment.
Leaf epidermis: Only DBAV-UERJ 93 Pb and MCNHBJ 176 show preserved epidermal features. Due to the preservation mode of the fossils, the epidermal cell layer is also three-dimentionally preserved and the cell luminae are filled with calcite. The cuticles are not preserved and so any potential microrelief on the outer cuticle surfaces is not visible. Only the abaxial leaf surface was visible, while the adaxial surface was not obtained.
The epidermal cell structure of the leaves could be only studied by SEM. The differently shaped ordinary epidermal cells are rectangular, rounded or oval in outline (Fig. 9A1–A3, B). They are ~10.6 µm wide and 22 µm long when rectangular, and arranged in longitudinally oriented rows at the leaf bases. The stomatal complexes are randomly oriented from the central part of the leaf to the apex, and distributed in ill-defined rows at the base of the leaf (Fig. 9A1–A3, B). The complexes do not share any subsidiary cells and are always separated from each other by at least two ordinary epidermal cells. The cyclocytic stomatal apparatuses measure between 33 and 40 µm in width and between 57 and 72 µm in length. Five or six subsidiary cells surround the slightly sunken guard cells. The stomatal apertures at the bases of the leaves are oriented longitudinally, although in rare cases, they can also be more or less obliquely oriented, as is mainly the case from the central part to the apex. The subsidiary cells are isodiametric at the poles and short rectangular (less than twice as long as wide) in shape on the lateral sides of the complexes and they show straight anticline walls. No trichomes, papillae or glands are visible.
Stem: It was only possible to observe the anatomical characteristics of the stem in MCNHBJ 176 and MCNHBJ 171. Both specimens have poorly preserved xylem structures, which only show the general shape of the tracheids (Fig. 9B2, C). No other features could be observed.
Stratigraphic and geographic range.—Aptian, Lower Cretaceous; Crato and Romualdo formations, Santana Group, Araripe Basin; NE Brazil (Assine et al. 2014; Nascimento et al. 2023; Gobo et al. 2023).
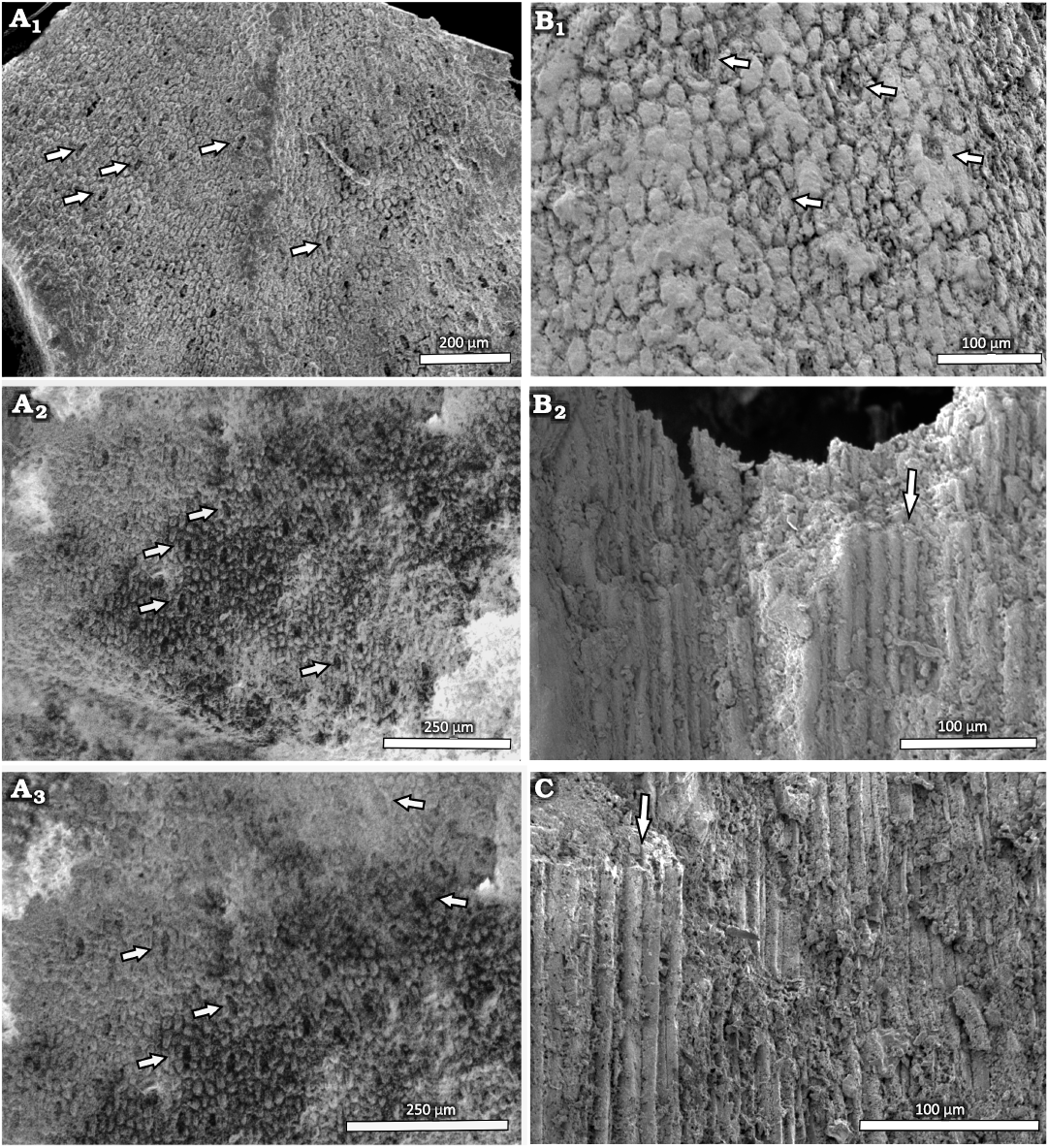
Fig. 9. Conifer Leliacladus castilhoi (Duarte, 1985) Batista & L.Kunzmann gen. nov. et comb. nov., from Aptian, Lower Cretaceous, Romualdo Formation, Araripe Basin, Ceará State, NE Brazil. A. Epidermis of DBAV-UERJ 92 Pb cyclocytic stomata in central part of the leaf (A1; arrows), basal part of the leaf with stomata (A2, A3; arrows). B. Anatomy of MCNHBJ 176, showing the epidermis with stomata (arrow) (B1) and longitudinal section of xylem with tracheids (arrow) (B2). C. Longitudinal section of xylem of MCNHBJ 171 with tracheid (arrow).
