


Deciphering the evolutionary history of early Mesozoic fossil corals
BERNARD LATHUILIÈRE, DANWEI HUANG, and THE CORALLOSPHERE GROUP
Lathuilière, B., Huang, D., and the Corallosphere Group. 2024. Deciphering the evolutionary history of early Mesozoic fossil corals. Acta Palaeontologica Polonica 69 (2): 249–262.
The morphology of stony corals (Scleractinia) remains the only means to reconstruct the most inclusive evolutionary history of the clade comprising both extant and extinct species. The definitions of morphological characters and their associated trait states are critical for assembling a dataset that could be analysed for phylogenetic reconstruction. Here, we present coral morphological data that consist of more than a hundred characters reviewed by the Corallosphere working group. These characters would eventually form the basis of a data matrix used to reconstruct the phylogeny of all extinct and extant scleractinian families. The initial results obtained by the working group comprise poorly resolved trees, which are biased by the complexity of the multiple character states and the multiplicity of researchers involved in the coding process. When the analysis is restricted to matrices consisting of families from the Triassic and Jurassic periods and coded by a single person, resolution increased, allowing for further exploration of various ingroups and outgroups. The results presented here represent analyses of (i) a data matrix with all families represented by their type genus; (ii) a data matrix with selected families represented by their solitary or phaceloid genera; (iii) a data matrix with only Triassic corals; (iv) a data matrix with only Jurassic corals; (v) a data matrix with Triassic and Jurassic corals; and (vi) data matrices with several outgroups. Well-resolved trees have been obtained in several cases. Phylogenetic relationships among basal, robust and complex groups established using molecular data are discussed in the context of the morphological phylogeny obtained here.
 This paper was presented during the 14th
Symposium of the International Fossil Coral and Reef Society (IFCRS),
Poland, 10–16 September 2023.
This paper was presented during the 14th
Symposium of the International Fossil Coral and Reef Society (IFCRS),
Poland, 10–16 September 2023. Key words: Anthozoa, Scleractinia, evolution, phylogeny, cladistics, Triassic, Jurassic.
Bernard Lathuilière [bernard.lathuiliere@univ-lorraine.fr; ORCID: https://orcid.org/0000-0001-5088-30571 ], Université de Lorraine, CNRS, lab. GeoRessources UMR 7359, BP 70239, 54506, Vandoeuvre-lès-Nancy Cedex, France.
Danwei Huang [huangdanwei@nus.edu.sg; ORCID: https://orcid.org/0000-0003-3365-5583 ], Lee Kong Chian Natural History Museum, National University of Singapore, 2 Conservatory Drive, Singapore, 117377, Singapore.
Received 15 January 2024, accepted 6 May 2024, published online 14 June 2024.
Copyright © 2024 B. Lathuilière et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
For two centuries of research on fossil corals, coral workers have studied the taxonomic classification of corals. The incredibly wide array of morphological possibilities of these organisms has resulted in an enormous diversity of nominal taxa, which is not always justified whatever the taxonomic rank. Reconstruction of the evolutionary history of Scleractinia began with some rare early attempts to understand phylogenetic relationships among corals at the family and superfamily levels. The drawing by Volz (1896) at the end of a monograph devoted to Late Triassic corals was a significant early hypothesis. Already in this depiction, the basic question of the evolutionary link between the Paleozoic Rugosa and the modern corals was considered. This question has been subsequently treated by various authors in different ways. Some of them assumed Rugosa to be the ancestor of Scleractinia (Schindewolf 1942; Cuif 1977), while others assumed a separated origin from non-skeletal cnidarians (Oliver 1980a,b; Stanley and Schootbrugge 2009) or an ancient common origin to Scleractinia and Rugosa (Stolarski et al. 2011).
The long-standing debate was mainly based on the septal insertion, the original mineralogy of the skeleton, and also on the Early Triassic gap in the fossil record. This debate was complicated by several issues, including the discovery of scleractinian-like corals in the Paleozoic (Kilbuchophyllida; Scrutton and Clarkson 1991), the discovery of calcitic scleractinians (Stolarski et al. 2007), the splitting of the order Scleractinia proposed by some authors with the appearance of the new order Hexanthiniaria (Montanaro-Gallitelli 1975), the new order Lemniscaterina (Montanaro-Gallitelli 1979), the Scleractiniamorph informal group characterised by the genus Furcophyllia (Stolarski et al. 2004), and the new incertae sedis family Hispaniastraeidae (Boivin et al. 2019), which has the shape of tabulate corals. The discovery that scleractinians can abandon their skeleton in some situations and that some soft corals appear to be derived from stony corals has also been explored for its phylogenetic consequences (Stanley and Fautin 2001; Medina et al. 2006).
