

Fossil caries in a Pliocene rodent with a plausible instance of in situ preservation of bacterial remains
MICHAŁ CZERNIELEWSKI, PAWEŁ BĄCAL, and BŁAŻEJ BŁAŻEJOWSKI
Czernielewski, M., Bącal, P., and Błażejowski, B. 2024. Fossil caries in a Pliocene rodent with a plausible instance of in situ preservation of bacterial remains. Acta Palaeontologica Polonica 69 (2): 217–225.
An interesting case of a caries-affected area where bacterial remains were plausibly preserved in situ was found in an isolated tooth of the Plio-Pleistocene dormouse Glis sackdillingensis (Rodentia, Gliridae). The sample is 2.9–2.6 million years old, and may be the only described case of a dental pathological condition preserved in a fossil together with the microbial pathogen responsible for its development. The tooth was investigated using various complementary techniques such as Scanning Electron Microscopy with Energy Dispersive Spectroscopy, Microtomography, and Light Microscopy. Available data on dietary habits and lifestyle of modern dormice are extrapolated to explain the origin of the infection. The results of the investigation are presented within the wider context of the fossil record of dental infections and other microbe-related diseases in vertebrates. Possible methodological reasons for the lack of similar reports are also discussed.
Key words: Rodentia, Gliridae, Plio-Pleistocene, enamel caries, bacterial infection.
Michał Czernielewski [m.czernielewski@int.pl; ORCID: https://orcid.org/0000-0001-8880-3964 ], Paweł Bącal [bacal@ twarda.pan.pl, ORCID: https://orcid.org/0000-0001-7935-639X ], and Błażej Błażejowski [bblazej@twarda.pan.pl; ORCID: https://orcid.org/0000-0001-6320-9870 ] (corresponding author), Institute of Paleobiology, Polish Academy of Sciences, Twarda 51/55, 00-818 Warszawa, Poland.
Received 12 December 2023, accepted 15 April 2024, published online 8 May 2024.
Copyright © 2024 M. Czernielewski et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Dental infections are thought to have been present in vertebrates since the Palaeozoic era (538.8–251.9 Mya), the earliest examples including cases of caries described in Devonian (419.2–358.9 Mya) lungfish (Kemp 2003; Reisz et al. 2011). In spite of that, relatively few pre-Holocene (older than ca. 12 kya) fossil specimens of bacteria-related tooth decay have been reported in scientific literature. Examples regarding occurrences of caries found in fossil hominids may be considered an exception (e.g., Grine et al. 1990; Tillier et al. 1995; Trinkaus et al. 2000; Humphrey et al. 2014; Fuss et al. 2018; Towle et al. 2021). The reason for that may be that hominid dental samples are studied more extensively with regard to caries due to their perceived immediate relevance to the topic of tooth decay in modern humans. Other pre-Holocene examples of conditions suspected to have resulted from dental infections were discovered in a Permian captorhinid reptile (Reisz et al. 2011), a Cretaceous ichthyosaur (Kear 2001), an early Eocene plesiadapiform primate (Selig and Silcox 2021), Miocene lungfishes (Kemp 2003), a Miocene artiodactyl (Sala Burgos et al. 2007) and a Pleistocene short-faced bear (Figueirido et al. 2017). Such studies are usually focused on recognizing and interpreting indirect evidence for the involvement of bacteria, i.e., pathological changes that are presumed to have been caused by bacteria because of their similarity to bacteria-induced conditions occurring in extant animals. This is also true for microbial infections which leave marks on bones (e.g., Wolff et al. 2009; Surmik et al. 2018; Moncunill-Solé et al. 2019).
More direct associations between bacteria and teeth do occur in the fossil record in the form of calcified dental plaque (also known as dental calculus), which is occasionally present in subfossil and fossil humans. It is known to preserve not only bacterial morphology but sometimes also a genetic information of the microbes responsible for the formation of the plaque (e.g., Preus et al. 2011; Adler et al. 2013; Weyrich et al. 2017). Bacterial body fossils preserved this way have also been recognized in the Miocene hominid Sivapithecus from Pakistan (Hershkovitz et al. 1997). However, what has been lacking in the fossil record is a clear instance of an association between a pathological condition and the microbial pathogen responsible for its development. To our knowledge, no such case has yet been convincingly documented.
In this paper we report a discovery of carious tooth damage of an isolated tooth of a Plio-Pleistocene dormouse with bacteria-like microstructures. We identified the microstructures as fossilized bacterial microflora associated with the enamel demineralization and dentine inflammation observed. The finding may represent an in situ preservation of cariogenic microbes that were causing the pathological condition during the animal’s life. The species in question, Glis sackdillingensis (Rodentia, Gliridae), is considered to be the direct ancestor of the modern European edible dormouse, Glis glis. The investigated tooth is ca. 2.9–2.6 My old and, to our knowledge, it is the only thus far described caries-affected fossil dental specimen with bacterial microflora preserved in clear association with the affected area. It is apparently the first described case of fossil caries in a rodent.
Institutional abbreviations.—ZPAL, Institute of Paleobiology, Polish Academy of Sciences, Warsaw, Poland.
Other abbreviations.—EDS, Energy Dispersive Spectroscopy; LM, Light Microscopy; mikro-CT, X-ray Microcomputed Tomography; SEM, Scanning Electron Microscopy.
Material and methods
The tooth specimen, ZPAL M. VIII/b/G2/1 is part of the fossil assemblage of Węże 2, a paleontological site located in the Wieluń Upland, southern Poland (Fig. 1). The described tooth is an isolated permanent left lower first molar representing an adult individual of the species Glis sackdillingensis (Fig. 2A1, B1). One of the roots of the tooth is missing (partly broken off) (SOM: fig. S1, Supplementary Online Material available at http://app.pan.pl/SOM/app69-Czernielewski_etal_SOM.pdf). It was recovered from the fossiliferous terra rossa sediments that were collected in a karst crevice during field surveys to the location organized in the late 50s and early 60s of the XX century. Based on its faunal composition, the age of the fossil assemblage is currently dated at the late Pliocene (2.9–2.6 Mya) (Sulimski 1962; Stefaniak et al. 2020). The specimen is housed at the Institute of Paleobiology, Polish Academy of Sciences in Warsaw.