Discussion
Comparison between the Crato Formation and Romualdo Formation specimens.—Based on overall gross-morphology, there is no doubt that the differently preserved specimens from the two formations belong to the same fossil-genus. All of the specimens exhibited conspicuously thick cylindrical leading axes and claviform lateral branchlets, arranged in two ranks. Slender branchlets, or their putative detachment scars, were not observed. Most of the branches are densely covered by the same uniform Brachyphyllum-type leaf. A minor difference between the specimens, i.e., the rare occurrence of the leaves of the Pagiophyllum morphotype, may have been due to individual variability in leaf morphology, which might be expected in fossil plants with predominantly Brachyphyllum-type leaves.
Major differences between the specimens were observed in the preserved branching modes. The leading axis of the comparatively small shoot portion of the holotype did not branch. Specimens MPPCN PL 6752 from the Crato Formation and MCNHBJ 176 and MCNHBJ 171 from the Romualdo Formation exhibited an isotomous trifurcate branching mode of the axes. The two specimens from the Crato Formation comprised larger shoot portions that also demonstrated dichotomous and anisotomous branching modes. The smaller of the Crato Formation specimens, MMG PB SAK 59, did not unambiguously exhibit a particular branching mode, with both isotomous (tri- and bifurcate) and anisotomous branching possible. Another difference is the occurrence of the shallow cup-shaped structures at the terminal ends of the axes. These were only seen in the larger Crato Formation specimen. This might be simply because the other specimens are smaller. It was also clear from both Crato specimens that not all the ends of the leading axes bore cup-shaped structures. Some ends of the axes are slightly tapering and rounded. It is likely that all the Romualdo Formation specimens represent smaller shoot portions of the axes carrying non-cup-shaped structures. Alternatively, if these structures are related to any reproductive organs, as discussed below, it might be assumed that the fossil plants represent both fertile and sterile portions of the shoots. However, this is only speculation. Compared to the holotype from the Romualdo Formation, lateral and terminal claviform branchlets in the two Crato Formation specimens are rare. However, the positions and arrangement of the claviform branchlets on the Crato Formation specimens suggested that some claviform branchlets had probably become detached. In addition, although the Crato Formation specimens did not unambiguously show the arrangement in two ranks, as can be seen in the holotype, the absence of detachment scars on the visible surfaces of the main axes clearly suggests to the same distichous arrangement. In conclusion, these gross-morphological differences could mainly be regarded as a relation to the size of the individual portions of the shoots. Consequently, we interpret these differences as not being meaningful enough to warrant the fossils from the two formations being placed in two distinct taxonomic units.
The leaf epidermal cell structures are very similar between the specimens from the two formations. The abaxial sides showed abundant cyclocytic stomatal complexes, loosely and randomly arranged in the median and apical parts of the leaves, but forming ill-defined rows in the basal part. All the epidermises are non-papillate and bore neither trichomes nor glands.
Anatomical details relating to the wood were only visible in one Crato Formation specimen, MPPCN PL 6752. Thus, xylotomical characters could not be used to distinguish between the Crato and Romualdo formations specimens.
Overall, none of the studied characters provided evidence for the presence of a distinct fossil-species in the Crato Formation. However, because not all characters of the fossil plant were represented in each specimen, e.g., reproductive organs, we cannot completely rule out the possibility of the fossils from the two formations being separate fossil-species or subspecies. Until new and better preserved material is found, we opted to include all the specimens in one fossil-species.
Fossil plant habit.—The relatively thick and woody axes of Leliacladus castilhoi (Duarte, 1985) Batista & L.Kunzmann gen. nov. et comb. nov., suggest a shrubby or tree-like habit with a rather rigid physical appearance. In particular, MPPCN PL 6752 exhibits a larger portion of a shoot system that is markedly sparsely branched, even if a number of potential lateral claviform branchlets are lacking. The branching mode is characterized by two features: (i) acute angles between the main and lateral axes; and (ii) partially isotomous trifurcate branching. Overall, these few characteristics suggest a candelabra-like growth habit, at least of the preserved shoot portion. The shallow cup-shaped structures could be interpreted as supports for reproductive organs, possibly cones. They could also be the basal most parts of cone-like structures, although there is currently no positive evidence for either.
Systematic position and affinity.—Although previously sometimes called into question, Leliacladus castilhoi (Duarte, 1985) Batista & L.Kunzmann gen. nov. et comb. nov., is a conifer based on a combination of leaf gross-morphology, leaf epidermal features, and xylotomical characters. The characteristics of the wood, such as the homoxylic vessel-less xylem formed by tracheids, point towards a gymnospermous seed plant, albeit some basal angiosperms also have vessel-less xylem (Carlquist and Schneider 2001). However, the other vegetative characters of this fossil plant are clearly distinct from those of angiosperms. Among gymnosperms, shoots with Brachyphyllum type leaves, in combination with a cyclocytic stomatal complex, are only known from conifers.