In parallel, the growing interest in cladistic methods has led to phylogenetic trees reconstructed based on molecular data of extant corals (Romano and Palumbi 1996; Kitahara et al. 2016; Quattrini et al. 2020; Quek et al. 2023). These trees demonstrated the necessity of a profound revision of the phylogeny of fossil corals (for instance those of Wells 1956; Krasnov 1970; Veron 1995). Previous attempts to apply such methods to morphological characters have been proposed for some restricted parts of the tree (for instance Fungiidae in Cairns 1984; Budd and Bosellini 2016; Budd et al. 2019) or at least to present the evolution of some groups with regards to synapomorphies (pachythecal corals in Stolarski and Russo 2001).
Analyses of the scleractinian phylogeny based on skeletal characters have had to grapple with the low numbers of stable characters. The homoplastic nature of many morphological characters resulting in iterative evolution (Lathuilière 1996a, b) is, of course, a challenge. The reticulate evolutionary pattern thought to have driven the diversification of scleractinians (Veron 1995) is also a challenge for cladistic approaches but it is assumed that this process is more pertinent at lower taxonomic levels (species, genera) than for higher levels (families, suborders). The revision of Scleractinia for the Treatise on Invertebrate Paleontology has been an opportunity to examine the definition of morphological terms by the Corallosphere working group (see the list of participants at the end of this paper). This work has led to the establishment of a list of 120 characters