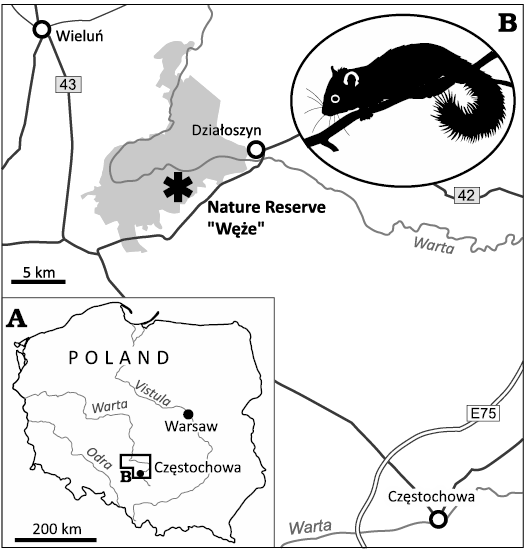
Fig. 1. Road map (A) with the location of the Nature Reserve “Węże” in the proximity to Wieluń in Central Poland, Załęcze Landscape Park marked in grey (B). A silhouette of modern dormouse is given as an inset.
To reveal the enamel structure the tooth was embedded in epoxy resin, polished perpendicularly to the vertical axis, treated with dilute (1%) orthophosphoric acid for ca. 60 seconds and dried. Subsequently, the internal structure of the enamel and dentin was examined using the Quattro S (Thermo Fisher) SEM apparatus. Energy dispersive X-ray spectroscopy (EDS) analyses were conducted using the Octane Elect (EDAX) detector. The procedure of both mechanical and chemical polishing was once repeated to enable observation of deeper parts of the tooth.
To visualize the affected area and build 3D models, X-ray microcomputed tomography (micro-CT) was performed. The digital processing and analysis of the tomographic data enabled the construction of isosurface-based and volume-based 3D “virtual fossils”, which can be manipulated and dissected interactively (Błażejowski et al. 2011; Zapalski et al. 2021). Micro-CT data were collected with the XRadia MicroXCT-200 (ZEISS) system in the Laboratory of Microtomography, Institute of Paleobiology, Polish Academy of Sciences, Warsaw, Poland. The reconstructed computed tomography data were converted into TIFF image stacks that were subsequently imported and segmented in VGStudio MAX version 3.0 (Volume Graphics Inc.).
Results
Light and Scanning Electron Microscopy.—Despite the small size of the sample (ca. 2×1×2 mm), physical damage to the enamel layer is visible at low magnification on the buccal side of the tooth (Fig. 2C). However, it can be distinguished from surrounding material only in cross-section and is not visible upon the investigation of the tooth’s surface. The damage is generally superficial and limited to the enamel tissue, but locally an indentation is visible, wedging into the dentine layer (Fig. 2D). We interpreted this damage as a cavity caused by an early stage of tooth decay, and the spot concerned as a caries-affected area. This area has become the focus of subsequent examinations. Detailed SEM inspections revealed that the dentine layer within the area was also damaged, which we consider to be caused by bacteria-inflicted inflammation and demineralization.
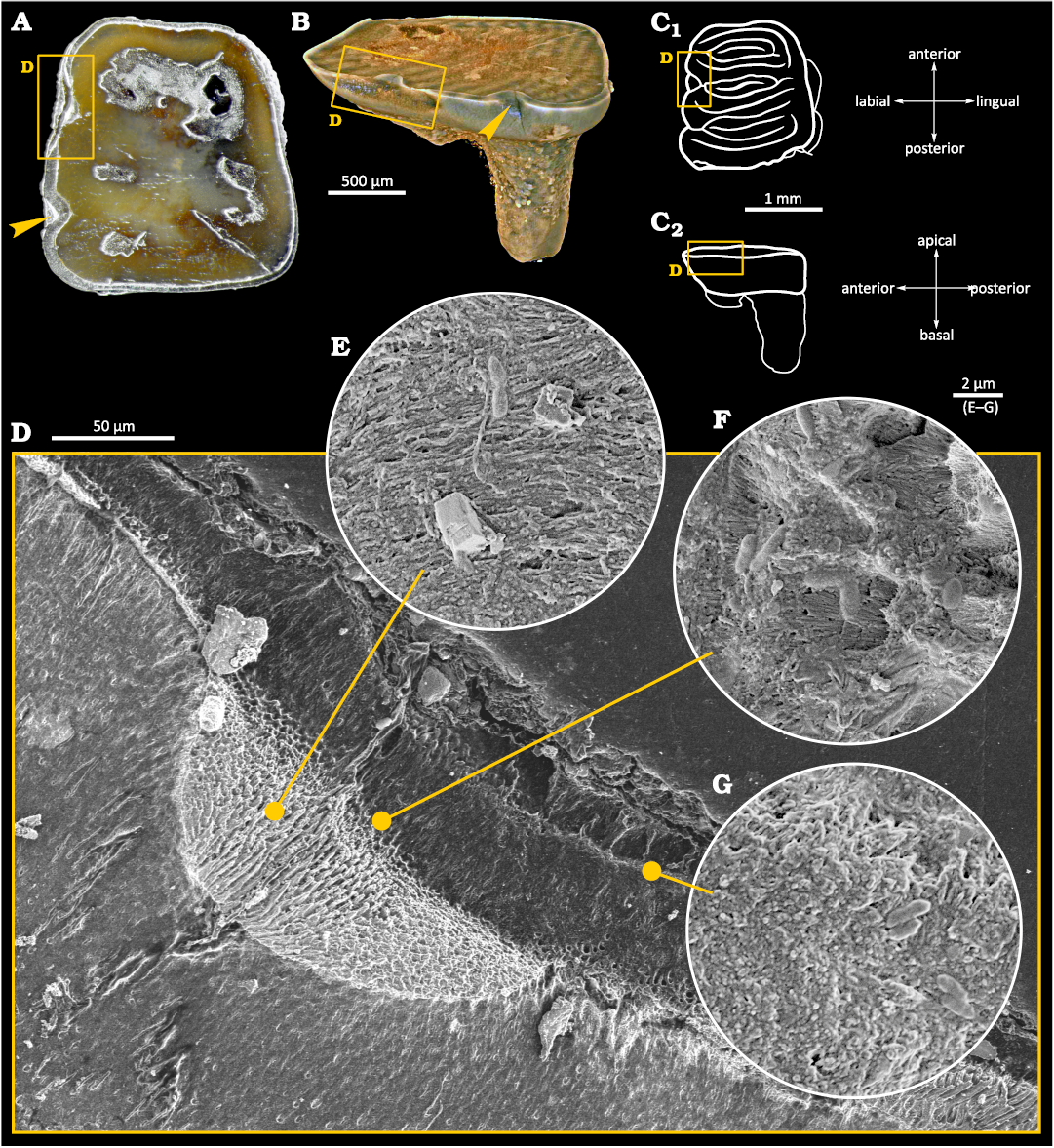
Fig. 2. Tooth of Glis sackdillingensis (Heller, 1930) (ZPAL M. VIII/b/G2/1) from Węże 2 (2.9–2.6 Mya), late Pliocene. A. Photography with the overview of the occlusal surface. The cavity area is indicated by yellow box. B. 3D CT model showing the cavity area. C. Drawings of occlusal (C1) and left lateral (C2) view, the upper surfaces created after each polishing marked with horizontal lines, the cavity area highlighted. Interpretation of the occlusal surface after Striczky and Pazonyi 2014. D. SEM photograph of the cavity area with the damage to the enamel and the dentin layers visible. E–G. SEM photographs showing fossilized bacteria-like microstructures.