This fossil conifer is very distinct from other conifers with Brachyphyllum-type leaves in having no slender (pen-)ultimate shoots. The claviform branchlets are obviously the youngest (ultimate) shoot portions but they are about 14 mm thick. Thus, this conifer differs, e.g., from B. obesum and B. sattlerae from the Crato Formation, both of which represent the typical Brachyphyllum morphotype (Kunzmann et al. 2004; Batista et al. 2017, 2020). Larger shoot portions of B. obesum exhibit thick leading branches similar to the cylindrical axes of our fossils, but the lateral twigs also have the slender ultimate branches (Kunzmann et al. 2004; Batista et al. 2017).
We propose the separation of L. castilhoi (Duarte, 1985) Batista & L.Kunzmann gen. nov. et comb. nov., from Brachyphyllum and the introduction of a new fossil-genus. The general habit of the shoot portions, with their bifurcated and trifurcated branching pattern, and the claviform lateral branchlets are unique among fossil conifers and are not covered by the emended diagnosis of Brachyphyllum (Harris 1979). In addition, the anatomical characters of the leaves and woody axes also fall outside this diagnosis.
In doing this, we applied the same taxonomic approach as proposed for the fossil conifer Duartenia araripensis from the same locality (Mohr et al. 2012). Duartenia was not included in a wood fossil-genus or into Brachyphyllum, even though its characters would allow for such affiliation. Instead, a whole-plant concept was applied, establishing a separate genus with a probable relationship to the Cheirolepidiaceae.
Even though L. castilhoi. has not been assigned to a family to date, we can discuss its systematic relationships based on our new anatomical data. Axes with densely and helically arranged scale leaves are know from several families, e.g., Araucariaceae, Cheirolepidiaceae, Cupressaceae, and Podocarpaceae. It should be noted that other macrofossil and microfossil records from the Santana Group have indicated the presence of these families in the Crato Formation flora (Lima 1978; Kunzmann et al. 2023), where the most informative specimen of L. castilhoi came from.
However, leaf gross-morphology and phyllotaxis only provide superficial hints as to its family affinity. The leaf epidermal structures observed in our fossils revealed clear information about which familial affinities could be excluded. In fossil and extant Araucariaceae, only the genus Araucaria possesses Brachyphyllum-like foliage. In Araucaria, the stomatal complexes are arranged in numerous uniform longitudinal rows on the abaxial surfaces, and these complexes are in contact with the neighboring complexes in each row (e.g., Stockey and Atkinson 1993). This pattern is known from another Brachyphyllum species in the Crato Formation flora, B. obesum, which has thus linked to the Araucariaceae (Kunzmann et al. 2004; Batista et al. 2017). The type species of Brachyphyllum, B. squamosum from the Jurassic of Yorkshire, UK, has also been assigned to the Araucariaceae based on its leaf epidermal cell structure (Harris 1979). In the Cretaceous of the Czech Republic, the same fossil-species also has stomata distributed in well-defined lines, as is typical in the Araucariaceae (Kvaček 2007). The fossil wood of the Araucariaceae, which is commonly assigned to the fossil-genus Agathoxylon, is characterized by araucarian pitting, which is clearly distinct from the abietinean pitting in the radial tracheid walls of our specimen. In short, L. castilhoi can not be assigned to the Araucariaceae.
In the extinct and extant Cupressaceae species with helically arranged scale leaves, the stomatal complexes are arranged either in two longitudinal bands or in two patches, which is quite distinct from the pattern observed in our material (e.g., Florin 1931). The same is the case for the extant Podocarpaceae with similar leaf types (e.g., Florin 1931).
By contrast, shoots with helically arranged scale leaves and stomata randomly distributed over the adaxial and abaxial surfaces are characteristic of fossil-species of the Cheirolepidiaceae (e.g., Watson 1988). Brachyphyllum crucis from the Jurassic of Yorkshire has been treated as a member of the Cheirolepidiaceae based on its association with male cones containing in situ Classopollis pollen, which is the synapomorphic character of the family (Harris 1979; Watson 1988). Brachyphyllum patens from the Cretaceous of Belgium and The Netherlands (Van der Ham et al. 2003) has also been attributed to the Cheirolepidiceae mainly based on the random distribution of its stomata. At least from the central to apical parts of the abaxial side of the leaves in our material, there is a random distribution of stomatal complexes. However, many Cheirolepidiaceae species are distinguished by papillate epidermides, i.e., the papillae are frequently present on ordinary epidermal cells and on the subsidiary cells of stomatal complexes (e.g., Watson 1988). The latter is also the case for B. crucis and for members of the fossil-genus Watsoniocladus (Harris 1979; Watson 1988; Kvaček and Mendes 2021).
Another rather enigmatic conifer from the Crato Formation flora, Duartenia araripensis is probably related to the Cheirolepidiaceae based on its scale leaves and wood anatomy (Mohr et al. 2012). However, its Brachyphyllum-like leaves are too poorly preserved to be able to see any distinctive characters (Mohr et al. 2012). Interestingly, its wood shares anatomical characteristics with the wood of L. castilhoi, such as uniseriate rays with only one big pit, but differs in the lateral tracheid walls. Duartenia wood is characterized as a mixed protopinaceous type, also known as the brachyoxylean type (Boura et al. 2021), while L. castilhoi is abietinean type. Mohr et al. (2012) reported several similarities between Duartenia wood and the wood of frenelopsid members of the Cheirolepidiaceae. Frenelopsid conifers are characterized by segmented shoots with minute adpressed leaves arranged in whorls, and these leaves are often completely (suturelessly) fused (Watson 1988; Sucerquia et al. 2015, Batista et al. 2017). This foliage type is very distinct from the leaf morphology of our fossils.