with their character states that will be used to create new phylogenetic reconstruction based on skeletal morphology, which can be contrasted with the molecular phylogeny (e.g., Huang et al. 2014, 2016; Kitahara et al. 2016). Due to poorly resolved results of our past unpublished attempts, we limit our investigation in this paper to the early history of corals (Jurassic and Triassic) analysed with a restricted set of characters.
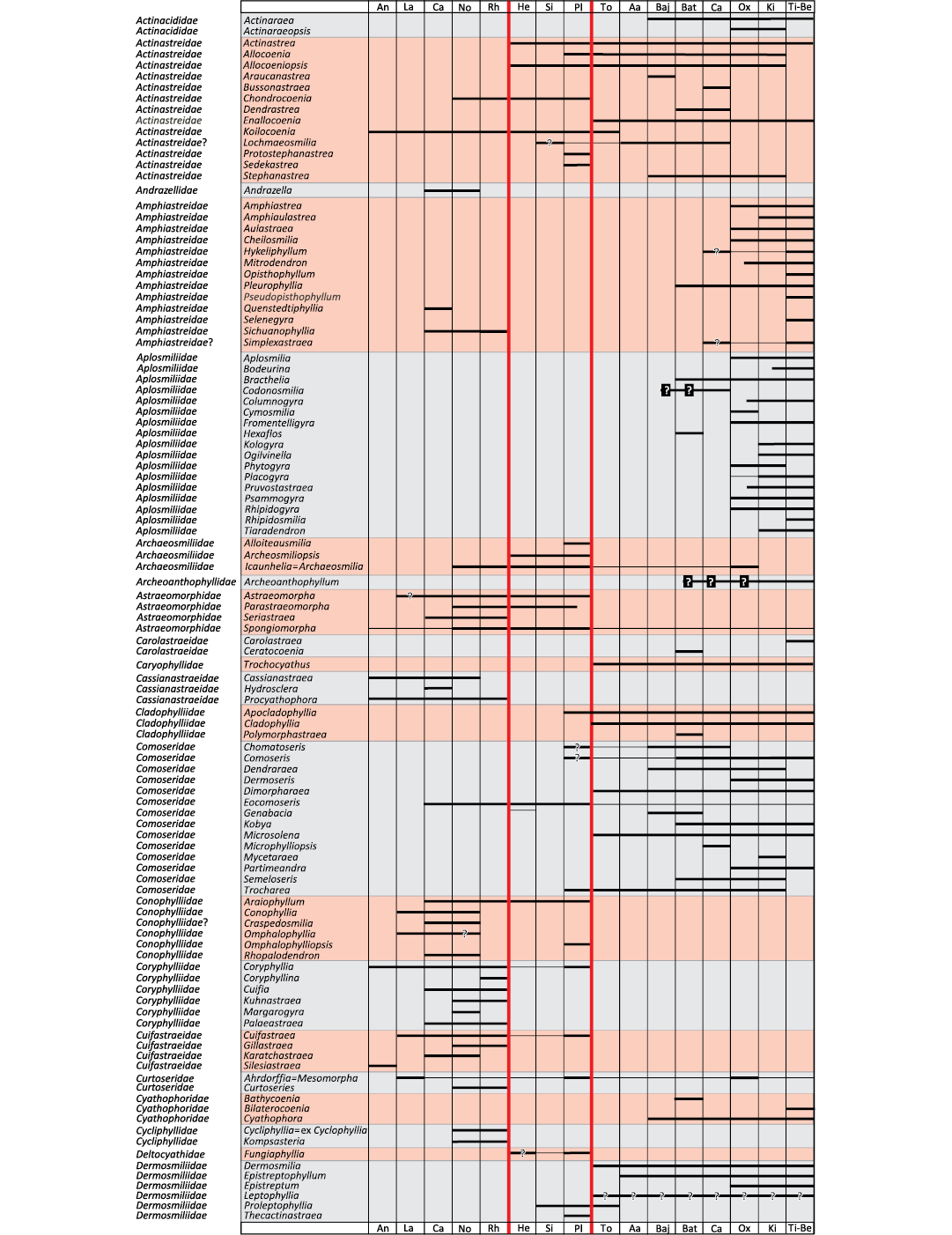
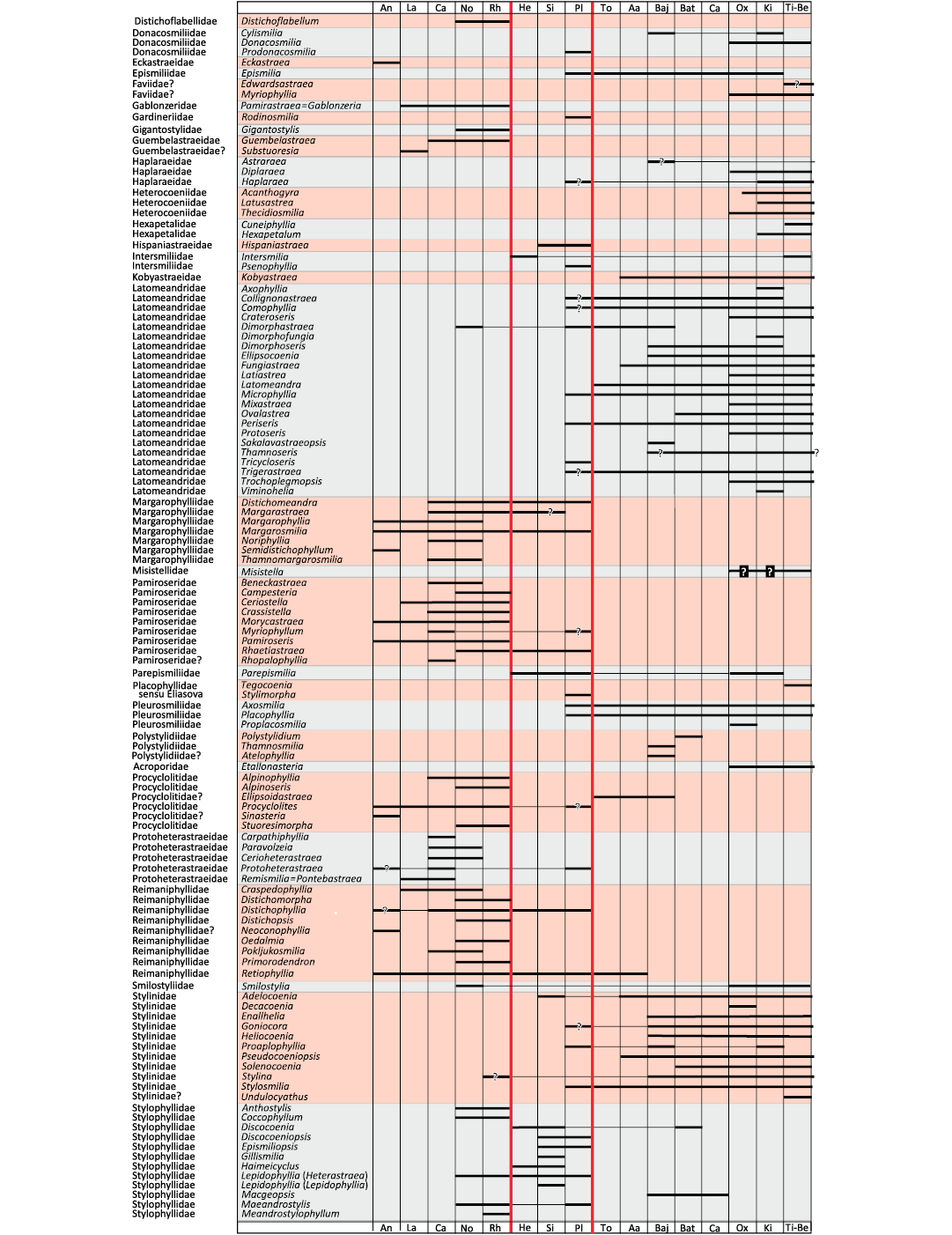
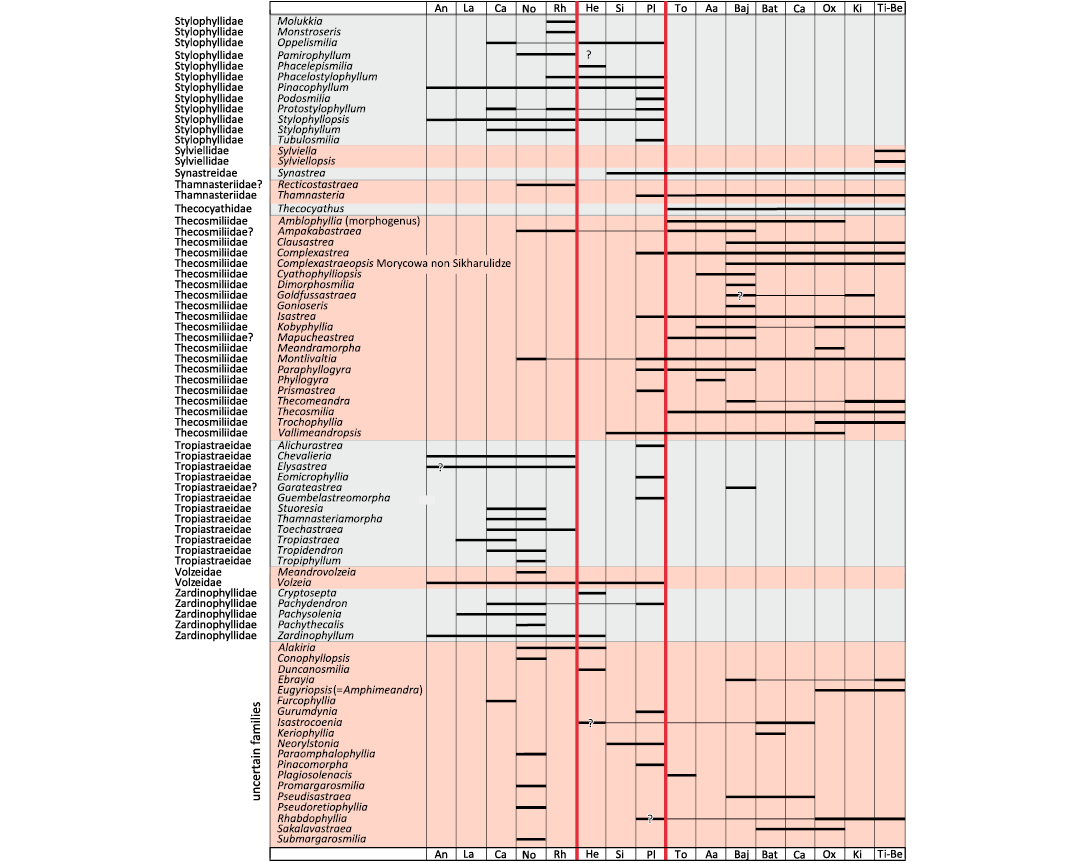
Fig. 1. Biostratigraphic chart of coral genera. Question marks with no background refer to taxonomic uncertainties. Question marks with black background refer to chronostratigraphic uncertainties. Major extinction events in vertical red lines.