Within the cavity area, cocci-like objects and bacilli-like objects were found. A compilation of SEM photographs showing examples of those various morphologies of plausible bacterial fossils is presented in Fig. 3A–D. Their presence in the tooth sample is limited to the tooth area with damaged enamel and near to the surface area of dentine as shown in Fig. 2D–G. Cocci-like structures (Figs. 2G, 3A) are c.a. 0.6 µm in diameter. Bacilli-like objects (Figs. 2D, G, 3B–D) are elongated with dimensions of c.a. 0.6–0.7 µm in width and up to 2 µm in length, with typical 1:2.5 aspect ratio. Structures resembling shapes like: diplococci (Fig. 3A), coccobacilli or diplobacilli (Figs. 2F, 3C), palisades (Fig. 3B) can be seen, however this organization can be illusory, and related to taphonomy issues. All mentioned bacteria-like structures were found in a wide range of morphologies from to being semi-flat to almost round in cross section, which can be related to different bacteria or results from the preservation processes.
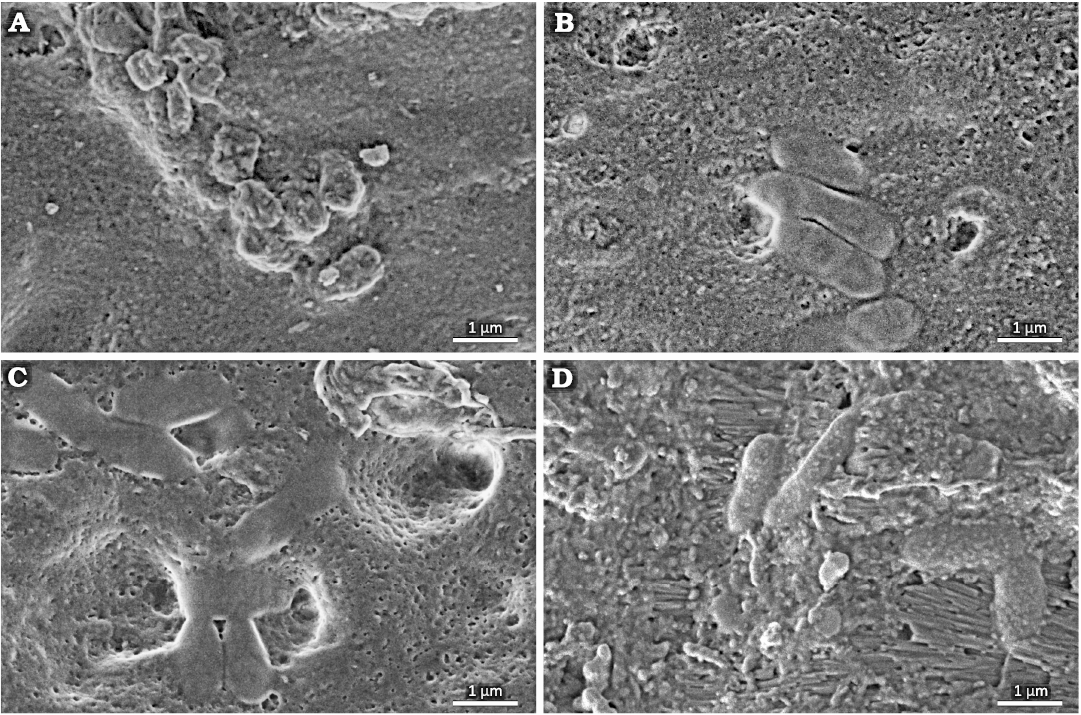
Fig. 3. Tooth of Glis sackdillingensis (Heller, 1930) (ZPAL M. VIII/b/G2/1) from Węże 2 (2.9–2.6 Mya), late Pliocene. SEM photographs showing various morphologies of probable bacterial fossils found in the tooth cavity. A. Cocci-like objects. B–D. Bacilli-like objects.
Energy Dispersive Spectroscopy.—Elemental distribution mapping was performed by the means of EDS (Fig. 4, SOM: fig. S2, S3 in electronic supplement information). Elemental mapping revealed that the content of carbon is relatively higher in the cavity, which is most likely caused by contamination with the epoxy resin used for fixing the sample (Fig. 4B, SOM: fig. S3B). At the same time, a slight decrease in the content of phosphorus and calcium was observed in the cavity area (Fig. 4C, D, SOM: fig. S3C, D). Contrarily, the content of fluorine was practically identical in the damaged area and in the non-affected enamel tissue (SOM: fig. S2H). Between the tooth (the cavity area) and the epoxy resin we detected clear indications of the presence of magnesium, chlorine, sulfur, sodium, oxygen, silicon, and aluminum (SOM: fig. S2B–G). This is indicative of residual soil, inorganic salts or the polishing agent, or a combination thereof. These elements are generally present neither in the section through the enamel, nor in the main mass of the tooth. Therefore, the observed elemental distribution within the caries-affected area should be interpreted as a filling of semi-porous material, i.e., enamel depleted in P and Ca (in vivo), by C-rich modern epoxy. The phosphorus and calcium contents within the cavity are lower than in other parts of the tooth where hydroxyapatite, the building material of enamel, remained uninfected (Fig. 4, SOM: fig. S3).