According to Thévenard et al. (2022), the Cheirolepidiaceae are not characterized by a uniform type of wood. Several types of fossil wood, such as Agathoxylon, Brachyoxylon, Protocupressinoxylon, and Protopodocarpoxylon have been ascribed to Cheirolepidiaceae (Alvin et al. 1981; Francis 1983; Falcon-Lang et al. 2001; Thévenard et al. 2022). Most of these attributions were proposed because these woods occured associated at the same lithostratigraphic level as other isolated and disarticulated organs of the family, including as Classopollis pollen and leaf twigs of the frenelopsid group (Alvin et al.1981; Francis 1983; Falcon-Lang et al. 2001; Thévenard et al. 2022). The few records demonstrating articulation of confirmed Cheirolepidiaceae members with the wood mainly have the anatomical characters of Brachyoxylon (Thévenard et al. 2022). However, other records, such as Pseudofrenelopsis sp. from the Crato Formation flora, have a distinct wood anatomy (Batista et al. 2017). In short, our fossils share leaf gross-morphology and epidermal cell structure with some non-frenelopsid members of the Cheirolepidiaceae. Members of this family have produced different wood types, making taxonomic affiliations based only on wood anatomy difficult. However, when using a combination of characters, such as leaf gross-morphology, epidermal cell structure, and wood anatomy, a tentative taxonomic assignment of L. castilhoi might be possible.
With its abietinean radial pitting and its cross-field pitting with one or two pits, the wood anatomy of L. castilhoi is dissimilar to both Brachyoxylon and Protocupressinoxylon, which have mixed pitting. It is also dissimilar to Agathoxylon, which has araucarian pitting (Vera and Loinaze 2022; Thevenard et al. 2022). However, despite some Protopodocarpoxylon woods are mixed type (Biondi 1983; Esteban et al. 2006) other Protopodocarpoxylon woods are similar in that it has abietinean radial pits and cross-field pitting with one or two pits (Philippe et al. 2002). Thus, if Protopodocarpoxylon wood can be demonstrably related to the Cheirolepidiaceae, then L. castilhoi could be affiliated with the same family. However, this relationship has not been reliably proven to date.
Among the extant conifer families, L. castilhoi shares some anatomical characteristics with the wood of members of the Podocarpaceae, i.e., the cross-fields with one or two large pits, predominantly one pit per field (Richter et al. 2004; Esteban et al. 2023); the presence of predominantly uniseriate and sparse radial pits (abietinean type) (Esteban et al. 2023; Philippe and Bamford 2008); and the presence of thick-walled rays (Evert 2013). However, the possible bases of reproductive structures in L. castilhoi, i.e., the terminal shallow cup-like structures, suggest that they may have supported comparatively large organs, whereas in the Podocarpaceae, the seed producing organs are reduced and often have a fleshy epimatium, and the pollen-producing cones are even smaller (e.g., Farjon 2017).
In short, some morphological and anatomical characters of L. castilhoi fit with certain characters of some non-frenelopsid members of the extinct Cheirolepidiaceae and with members of the extant Podocarpaceae but none of these is clear evidence for assignment to one of these families. It seems that any progress in this respect will depend on new findings of unambiguous reproductive organs of our fossil plant.
Paleoecology.—Deposition of the Crato and Romualdo formations took place under arid to semiarid paleoclimatic conditions (Ribeiro et al. 2021; Lacerda et al. 2023), which were common in the Early Cretaceous in the equatorial part of northern Gondwana (e.g., Chumakov 1995). Fossil plants, as excellent paleoclimatic indicators, can support the general reconstruction of paleoclimatic and paleoenvironmental conditions for both formations during the Aptian. In the Crato Formation, there is a diversity of fossil plants with xeromorphic features, among which are several conifers, such as B. obesum, B. sattlerae, D. araripensis, and Pseudofrenelopsis capillata (Kunzmann et al. 2004, 2006; Mohr et al. 2012; Sucerquia et al. 2015; Batista et al. 2017, 2020, 2021, 2022). Only a few fossil plant species have been found in the Romualdo Formation to date, Pseudofrenelopsis salesii being one of these, which exhibits xeromorphic characteristics (Batista et al. 2018). The morphological and/or anatomical features of fossil plants can be interpreted in terms of adaptations to the presumed paleoclimate. For example, scales and fleshy leaves with sunken stomata, thick cuticles, lignified hypodermides, and xylem with thick-walled tracheal elements are the most commonly shared anatomical features in paleoenvironment where water is scarce (Kunzmann et al. 2004, 2006; Sucerquia et al. 2015; Bernardes-de-Oliveira et al. 2014; Batista et al. 2017, 2018, 2020). Additionally, Mohr et al. (2012) reported a distinct anisotomous branching pattern in D. araripensis that is only known from angiosperm trees growing in tropical arid environments. Thus, Duartenia has been interpreted as a drought resistent conifer.
Leliacladus castilhoi (Duarte, 1985) Batista & L.Kunzmann gen. nov. et comb. nov., has minute leaves with their tiny adaxial sides closely adpressed to the axis, and abaxial stomatal complexes with sunken guard cells. These characteristics could indicate a strategy to reduce water loss from the plant (Lewis 1972; Bernardes-de-Oliveira et al. 2014; Batista et al. 2017, 2018). In addition, thick-walled tracheids and reduced tracheid cell lumina in the wood allow greater hydraulic safety in periods of water scarcity (Bouche et al. 2014; Carlquist 1975; Sperry 1990; Batista et al. 2017). We observed a large number of parenchyma cells at the bases of the putative reproductive structures, which may be associated with water or nutrient storage. However, our expectation that the overall comparatively thick axes of our fossils might indicate specialized water-storage tissue, as seen in succulents, was not met. Nevertheless, the total absence of slender ultimate axes could be hypothesized as being a xeromorphic feature.
Conclusions
Our study confirms the presence of the enigmatic Leliacladus castilhoi (Duarte, 1985) Batista & L.Kunzmann gen. nov. et comb. nov., conifer in the Crato Formation. We demonstrated that all plant specimens from the Romualdo and Crato formations should be treated as a single fossil-species. Based on the anatomical characters of the wood and leaf epidermises, which we described for the first time, the fossil plant should be assigned to a separate fossil-genus, Leliacladus Batista & L.Kunzmann gen. nov. The higher taxonomic affiliation of this conifer is still unresolved, however, a relationship with the extinct Cheirolepidiaceae or Podocarpaceae is possible. From the morphological features we have hypothesized a candelabra-like growth habit, and the exclusive presence of thick axes suggests a xeromorphic adaptation to the hot and semiarid paleoclimate.