Material and methods
A total of 120 characters (with two or more character states) for scleractinian families were consolidated from literature and processed in Mesquite 3.6 (Maddison and Maddison 2021). A first subset of 98 characters that apply to Jurassic and Triassic was extracted from the full dataset for analysis. A second subset of 75 characters that apply only to solitary or phaceloid corals was also extracted for separate analysis. These characters are given in SOM 1: table 1 (Supplementary Online Material available at http://app.pan.pl/SOM/app69-Lathuiliere_etal_SOM.pdf). Triassic and Jurassic families were coded by a single researcher (BL) to avoid discrepancies in the interpretation of morphological terms. In the first series of analyses, all Triassic and Jurassic families were coded based on their type genera. This may be a simplification that can obscure the intra-familial variability. Choosing the type genus is just a way to avoid further nomenclatural confusion in an already complex taxonomic situation. In a second series of runs, only solitary and phaceloid forms were considered. Because of this limitation, the type genus of the family could not always be coded. For instance, Thamnasteriidae, a family devoid of solitary or phaceloid forms, were coded , to a young specimen of Thamnasteria mettensis. In addition, several families could not be included in this second series of runs. Matrices are given in SOM 2.
Maximum parsimony (MP) trees were reconstructed using tree searches performed in TNT 1.5 (Goloboff 1999; Nixon 1999; Goloboff et al. 2008). Gardineria (the type genus of the Gardineriidae for its place as the basal branch in molecular trees), Numidiaphyllum (as a good candidate for ancestry among Rugosa) and Kilbuchophyllia (as a Paleozoic scleractinian-like possible ancestor) were coded as outgroups independently in separate datasets. Each dataset was analysed with 10 000 random additions, with each utilising 100 cycles of sectorial searches, ratcheting, drifting, and tree fusing (Goloboff et al. 2008). Gaps were treated as missing data. Node supports were assessed using 10 000 bootstrap pseudoreplicates.