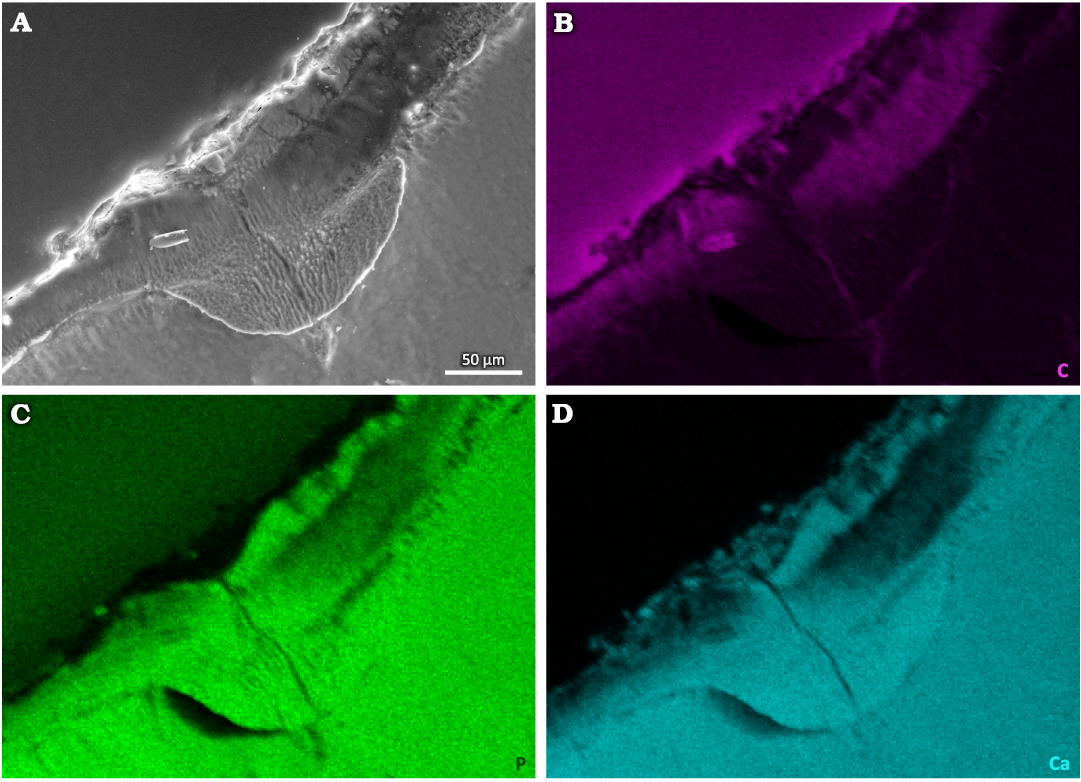
Fig. 4. Tooth of Glis sackdillingensis (Heller, 1930) (ZPAL M. VIII/b/G2/1) from Węże 2 (2.9–2.6 Mya), late Pliocene. SEM photograph (A) and EDS maps of distributions of carbon (B), phosphorus (C), and calcium (D) within the tooth and in the cavity area.
X-ray Microcomputed Tomography.—Visualization of the 3D structure of the specimen was done using microtomography, which allowed to locate the caries within the tooth in respect to its morphological details (SOM: fig. S1). The caries-affected area was situated in one of the tooth’s sinuses (Fig. 2A–D). Considering that, as well as the elongated shape of the lesion, we deduce that we found an early stage of enamel caries where no hollow structure had been created yet, but instead there was a straight, sharp breakage formed in vivo in the enamel tissue, surrounded by partially demineralized (and therefore porous) enamel that was colonized by pathogens. Although we chose to apply the term “cavity”, generally accepted when dealing with caries-induced dental traumas, this structure is not a hollow or a gap, and should rather be imagined as demineralized porous material contrasting with the surrounding enamel. The changes in the dentin structure may be interpreted as tertiary dentin. Such tissue is formed by odontoblasts or odontoblast-like cells as a reaction against physical damage or development of caries (Goldberg et al. 2011).
Other samples.—Other dental specimens belonging to the dormouse assemblage of Węże 2 were studied using the same methodology and techniques to determine whether more cases of bacterial fossilization could be found. No other cases of caries with remains of fossilized bacteria were discovered.
Discussion
Caries are caused by cariogenic bacteria (most notably Streptococcus mutans) that metabolize carbohydrates and create acidic conditions in the mouth, which leads to enamel demineralization and favors their further growth at the expense of microorganisms neutral to oral health. The relatively recent global dispersal of S. mutans was apparently facilitated by evolutionary changes caused by positive selection of genes involved in acid tolerance and sugar metabolism. Such genetic transformations accompanied the spread of agriculture (and thus the worldwide shift towards carbohydrate-rich diets) which resulted in caries becoming almost ubiquitous in human populations (Burne 1998; Ajdić et al. 2002; Cornejo et al. 2013; Adler et al. 2017; Larsen et al. 2019). However, dental caries or, more broadly speaking, dental infections, are not confined to modern-day humans, but have been present also in many other toothed animals. Despite this fact, earlier evolutionary history of the parasitic relationship between cariogenic pathogens and their hosts remains unclear (Bowen 2013; Poinar 2014).
As a result of the analyses of bacteria-like objects found during the SEM examination of the internal structure in the described dental specimen, we suggest that they are in fact cariogenic bacteria due to their morphology, dimensions, and the context in which they were found (Watanabe et al. 2013; You et al. 2017; Ortega-Cuadros et al. 2018). Unfortunately, the methods commonly used to determine taxonomic identity of extant bacteria (such as comparative analysis of 16S rRNA sequences, DNA-DNA hybridization or chemotaxonomic methods) are obviously not available for such ancient fossilized bacterial specimens (Neefs et al. 1990; Raina et al. 2019). Therefore, when dealing with such material we are forced to rely only on the shape and size of bacterial structures.