Acknowledgements
We thank preparator Carola Kunzmann (Senckenberg Naturhistorische Sammlungen Dresden, Germany) for SEM preparation and imaging of a Crato Formation specimen MMG PB SAK 59, and to Jiří Kvaček (National Museum Prague, Czech Republic), Marion Bamford (University of Witwatersrand, Johannesburg, South Africa) and two anonymous reviewers for their suggestions to improve the manuscript. Jackie Lees supported us improving the English language. We sincerely thank the Museu de Paleontologia Plácido Cidade Nuvens (Santana do Cariri, Ceará, Brazil) and Museu de Ciências Naturais e de História Barra do Jardim (Jardim, Ceará, Brazil) for providing the fossil specimens analyzed here. MEPB thanks Domingas M. Conceição (Museu de Paleontologia Plácido Cidade Nuvens, Santana do Cariri, Brazil) for her help and discussions during the work. The investigation was partly funded by the CAPES-DAAD PROBRAL mobility program (CAPES ID 88881.198776/2018-01; DAAD ID-57446885) and FUNCAP, through the support grant to MEPB (BP5-0197-00135.01.00/22 and UNI-0210-00102.01.00/23) AAF (#BP3-013900202.01.00/18), and RAMB Bantim (#BMD-0124-00302.01.01/19).
Author’s contributions
Conceptualization MEPB, LK; Data curation MEPB, LK; Formal analysis MEPB, LK, APP, RAMB, FJL, DBS; Funding acquisition MEPB, LK, AAFS; Investigation MEPB, LK; Methodology MEPB, LK, APP, FJL, DBS; Project administration MEPB; Resources MEPB, LK, APP, DBS; AAFS; Software MEPB, LK, RAMB; Supervision MEPB, LK; Validation MEPB, LK; Visualization MEPB, LK; Writing—original draft MEPB, LK, RAMB; Writing—review and editing MEPB, LK.
References
APG IV 2016. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Botanical Journal of the Linnean Society 181: 1–20 [online access: Angiosperm Phylogeny Website. Version 14, http://www.mobot.org/MOBOT/research/APweb/]. Crossref
Alvin, K.L. 1982. Cheirolepidiaceae: biology, structure and paleoecology. Review of Palaeobotany and Palynology 37: 71–98. Crossref
Alvin, K.L., Fraser, C.J., and Spicer, R.A. 1981. Anatomy and palaeoecology of Pseudofrenelopsis and associated conifers in the English Wealden. Palaeontology 24: 759–778.
Assine, M.L. 2007. Bacia do Araripe. Boletim de Geociências da Petrobras 15: 371–389.
Assine, M.L., Perinotto, J.A.J., Andriolli, M.C., Neumann, V.H., Mescolotti, P.C., and Varejão, F.G. 2014. Sequências deposicionais do Andar Alagoas da Bacia do Araripe, Nordeste do Brasil. Boletim de Geociências da Petrobras 22: 3–28. Crossref
Barreto, A.M.F., Bertotti, A.L., Sylvester, P.J., Prado, L.A.C., Araripe, R.C., Oliveira, D.H., Tomé, M.E.T.R., Lemos, F.A.P., Nascimento, L.R.L., Pereira, P.A., and Albayrak, A.I. 2022. U/Pb geochronology of fossil fish dentine from Romualdo Formation, Araripe Basin, northeast of Brazil. Journal of South America Earth Science 116: 103774. Crossref
Batista, M.E.P., Kunzmann, L., Bezerra, F.I., De Andrade, J.A.F., Sá, A.A., and Loiola, M.I.B. 2018. A new cheirolepidiaceous conifer Pseudofrenelopsis salesii sp. nov. from the Early Cretaceous of Brazil (Romualdo Formation, Araripe Basin): Paleoecological and taphonomic significance. Review of Palaeobotany and Palynology 258: 154–162. Crossref
Batista, M.E.P., Kunzmann, L., Sá, A.A., Saraiva, A.Á.F., and Loiola, M.I.B. 2020. A New Species of Brachyphyllum from the Crato Formation (Lower Cretaceous), Araripe Basin, Brazil. Ameghiniana 57: 519–533. Crossref
Batista, M.E.P., Loiola, M.I.B., Soares, A.A., Mastroberti, A.A., Sá, A.A., Nascimento, D.R., Jr., Silva Filho, W.F., and Kunzmann, L. 2022. New insights into the evolution of mucilage cells in Araucariaceae: Araucaria violetae sp. nov. from the Early Cretaceous Araripe Basin (Northeast Brazil). International Journal of Plant Sciences 183 (1): 43–60. Crossref
Batista, M.E.P., Martine, A.M., Saraiva, A.Á.F., Lima, F.J., Barros, O.A., Sá, A.A., and Loiola, M.I.B. 2021. Brachyphyllum: State of the art and new data regarding B. obesum, the most representative fossil plant in the Araripe Basin, Brazil. Journal of South American Earth Sciences 110: 103405. Crossref
Batista, M.E.P., Silva, D.D.C., Sales, M.A., Sá, A.A., Saraiva, A.A.F., and Loiola, M.I.B. 2017. New data on the stem and leaf anatomy of two conifers from the Lower Cretaceous of the Araripe Basin, northeastern Brazil, and their taxonomic and paleoecological implications. PloS ONE 12 (3): e0173090. Crossref
Batten, D.J. 2007. Spores and pollen from the Crato Formation: biostratigraphic and palaeoenvironmental implications. In: D.M. Martill, G.Bechly, and R.F. Loveridge (eds.), The Crato Fossil Beds of Brazil: Window into an Ancient World, 566–573, Cambridge University Press, Cambridge. Crossref
Bernardes-de-Oliveira, M.E.C., Sucerquia, P.A., Mohr, B., Dino, R., Antonioli, L., and Garcia, M.J. 2014. Indicadores paleoclimáticos na paleoflora do Crato, final do Aptiano do Gondwana Norocidental. In: I.S. Carvalho, M.J. Garcia, C.C. Lana, and O. Strohschoen Jr. (eds.), Paleontologia: Cenários de Vida: Paleoclimas, 99–118. Interciência, Rio de Janeiro.