To date the divergence of branches, a biostratigraphic chart (Fig. 1) for all Triassic and Jurassic genera (around 300 genera) was constructed to determine the first occurrence of each family. As it is often a matter of debate, for traceability requirements, the first and last occurrences taken into consideration are presented here with the corresponding bibliographical references in SOM 1: table 2. Uncertainties in identifications were distinguished from chronological uncertainties. Long absences of occurrences between two occurrences were graphically marked. The resolution was that of a geological stage. In other words, a genus considered to occur in the Domerian substage was considered present throughout the whole Pliensbachian stage.
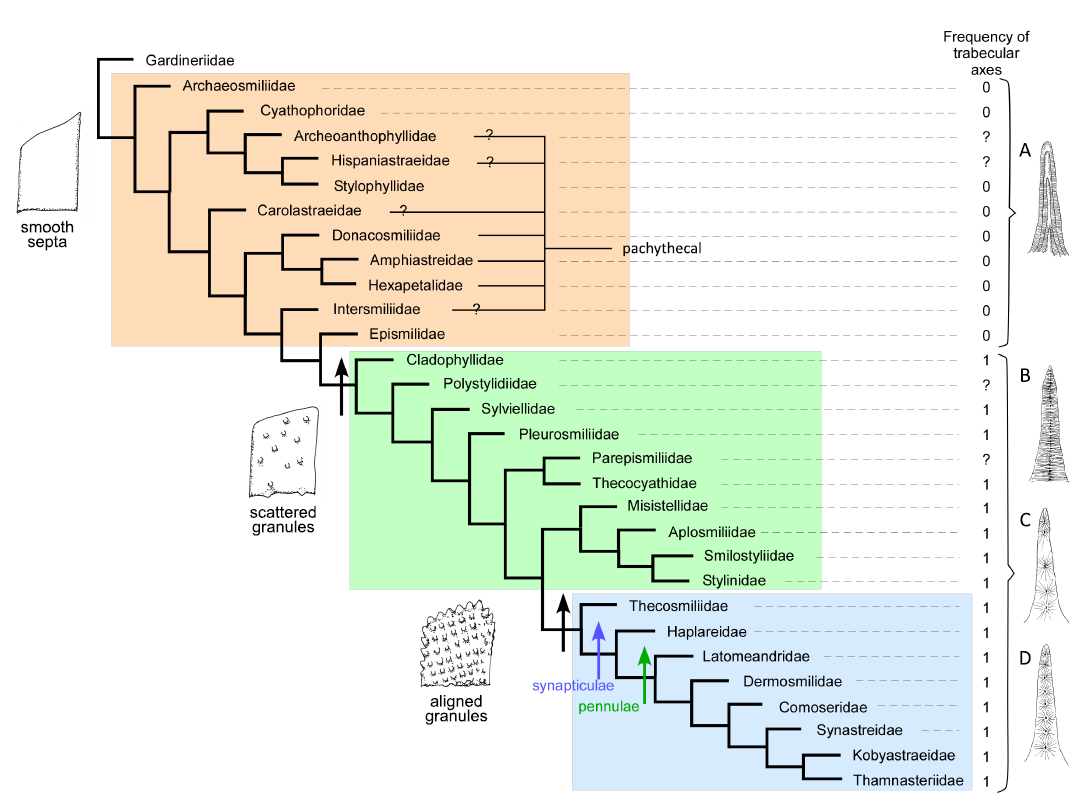
Fig. 2. Maximum parsimony cladogram of Jurassic families inferred with 98 characters and Gardineriidae as outgroup. The four drawings at right illustrate character states: septum dominated by thickening deposits (A), septum with close trabecular axes (B), septum with irregularly placed trabecular axes (C), and septum with regularly placed trabecular axes (D). Coloured frames represent the groups defined by the type of ornamentation of septa. Black arrows are characters states of septal ornamentation (illustrated) the blue arrow corresponds to the first appearance of synapticulae and green arrow to the first appearance of pennulae (both not illustrated).
Results