Although we consider the case here described as the most compelling instance of a possible in situ preservation of a pathogen-host association in a fossil, some kind of contamination cannot be completely ruled out. While the bacteria are spatially associated with the caries-affected area, their incorporation into plaque would make a stronger case against in vivo or postmortem contamination (Bruce M. Rothschild, personal communication 2024). It is also worth noting that even though in our opinion Streptococcus-like bacteria are clearly present in the affected area, at last two, if not three, distinctive bacterial morphotypes may be distinguished, which may suggest contamination of the wound by different organisms (“in vivo contamination”) or post-portem contamination (Bruce M. Rothschild, personal communication 2024). However, such “in vivo contamination” of a mechanically injured tooth is one of several scenarios that we consider plausible (see below) and, in itself, it does not undermine our interpretation of the fossilized microbes as representing pathogenic flora that was causing enamel demineralization and defense reaction of the dentin. On the contrary, we still deem it to be perhaps the most parsimonious explanation for the spatial association between the observed non-mechanical damage to the enamel and dentin and the fossilized microbes. Although bacteria colonizing cracks in teeth are indeed supposed to create a biofilm structure, described instances of in vivo and in vitro contamination of damaged teeth demonstrate that such structure is not always present, with some samples showing only few microorganisms or small clusters of them (Michelich 1980; Kahler et al. 2000; Love and Jenkinson 2002; Ricucci et al. 2015). The supposed taxonomic diversity of the bacteria-like organisms present in the sample does not necessarily weaken our interpretation either as not only S. mutans but also several other species of microorganisms are often found in caries-affected dentin and are supposed to induce caries as well as contribute to its progression. These include species of the genera Lactobacillus, Propionibacterium, Bifidobacterium, Veillonella, Actinomyces, Atopobium (Aas et al. 2008), Candida (Klinke et al. 2011), and Scardovia (Fakhruddin et al. 2019)
Although there seems to be no systematic research on the incidence of caries in extant dormice, one can suspect that they may be prone to dental infections owing to their low-cellulose diet with a relatively high sugar intake connected to the regular consumption of fruit. The low tolerance for dietary fiber exhibited by modern glirids is caused by their lack of a caecum, which is a seemingly unique feature among rodents. The loss of the caecum has been hypothesized to have occurred during the middle Miocene, as a consequence of increasing specialization. Another factor potentially contributing to the onset of caries in dormice is the presence of hard-shelled fruits in their diet, including beechnuts (Fagus spp.), acorns (Quercus spp.) and hazelnuts (Corylus spp.). (Daams and de Bruijn 1994; Freudenthal and Martín-Suárez 2013; Jurczyszyn 2018). This increases the chances of mechanical damage to the enamel (e.g., cracks and breaks) which would then make the colonization of the tooth by cariogenic pathogens easier. Moreover, the complicated enamel patterns of the occlusal surfaces of the teeth (Daoud 1993; Daams and de Bruijn 1994; Lu et al. 2021) may facilitate the accumulation of food material and the growth of bacterial biofilm. These patterns may represent an adaptation driven by consumption of hard-shelled fruits. Although the earliest known dormouse, Eogliravus wildi (known from the lower and middle Eocene of Europe), had a simpler occlusal morphology (Storch and Seiffert 2007), more complicated enamel patterns appeared already in late Eocene forms and resembled those discernible in modern dormice (Freudenthal 2004).
The fact that dormice are hibernating animals (an ability that could have been acquired as early as in the early Oligocene) (Bieber and Ruf 2009; Lu et al. 2021) can also be of significance as, according to some studies, hibernation may have an adverse effect on dental and peridental tissues (Richardson et al. 1961; Mayer and Bernick 1963). It is however also worth noting that the average lifespan of the extant edible dormouse Glis glis may be as much as nine (or even 12) years in wild populations. This is remarkably long for a small rodent but still relatively short for a mammal and thus a high incidence of diseases which require a longer time to develop is not to be expected owing to this factor alone (Hoelzl et al. 2016; Lemaître et al. 2020).
As far as we know, the described tooth is to date the only recognized pre-Holocene specimen which possibly provides direct evidence of bacterial involvement in the formation of a pathological condition. Potentially pathogenic actinomycetous bacteria (which are present in soil and are normal part of oral microbiomes) (Könönen and Wade 2015; Gajdács and Urbán 2020) have been discovered fossilized within the dental tissue of the Eocene lagomorph Megalagus brachyodon from the United States (Fostowicz-Frelik and Frelik 2010), but unlike the case here reported, no mutual association between the bacteria and any tissue damage could be demonstrated. Similarly, the report on the Pleistocene australopithecine Paranthropus boisei (involving a microbe reminiscent of Clostridium) most probably describes a postmortem colonization (Furseth Klinge et al. 2009).
In the case of the described Glis sackdillingensis specimen (ZPAL M. VIII/b/G2/1), the bacteria are spatially associated with damaged tissue (enamel and dentine) and are not found anywhere else in any of the studied samples. We consider it unlikely that the fossilized microflora represents a subsequent postmortem colonization of caries-affected tissue by bacteria but with the original pathogens having left no trace. Therefore, we tentatively deduce the specimen to be a unique example of an in situ fossil preservation of the actual pathogens that were causing damage to a tissue during the animal’s life. The enamel had supposedly been physically/mechanically injured before the infection developed, facilitating the colonization by cariogenic bacteria which, in turn, worsened the condition. We assume this based on the shape of the cavity, which makes us suspect that the most plausible scenario of caries development in this case was that the tooth had been subjected to a mechanical trauma and that a resulting longitudinal crack in the enamel initiated the process of cariogenesis. According to a less probable scenario, initial damage to the enamel had been caused by the microbes themselves (that could be driven by especially cariogenic diet based on fruits).
Such fossil pathogen-host associations as the one documented here have not yet been reported in the literature. This can be explained by the fact that bacteria are rarely fossilized (however in some settings bacterial remains may undergo rapid biomineralization) or fossilize in far different conditions (Westall 1999; Ushatinskaya 2009). Another factor contributing to the lack of such reports and the relative scarcity of the described cases of caries in fossil assemblages is that teeth that have already been affected by caries, and thus are partially demineralized, may be more prone to degradation due to diagenetic processes after the deposition in the soil. Furthermore, it is possible that the same bacteria that were causing caries during the animal’s life continue to feed on the affected tooth after the death of the animal, thereby reducing the chances of fossilization of such a specimen. In addition, fossilized carious changes may be inconspicuous, especially those present in the teeth of small animals.