Biondi, E. 1983. Étude d’un bois fossile du Jurassiquedes Préalpes italiennes: Protopodocarpoxylon dariae nov. sp. Geobios 16: 363–369. Crossref
Bouche, P.S., Larter, M., Domec, J.C., Burlett, R., Gasson, P., Jansen, S., and Delzon, S. 2014. A broad survey of hydraulic and mechanical safety in the xylem of conifers. Journal of Experimental Botany 65: 4419–4431. Crossref
Boura, A., Bamford, M., and Philippe, M. 2021. Promoting a standardized description of fossil tracheidoxyls. Review of Palaeobotany and Palynology 295: 104525. Crossref
Brongniart, A. 1828. Prodrome d’une histoire des végétaux fossiles. 223 pp. Levrault, Strasbourg. Crossref
Carlquist, S.J. 1975. Ecological Strategies of Xylem Evolution. 259 pp. University of California Press, Berkeley. Crossref
Carlquist, S.J. and Schneider, E.L. 2001. Vegetative anatomy of the New Caledonian endemic Amborella trichopoda: relationships with the Illiciales and implications for vessel origin. Pacific Science 55: 305–312. Crossref
Chumakov, N.M. 1995. Climatic zones in the middle of the Cretaceous period. Stratigraphy and Geological Correlation 3: 3–14.
Coimbra, J.C. and Freire, T.M. 2021. Age of the Post-rift Sequence I from the Araripe Basin, Lower Cretaceous, NE Brazil: Implications for spatio-temporal correlation. Revista Brasileira Paleontologia 24: 37–46. Crossref
Corecco, L., Bezerra, F.I., Da Silva Filho, W.F., Júnior, D.N., Da Silva, J.H., and Félix, J. 2022. Petrological meaning of ethnostratigraphic units: laminated limestone of the Crato Formation, Araripe Basin, NE Brazil. Pesquisas em Geociências 49 (1): e121139. Crossref
Du, B.X., Sun, B.N., Ferguson, D.K., Yan, D.F., Dong, C., and Jin, P.H. 2013. Two Brachyphyllum species from the Lower Cretaceous of Jiuquan Basin, Gansu Province, NW China and their affinities and palaeoenvironmental implications. Cretaceous Research 41: 242–255. Crossref
Duarte L. 1985. Vegetais fósseis da Chapada do Araripe. In: D.A. Campos (ed.), Congresso Brasileiro de Paleontologia, Coletaânea de Trabalhos Paleontológicos. Série Geologia, 557–568. Departamento Nacional de Produção Mineral, Rio de Janeiro.
Esteban, L.G., De Palacios, P., Heinz, I., Gasson, P., García-Iruela, A., and García-Fernández, F. 2023. Softwood anatomy: a review. Forests 14 (2): 323. Crossref
Esteban, L.G., De Palacios, P., Philippe, M., Guindeo, A., and Fernández, F.G. 2006. New xylological data and the biogeography of the Iberian Peninsula during the Early Cretaceous. Geobios 39: 805–816. Crossref
Evert, R.F. 2013. Anatomia das plantas de Esau: meristemas, células e tecidos do corpo da planta: sua estrutura, função e desenvolvimento. 648 pp. Blucher, São Paulo.
Falcon-Lang, H.J., Kvaček, J., and Uličný, D. 2001. Fire-prone plant communities and palaeoclimate of a Late Cretaceous fluvial to estuarine environment, Pecínov quarry, Czech Republic. Geological Magazine 138: 563–576. Crossref
Fara, E., Saraiva, A.Á., Almeida Campos, D., Moreira, J.K., Carvalho Siebra, D., and Kellner, A.W. 2005. Controlled excavations in the Romualdo Member of the Santana Formation (Early Cretaceous, Araripe Basin, northeastern Brazil): stratigraphic, palaeoenvironmental and palaeoecological implications. Palaeogeography, Palaeoclimatology, Palaeoecology 218 (1–2): 145–160. Crossref
Farjon, A. 2017. A Handbook of the World’s Conifers (2 Vols.), Revised and updated edition. 1154 pp. Brill, Leiden. Crossref
Fauth, G., Kern, H.P., Villegas-Martín, J., Mota, M.A.L., Santos Filho, M.A.B., Catharina, A.S., Leandro, L.M., Luft-Sousa, F., Strohschoen Jr., O., Nauter-Alves, A., Tungo, E.J.F., Bruno, M.D.R., Ceolin, D., Baecker-Fauth, S., Bom, M.H.H., Lima, F.H.O., Santos, A., and Assine, M.L. 2023. Early Aptian marine incursions in the interior of northeastern Brazil following the Gondwana breakup. Scientifc Reports 13: 6728. Crossref
Florin, R. 1931. Untersuchungen zur Stammesgeschichte der Coniferales und Cordaitales. I. Morphologie und Epidermisstruktur des Assimilationsorgane bei den regenten Koniferen. Kungliga Svenska Vetenskapsakademiens Handlingar 10: 1–588.
Francis, J.E. 1983. The dominant conifer of the Jurassic Purbeck formation, England. Palaeontology 26: 277–294.