The first result involves a set of 30 Jurassic families represented by their type genus and analysed with 98 characters. The outgroup is Gardineriidae. The resulting cladogram, presented in Fig. 2 is generally well-resolved. The cladogram can be interpreted by the delimiting three groups: (i) a basal group characterised by smooth septa and including pachythecal corals and corals dominated by thickening deposits; (ii) a middle group characterised by scattered granules with closely packed trabecular axes; (iii) an apical well-defined group with spaced trabecular axes generally producing small columns referred to as “trabeculae” in the past literature, accompanied by the gains of synapticulae and pennulae.
To test the extent to which colonial structures may influence taxon placement on the cladograms, a dataset with only solitary and phaceloid forms was analysed independently with fewer characters and a more limited number of families (Fig. 3).
To illustrate the impact of the choice of outgroup, an unrooted tree is also presented (Fig. 4).
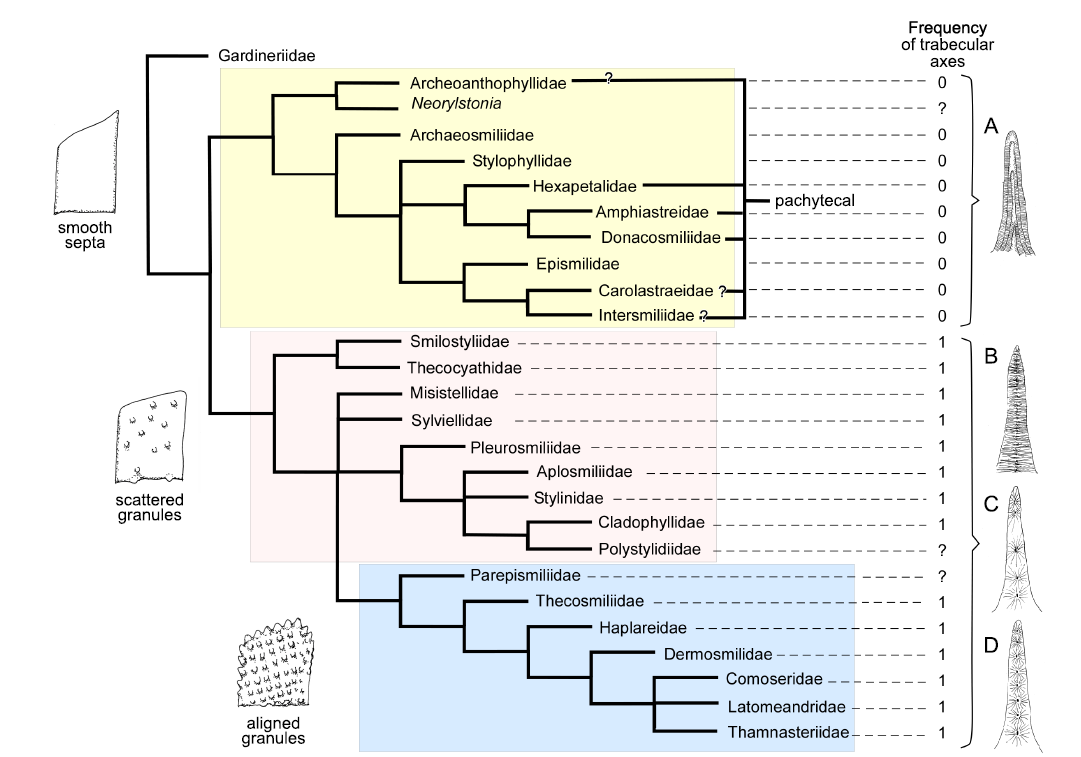
Fig. 3. Strict consensus of eight maximum parsimony cladograms of Jurassic families represented by solitary or phaceloid genera inferred with 75 characters and Gardineriidae as outgroup. The four drawings at right illustrate character states: septum dominated by thickening deposits (A), septum with close trabecular axes (B), septum with irregularly placed trabecular axes (C), and septum with regularly placed trabecular axes (D). Coloured frames represent the groups defined by the type of ornamentation of septa. colours are independent between figures because analyses are independent. Coloured frames represent the groups defined by the type of ornamentation of septa. Colours are independent between figures because analyses are independent.
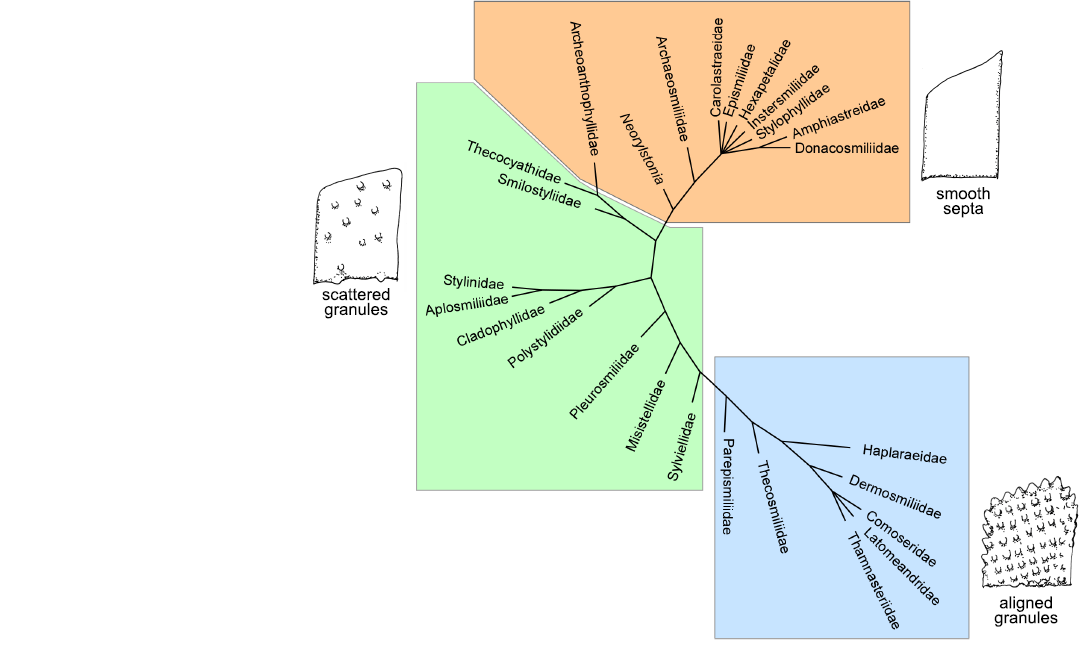
Fig. 4. Strict consensus of four maximum parsimony cladograms of 26 Jurassic families represented by solitary or phaceloid genera inferred with 75 characters and without outgroup.