Nevertheless, even if these changes are observed and subjected to an examination, the actual bacterial body fossils may be hard to detect or interpret. In laboratory conditions, the preparation of bacterial samples for SEM investigation consists of several steps, usually including fixing (e.g., with glutaraladehyde), staining and finally drying (dehydration of the bacteria). These procedures promote the maintenance of cell shapes and their three-dimensional organization within a bacterial biofilm and permit to achieve satisfactory material contrast. Obviously, these steps do not occur during natural preservation of bacterial specimens. Therefore, the observed bacteria may differ significantly from the samples prepared in laboratory conditions. We found that when investigating such naturally prepared bacterial samples it is a good strategy to ensure that the surface of the cross-section is coated with a conductive layer (e.g., carbon, platinum) as thinly as possible, or non-sputtered at all. This is because the thin bacterial remains can be flattened and therefore easily covered even with thin coating, becoming indistinguishable from their surroundings. Besides, the observed bacterial structures were found to have merely little to no material contrast vs. the surrounding material. Moreover, as such bacteria are thin and rather flat, the applied high tension of the SEM beam should be kept low so that the volume interaction of the incident electron beam is as little as possible. Perhaps the factors mentioned above may be the reason why such bacterial traces have often been overlooked and therefore not yet properly described in the literature.
Conclusions
The described dental specimen belonging to a fossil dormouse may document a case of bacterial infection that was developing in vivo in a rodent living around 2.9–2.6 Mya. Discovering fossil examples of bacterial microflora linked to specific pathological conditions may prove indispensable in deciphering the profound evolutionary origins of pathogen-host associations and provide the means to better comprehend the dynamics behind their formation. In turn, we can acquire a more in depth understanding of pathogens immediately relevant to human health and economy, and of the diseases caused by them. The described case of a plausible bacterial dental infection preserved in a fossil is also exceptional when considered in the wider context of the paleopathological record of hard-tissue infections in general, as we are also not aware of any examples of fossilized bacteria associated with signs of bone disease. Although it can very well be caused by objective reasons (both bacteria themselves and the affected tissues may not be sufficiently prone to fossilization), methodological issues, such as the difficulty of working with minute samples, may also play an important role.
Regardless to the provenance of the fossilized bacteria, the specimen here discussed is apparently the first case of fossil caries described in a rodent. Whether there is a correlation between the diet and lifestyle of the Plio-Pleistocene dormouse Glis sackdillingensis and the chances of dental pathologies occurring in this species remains an open question. It is conceivable that the low-fiber and carbohydrate-rich diet, the consumption of hard-shelled fruits, the ability to hibernate, as well as an extremely long lifespan (for an animal of this size) contributed to a relatively high risk of dental trauma and bacterial dental infections as exemplified by the specimen described in this paper.
Authors’ contributions
PB and MC are equal contributors to this work and designated as co-first authors.
Acknowledgements
We would like to thank Łukasz Skomorucha (Veterinary Clinic, Warsaw) and Emilia Klim (STOMAVET, Warsaw) for many helpful suggestions during the early phase of this investigation. We thank the reviewers, Alejandro Pérez-Ramos (Departamento de Ecología y Geología, Facultad de Ciencias, Universidad de Málaga) and Bruce M. Rothschild (Carnegie Museum of Natural History, Pittsburgh, Pennsylvania), for their useful and insightful comments. No additional external funding was received for this study.
References
Aas, J.A., Griffen, A.L., Dardis, S.R., Lee, A.M., Olsen, I., Dewhirst, F.E., Leys, E.J., and Paster, B.J. 2008. Bacteria of dental caries in primary and permanent teeth in children and young adults. Journal of Clinical Microbiology 46: 1407–1417. Crossref
Adler, C.J., Browne, G.V., Sukumar, S., and Hughes, T. 2017. Evolution of the oral microbiome and dental caries. Current Oral Health Reports 4: 264–269. Crossref
Adler, C.J., Dobney, K., Weyrich, L.S., Kaidonis, J., Walker, A.W., Haak, W., Bradshaw, C.J.A., Townsend, G., Sołtysiak, A., Alt, K.W., Parkhill, J., and Cooper, A. 2013. Sequencing ancient calcified dental plaque shows changes in oral microbiota with dietary shifts of the Neolithic and Industrial revolutions. Nature Genetics 45: 450–455. Crossref
Ajdić, D, McShan, W.M., McLaughlin, R.E., Savić, G., Chang, J., Carson, M.B., Primeaux, C., Tian, R., Kenton, S., Jia, H., Lin, S., Qian, Y., Li, S., Zhu, H., Najar, F., Lai, H., White, J., Roe, B.A., and Ferretti, J.J. 2002. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proceedings of the National Academy of Sciences USA 99: 14434–14439. Crossref
Bieber, C. and Ruf, T. 2009. Summer dormancy in edible dormice (Glis glis) without energetic constraints. Naturwissenschaften 96: 165–171. Crossref
Błażejowski, B., Binkowski, M., Bitner, M.A., and Gieszcz, P. 2011. X-ray microtomography (XMT) of fossil brachiopod shell interiors for taxonomy. Acta Palaeontologica Polonica 56: 439–440. Crossref
Bowen, W.H. 2013. Rodent model in caries research. Odontology 101: 9–14. Crossref
Burne, R. 1998. Oral Streptococci: products of their environment. Journal of Dental Research 77: 445–452. Crossref
Cornejo, O.E., Lefébure, T., Pavinski Bitar, P.D., Lang, P., Richards, V.P., Eilertson, K., Do, T., Beighton, D., Zeng, L., Ahn, S.-J., Burne, R.A., Siepel, A., Bustamante, C.D., and Stanhope, M.J. 2013. Evolutionary and population genomics of the cavity causing bacteria Streptococcus mutans. Molecular Biology and Evolution 30: 881–893. Crossref
Daams, R. and de Bruijn, H. 1994. A classification of the Gliridae (Rodentia) on the basis of dental morphology. Hystrix (n.s.) 6: 3–50.
Daoud, A. 1993. Evolution of Gliridae (Rodentia, Mammalia) in the Pliocene and Quaternary of Poland. Acta Zoologica Cracoviensia 36: 199–231.