Fürsich, F.T., Custódio, M.A., Matos, S.A., Hethke, M., Quaglio, F., Warren, L.V., Assine, M.L., and Simões, M.G. 2019. Analysis of a Cretaceous (late Aptian) high-stress ecosystem: The Romualdo Formation of the Araripe Basin, northeastern Brazil. Cretaceous Research 95: 268–296. Crossref
Gobo, W.V., Kunzmann, L., Iannuzzi, R., Santos, T.B., Conceição, D.M., Nascimento, D.R., Jr., Silva Filho, W.F., Bachelier, J.B., and Coiffard, C. 2023. A new remarkable Early Cretaceous nelumbonaceous fossil bridges the gap between herbaceous aquatic and woody protealeans. Scientific Reports 13 (1): 8978. Crossref
Harris, T.M. 1979. The Yorkshire Jurassic Flora. V. Coniferales. 166 pp. British Museum (Natural History), London.
Kendall, M.W. 1947. On five species of Brachyphyllum from the Jurassic of Yorkshire and Wiltshire. Annals and Magazine of Natural History 14: 225–251. Crossref
Kunzmann, L., Westerkamp, A.P.A.O., Batista, M.E.P., and Rydin, C. 2023. Gymnosperms from the Early Cretaceous Crato flora—competitors for the nascent flowering plants. In: R. Iannuzzi, R. Rossler, and L. Kunzmann (eds.), Brazilian Paleofloras: From Paleozoic to Holocene, 1–46. Springer, Cham. Crossref
Kunzmann, L., Coiffard, C., Westerkamp, A.P.A.O, Batista, M.E.P., Uhl, D., Solórzano-Kraemer, M.M., Mendes, M., Nascimento, D.R., Jr., Ianuzzi, R., and Da Silva Filho, W.F. 2021. Crato flora: a 115-million-year-old window into the Cretaceous world of Brazil. In: R. Iannuzzi, R. Rossler, and L. Kunzmann (eds.), Brazilian Paleofloras: From Paleozoic to Holocene, 1–40. Springer, Cham. Crossref
Kunzmann, L., Mohr, B.A., and Bernardes‐de‐Oliveira, M.E.C. 2004. Gymnosperms from the Lower Cretaceous Crato Formation (Brazil). I. Araucariaceae and Lindleycladus (incertae sedis). Fossil Record 7: 155–174. Crossref
Kunzmann, L., Mohr, B.A., Bernardes‐de‐Oliveira, M.E., and Wilde, V. 2006. Gymnosperms from the Early Cretaceous Crato Formation (Brazil). II. Cheirolepidiaceae. Fossil Record 9: 213–225. Crossref
Kunzmann, L., Mohr, B.A., Wilde, V., and Bernardes-de-Oliveira, M.E. 2011. A putative gnetalean gymnosperm Cariria orbiculiconiformis gen. nov. et spec. nov. from the Early Cretaceous of northern Gondwana. Review of Palaeobotany and Palynology 165 (1–2): 75–95. Crossref
Kvaček, J. 2007. The conifer Brachyphyllum squammosum. Acta Palaeobotanica 47: 25–35.
Kvaček, J. and Mendes, M.M. 2021. A new Cheirolepidiaceae conifer Watsoniocladus cunhae sp. nov. from the Early Cretaceous (late Aptian–early Albian) of western Portugal. Review of Palaeobotany and Palynology 295: 104519. Crossref
Lacerda, J.N.L. and Barreto, A.M.F. 2023. Paleoecology and paleoenvironmental inferences based on palynomorphs from the Romualdo Formation of the Araripe Basin, Serrolândia Mine, Pernambuco, northeastern Brazil. Journal of South American Earth Sciences 129: 104526. Crossref
Lewis, M.C. 1972. The physiological significance of variation in leaf structure. Science Progress 60: 25–51.
Lima, M.R.D. 1978. Palinologia da Formação Santana (Cretáceo do Nordeste do Brasil). 335 pp. Universidade de São Paulo, São Paulo.
Lindley, J. and Hutton, W. 1836. The Fossil Flora of Great Britain; or, Figures and Descriptions of the Vegetable Remains Found in a Fossil State in This Country, Part 3. 208 pp. J. Ridgway, London.
Lúcio, T., Souza Neto, J.A., and Selby, D. 2020. Late Barremian/ Early Aptian Re-Os age of the Ipubi Formation black shales: stratigraphic and paleoenvironmental implications for Araripe Basin, northeastern Brazil. Journal of South American Earth Sciences 102: 102699. Crossref
Martill, D.M. 2007. The age of the Cretaceous Santana Formation fossil Konservat Lagerstätte of north-east Brazil: a historical review and an appraisal of the biochronostratigraphic utility of its palaeobiota. Cretaceous Research 28: 895–920. Crossref
Martill, D.M. Bechly, G., and Loveridge, R.F. 2007. The Crato Fossil Beds of Brazil: Window into an Ancient World. 625 pp. Cambridge University Press, Cambridge. Crossref
Melo, R.M., Guzmán, J., Almeida-Lima, D., Piovesan, E.K., Neumann, V.H.D.M.L., and Sousa, A.D.J.E. 2020. New marine data and age accuracy of the Romualdo Formation, Araripe Basin, Brazil. Scientific Reports 10 (1): 15779. Crossref
Mohr, B.A., Bernardes-de-Oliveira, M.E.C., and Loveridge, R.F. 2007. The macrophyte flora of the Crato Formation. In: D.M. Martill, G. Bechly, and R.F. Loveridge (eds.), The Crato Fossil Beds of Brazil: Window into an Ancient World, 537–566, Cambridge University Press, Cambridge. Crossref
Mohr, B.A., Schultka, S., Süss, H., and Bernardes-de-Oliveira, M.E. 2012. A new drought-resistant gymnosperm taxon Duartenia araripensis gen. nov. et sp. nov. (Cheirolepidiaceae?) from the Early Cretaceous of Northern Gondwana. Palaeontographica Abteilung B 289 (1–3): 1–25. Crossref
Nascimento, D.R., Da Silva Filho, W.F., and Erthal, F. 2023. Crato Lake deposits. Rocks to preserve an extraordinary fossil Lagerstätte. In: R. Iannuzzi, R. Rossler, and L. Kunzmann (eds.), Brazilian Paleofloras: from Paleozoic to Holocene, 1–53. Springer, Cham. Crossref
Neumann, V.H.M.L. and Assine, M.L. 2015. Stratigraphical proposal to the post-rift I tectonic-sedimentary sequence of Araripe Basin, Northeastern Brazil. In: Proceedings of the 2nd International Congress on Stratigraphy, Graz, Austria, 19–23 July 2015, 274–274. Berichte des Institutes für Erdwissenschaften der Universität Graz.
Neumann, V.H.M.L. and Cabrera, L. 1999. Uma nueva propuesta estratigráfica para la tectonosecuencia post-rifte de la cuenca de Araripe, Nordeste de Brasil. Boletim do Quinto Simpósio sobre o Cretáceo do Brasil/Primero Simposio sobre el Cretácico de América del Sur. 279–285. UNESP, Rio Claro.
Noll, R. and Kunzmann, L. 2020. Diversity in fossil Araucaria Juss.: new species from the Middle Jurassic Jaramillo Petrified Forests in Santa Cruz province, Argentina. Palaeontographica Abteilung B 301 (1–3): 3–75. Crossref
Passalia, M.G. 2009. Cretaceous pCO2 estimation from stomatal frequency analysis of gymnosperm leaves of Patagonia, Argentina. Palaeogeography, Palaeoclimatology, Palaeoecology 273: 17–24. Crossref
Philippe, M. and Bamford, M.K. 2008. A key to morphogenera used for Mesozoic conifer-like woods. Review of Palaeobotany and Palynology 148: 184–207. Crossref
Philippe, M., Bamford, M., and Zijlstra, G. 2002. Proposal to conserve the name Protopodocarpoxylon (fossil Gymnospermae, Coniferales) with a conserved type. Taxon 51: 207–208. Crossref
Ribeiro, A.C., Ribeiro, G.C., Varejão, F.G., Battirola, L.D., Pessoa, E.M., Simões, M.G., Warren, L.V., Riccomini, C., and Poyato-Ariza, F.J. 2021. Towards an actualistic view of the Crato Konservat-Lagerstätte paleoenvironment: a new hypothesis as an Early Cretaceous (Aptian) equatorial and semi-arid wetland. Earth-Science Reviews 216: 103573. Crossref
Richter, H.G., Grosser, D., Heinz, I., and Gasson, P.E. 2004. IAWA list of microscopic features for softwood identification. IAWA Journal 25: 1–70. Crossref
Seward, A.C. 1895. Catalogue of the Mesozoic Plants in the Department of Geology British Museum (Natural History). Vol. 2. Gymnospermae. 252 pp. Printed by Order of the Trustees, London.
Sperry, J.S. and Tyree, M.T. 1990. Water-stress-induced xylem embolism in three species of conifers. Plant Cell Environment 13: 427–236. Crossref
Stockey, R.A. 1982. The Araucariaceae: an evolutionary perspective. Review of Palaeobotany and Palynology 37: 133–154. Crossref
Stockey, R.A. 1994. Mesozoic Araucariaceae: morphology and systematic relationships. Journal of Plant Research 107: 493–502. Crossref
Stockey, R.A. and Atkinson, I.J. 1993. Cuticle micromorphology of Agathis salisbury. International Journal of Plant Science 154: 187–225. Crossref
Sucerquia, P.A., Bernardes-de-Oliveira, M.E., and Mohr, B.A. 2015. Phytogeographic, stratigraphic, and paleoclimatic significance of Pseudofrenelopsis capillata sp. nov. from the Lower Cretaceous Crato Formation, Brazil. Review of Palaeobotany and Palynology 222: 116–128. Crossref
Thevenard, F., Chernomorets, O., Moreau, J.D., Neraudeau, D., and Philippe, M. 2022. A review of the Hirmeriellaceae (Cheirolepidiaceae) wood. IAWA Journal 43: 428–447. Crossref
Van der Ham, R.W.J.M., Van Konijnenburg-Van Cittert, J.H.A., Dortangs, R.W., Herngreen, G.F.W., and Van der Burgh, J. 2003. Brachyphyllum patens (Miquel) comb. nov. (Cheirolepidiaceae?): remarkable conifer foliage from the Maastrichtian type area (Late Cretaceous, NE Belgium, SE Netherlands). Review of Palaeobotany and Palynology 127: 77–97. Crossref
Turland, N.J., Wiersema, J.H., Barrie, F.R., Greuter, W., Hawksworth, D.L., Herendeen, P.S., Knapp, S., Kusber, W.-H., Li, D.-Z., Marhold, K., May, T.W., McNeill, J., Monro, A.M., Prado, J., Price, M.J., and Smith, G.F. (eds.) 2018. International Code of Nomenclature for Algae, Fungi, and Plants (Shenzhen Code) Adopted by the Nineteenth International Botanical Congress Shenzhen, China, July 2017. 254 pp. Koeltz Botanical Books, Glashütten. Crossref
Vera, E.I. and Loinaze, V.S.P. 2022. Ecological interactions in conifers (Agathoxylon and Protocupressinoxylon) from the Punta del Barco Formation (Baqueró Group, upper Aptian), Patagonia, Argentina. Cretaceous Research 129: 105035. Crossref
Vila Nova, B.C., Saraiva, A.A., Moreira, J.K., and Sayão, J.M. 2011. Controlled excavations in the Romualdo Formation lagerstatte (Araripe basin, Brazil) and pterosaur diversity: Remarks based on new findings. Palaios 26: 173–179. Crossref
Watson, J. 1988. The Cheirolepidiaceae. In: C.B. Beck (ed.), Origin and Evolution of Gymnosperms, 382–447. Columbia University Press, New York.
Acta Palaeontol. Pol. 69 (3): 375–393, 2024
https://doi.org/10.4202/app.01116.2023