Fakhruddin, K.S., Ngo, H.C., and Samaranayake, L.P. 2019. Cariogenic microbiome and microbiota of the early primary dentition: a contemporary overview. Oral Diseases 25: 982–995. Crossref
Figueirido, B., Pérez-Ramos, A., Schubert, B.W., Serrano, F., Farrell, A.B., Pastor, F.J., Nevesm, A.A., and Romero, A. 2017. Dental caries in the fossil record: a window to the evolution of dietary plasticity in an extinct bear. Scientific Reports 7: 17813. Crossref
Fostowicz-Frelik, Ł., and Frelik, G. 2010. Earliest record of dental pathogen discovered in a North American Eocene rabbit. Palaios 25: 818–822. Crossref
Freudenthal, M. 2004. Gliridae (Rodentia, Mammalia) from the Eocene and Oligocene of the Sierra Palomera (Teruel, Spain). Treballs del Museu de Geologia de Barcelona 12: 97–173.
Freudenthal, M. and Martín-Suárez, E. 2013. New ideas on the systematics of Gliridae (Rodentia, Mammalia). Spanish Journal of Paleontology 28: 239–252. Crossref
Furseth Klinge, R., Dean, M.C., Risnes, S., Erambert, M., and Gunnaes, A.E. 2009. Preserved microstructure and mineral distribution in tooth and periodontal tissues in early fossil hominin material from Koobi Fora, Kenya. In: T. Koppe, G. Meyer, and K.W. Alt (eds.), Comparative Dental Morphology. Frontiers of Oral Biology 13: 30–35. Crossref
Fuss, J., Uhlig, G., and Böhme, M. 2018. Earliest evidence of caries lesions in hominids reveal sugar-rich diet for a Middle Miocene dryopithecine from Europe. PloS One 13: e0203307 Crossref
Gajdács, M. and Urbán, E. 2020. The pathogenic role of Actinomyces spp. and related organisms in genitourinary infections: discoveries in the new, modern diagnostic era. Antibiotics 2020 (9): 524. Crossref
Goldberg, M., Kulkarni, A.B., Young, M., and Boskey, A. 2011. Dentin: structure, composition and mineralization. Frontiers in Bioscience (Elite Edition) 2011: 711–735. Crossref
Grine, F.E., Gwinnett, A.J., and Oaks, J.H. 1990. Early hominid dental pathology: interproximal caries in 1.5 million-year-old Paranthropus robustus from Swartkrans. Archives of Oral Biology 35: 381–386. Crossref
Hershkovitz, I., Kelly, J., Latimer, B., Rothschild, B.M., Simpson, S., Polak, J., and Rosenberg, M. 1997. Oral bacteria in Miocene Sivapithecus. Journal of Human Evolution 33: 507–512. Crossref
Hoelzl, F., Smith, S., Cornils, J.S., Aydinonat, D., Bieber, C., and Ruf, T. 2016. Telomeres are elongated in older individuals in a hibernating rodent, the edible dormouse (Glis glis). Scientific Reports 6: 36856. Crossref
Humphrey, L.T., De Groote, I., Morales, J., Barton, N., Collcutt, S., Bronk Ramsey, C., and Bouzouggar, A. 2014. Earliest evidence for caries and exploitation of starchy plant foods in Pleistocene hunter-gatherers from Morocco. Proceedings of the National Academy of Sciences USA 111: 954–959. Crossref
Jurczyszyn, M. 2018. Food and foraging preferences of the edible dormouse Glis glis at two sites in Poland. Folia Zoologica 67: 83–90. Crossref
Kahler, B., Moule, A., and Stenzel, D. 2000. Bacterial contamination of cracks in symptomatic vital teeth. Australian Endodontic Journal 26: 115–118. Crossref
Kear, B.P. 2001. Dental caries in an Early Cretaceous ichthyosaur. Alcheringa 25: 987–390. Crossref
Kemp, A. 2003. Dental and skeletal pathology in lungfish jaws and tooth plates. Alcheringa 27: 155–170. Crossref
Klinke, T., Guggenheim, B., Klimm, W., and Thurnheer, T. 2011. Dental caries in rats associated with Candida albicans. Caries Research 45: 100–106. Crossref
Könönen, E. and Wade, W.G. 2015. Actinomyces and related organisms in human infections. Clinical Microbiology Reviews 28: 419–442. Crossref
Larsen, C.S., Knüsel, C.J., Haddow, S.D., Pilloud, M.A., Milella, M., Sadvari, J.W., Pearson, J., Ruff, C.B., Garofalo, E.M., Bocaege, E., Betz, B.J., Dori, I., and Glencross, B. 2019. Bioarchaeology of Neolithic Çatalhöyük reveals fundamental transitions in health, mobility, and lifestyle in early farmers. Proceedings of the National Academy of Sciences USA 116: 12615–12623. Crossref
Lemaître, J.-F., Ronget, V., Tidière, M., Allainé, D., Berger, V., Cohas, A., Colchero, F., Conde, D.A., Garratt, M., Liker, A., Marais, G.A.B., Scheuerlein, A., Székely, T., and Gaillard, J.M. 2020. Sex differences in adult lifespan and aging rates of mortality across wild mammals. Proceedings of the National Academy of Sciences USA 117: 8546–8553. Crossref
Love, R.M. and Jenkinson, H.F. 2002. Invasion of dentinal tubules by oral bacteria. Critical Reviews in Oral Biology and Medicine 13: 171–183. Crossref
Lu, X., Costeur, L., Hugueney, M., and Maridet, O. 2021. New data on early Oligocene dormice (Rodentia, Gliridae) from southern Europe: phylogeny and diversification of the family. Journal of Systematic Palaeontology 19: 169–189. Crossref
Mayer, W.V. and Bernick, S. 1963. Effect of hibernation on tooth structure and dental caries. In: R.F. Sognnaes (ed.), Mechanisms of Hard Tissue Destruction, 285–296. Association for the Advancement of Science, Washington D. C.
Michelich, V.J., Schuster, G.S., and Pashley, D.H. 1980. Bacterial penetration of human dentin in vitro. Journal of Dental Research 59: 1398–1403. Crossref
Moncunill-Solé, B., Isidro, A., Blanco, A., Angelone, C., Rössner, G., and Jordana, X. 2019. The most ancient evidence of a diseased lagomorph: Infectious paleopathology in a tibiofibular bone (Middle Miocene, Germany). Comptes Rendus Palevol 18: 1011–1023. Crossref
Neefs, J.-M., Van de Peer, Y., Hendriks, L., and De Wachter, R. 1990. Compilation of small ribosomal subunit RNA sequences. Nucleic Acids Research 18, Supplement: 2237–2317. Crossref
Ortega-Cuadros, M., Tofiño-Rivera, A.-P., Merini, L.J., and Martínez-Pabón, M.C. 2018. Antimicrobial activity of Cymbopogon citratus (Poaceae) on Streptococcus mutans biofilm and its cytotoxic effects. Revista de Biología Tropical 66: 1519–1529. Crossref
Poinar, G., Jr. 2014. Evolutionary history of terrestrial pathogens and endoparasites as revealed in fossils and subfossils. Advances in Biology 2014: 181353. Crossref
Preus, H., Marvik, O.J., Selvig, K.A., and Bennike, P. 2011. Ancient bacterial DNA (aDNA) in dental calculus from archaeological human remains. Journal of Archaeological Science 38: 1827–1831. Crossref
Raina, V., Nayak, T., Ray, L., Kumari, K., and Suar, M. 2019. A polyphasic taxonomic approach for designation and description of novel microbial species. In: S. Das and H.R. Dash (eds.), Microbial Diversity in the Genomic Era, 137–152. Academic Press, London. Crossref
Reisz, R.R., Scott, D.M., Pynn, B.R., and Modesto, S.P. 2011. Osteomyelitis in a Paleozoic reptile: ancient evidence for bacterial infection and its evolutionary significance. Naturwissenschaften 98: 551–555. Crossref
Richardson, R.L., Fisher, A.K., and Folk, G.E., Jr. 1961. The dental tissues of wild and laboratory-raised hibernating and non-hibernating 13-lined ground squirrels. Journal of Dental Research 40: 1029–1035. Crossref
Ricucci, D., Siqueira, J.F., Jr., Loghin, S., and Berman, L.H. 2015. The cracked tooth: histopathologic and histobacteriologic aspects. Journal of Endodontics 41: 343–352. Crossref
Sala Burgos, N., Cuevaz González, J., and López Martínez, N. 2007. Estudio paleopatológico de una hemimandíbula de Tethytragus (Artiodactyla, Mammalia) del Mioceno Medio de Somosaguas (Pozuelo de Alarcón, Madrid). Coloquios de Paleontología 57: 7–14.
Selig, K.R. and Silcox, M.T. 2021. The largest and earliest known sample of dental caries in an extinct mammal (Mammalia, Euarchonta, Microsyops latidens) and its ecological implications. Scientific Reports 11: 15920. Crossref
Stefaniak, K., Ratajczak, U., Kotowski, A., Kozłowska, M., and Mackiewicz, P. 2020. Polish Pliocene and Quaternary deer and their biochronological implications. Quaternary International 546: 64–83. Crossref
Storch, G. and Seiffert, C. 2007. Extraordinarily preserved specimen of the oldest known glirid from the Middle Eocene of Messel (Rodentia). Journal of Vertebrate Paleontology 27: 189–194. Crossref
Striczky, L. and Pazonyi, P. 2014. Taxonomic study of the dormice (Gliridae, Mammalia) fauna from the late Early Pleistocene Somssich Hill 2 locality (Villány Hills, South Hungary) and its palaeoecological implications. Fragmenta Palaeontologica Hungarica 31: 51–81. Crossref
Sulimski, A. 1962. O nowym znalezisku kopalnej fauny kręgowców w okolicy Działoszyna. Przegląd Geologiczny 10: 291–223.
Surmik, D, Szczygielski, T., Janiszewska, K., and Rothschild, B.M. 2018. Tuberculosis-like respiratory infection in 245-million-year-old marine reptile suggested by bone pathologies. Royal Society Open Science 5: 180225. Crossref
Tillier, A.M., Arensburg, B., Rak, Y., and Vandermeersch, B. 1995. Middle Palaeolithic dental caries: new evidence from Kebara (Mount Carmel, Israel). Journal of Human Evolution 29: 189–192. Crossref
Towle, I., Irish, J.D., De Groote, I., Fernée, C., and Loch, C. 2021. Dental caries in South African fossil hominins. South African Journal of Science 117: 8705. Crossref
Trinkaus, E., Smith, R.J., and Lebel, S. 2000. Dental caries in the Aubesier 5 Neandertal primary molar. Journal of Archaeological Science 27: 1017–1021. Crossref
Ushatinskaya, G.T. 2009. Preservation of living organic structures in unicellular and multicellular organisms in the fossil state. Paleontological Journal 43: 928–939. Crossref
Watanabe, I.-S., Ogawa, K., Cury, D.P., Dias, F.J., Consentino Kronka Sosthenes, M., Mardegan Issa, J.P., and Iyomasa M.M. 2013. Fine structure of bacterial adhesion to the epithelial cell membranes of the filiform papillae of tongue and palatine mucosa of rodents: a morphometric, TEM, and HRSEM study. Microscopy Research and Technique 76: 1226–1233. Crossref
Westall, F. 1999. The nature of fossil bacteria: a guide to the search for extraterrestrial life. Journal of Geophysical Research 104: 437–451. Crossref
Weyrich, L.S., Duchene, S., Soubrier, J., Arriola, L., Llamas, B., Breen, J., Morris, A.G., Alt, K.W., Caramelli, D., Dresely, V., Farrell, M., Farrer, A.G., Francken, M., Gully, N., Haak, W., Hardy, K., Harvati, K., Held, P., Holmes, E.C., Kaidonis, J., Lalueza-Fox, C., de la Rasilla, M., Rosas, A., Semal, P., Soltysiak, A., Townsend, G., Usai, D., Wahl, J., Huson, D.H., Dobney, K., and Cooper, A. 2017. Neanderthal behaviour, diet, and disease inferred from ancient DNA in dental calculus. Nature 544: 357–361. Crossref
Wolff, E.D.S., Salisbury, S.W., Horner, J.R., and Varricchio, D.J. 2009. Common avian infection plagued the tyrant dinosaurs. PLoS ONE 4 (9): e7288. Crossref
You, M.S., Lee, S.Y., and Ma, D.S. 2017. In vitro antimicrobial activity of different mouthwashes available in Korea. Journal of Korean Academy of Oral Health 41: 188–193. Crossref
Zapalski, M.K., Kise, H., Dohnalik, M., Yoshida, R., Izumi, T., and Reimer, J.D. 2021. Hexacoral-crinoid associations from the modern mesophotic zone: ecological analogues for Palaeozoic associations. Palaeogeography, Palaeoclimatology, Palaeoecology 572: 110419. Crossref
Acta Palaeontol. Pol. 69 (2): 217–225, 2024
https://doi.org/10.4202/app.01125.2023