Triassic families treated with the whole set of 98 characters with the same outgroup do not produce a well-resolved tree (Fig. 5). Pachythecal corals represented by Zardinophyllidae appear to be basal. A small group of pennular corals appear as apical (Astreomorphidae, Pamiroseriidae, and Cuifastraeidae) but ornamented septa appear in mixed sequences with smooth septa families.
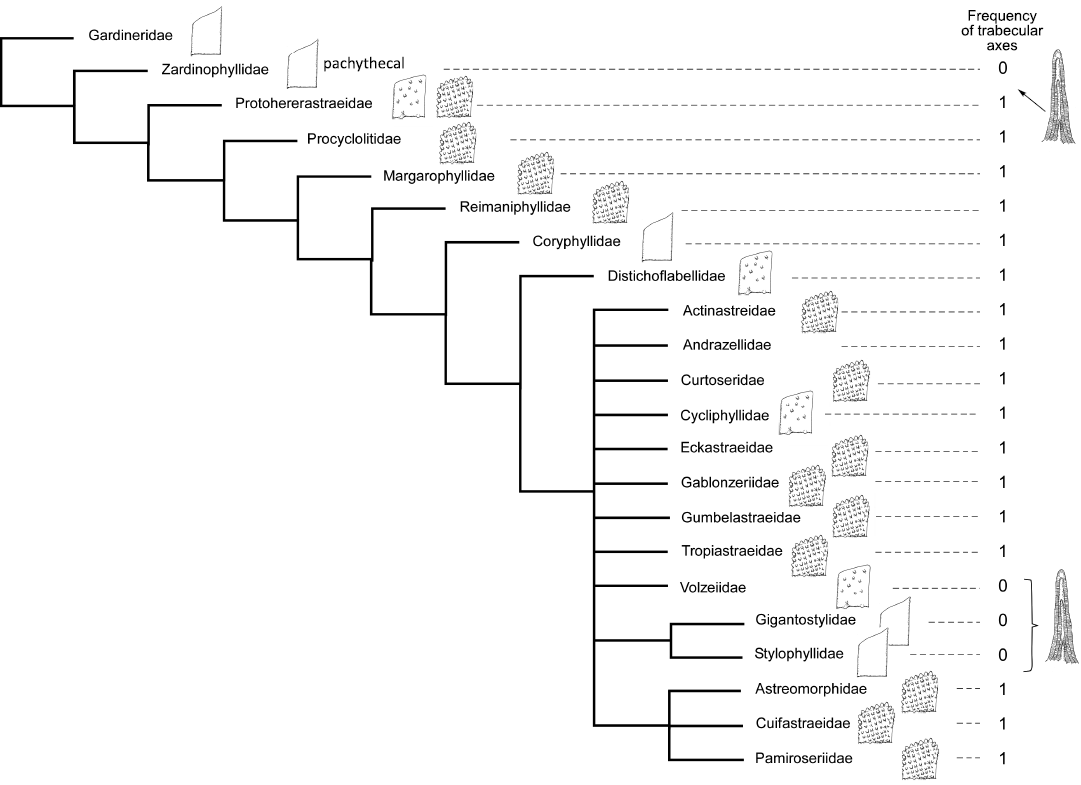
Fig. 5. Strict consensus of 12 maximum parsimony cladograms of 21 Triassic families inferred with 98 characters and Gardineriidae as outgroup. Schematic septa (smooth, with non-aligned granules and with aligned granules) are presented. The drawings at right illustrate character state: septum dominated by thickening deposits.
Another cladogram obtained with poor resolution is a Triassic tree with the families represented by their type genera and with the subset of 98 characters in which Kilbuchophyllia is used as the outgroup. Here the tree only shows the apical group characterised by highly ornamented septa with spaced trabecular axes (with an exception for Astraeomorphidae that show pennular ornamentation but not these microstructural features).
The tree obtained with only solitary and phaceloid forms is much more resolved and shows different groups according to the morphology and structure of septa. The apical group characterised by a structured ornamentation and most derived character states is well-defined when a basal group seems also well-resolved with Zardinophyllidae (a family excluded from the Scleractinia by some authors, e.g., Montanaro Gallitelli 1975) near the base of the phylogeny.
Finally, we present a comprehensive cladogram with both Triassic and Jurassic families based on 75 characters for a total set of 39 families each represented by a solitary/phaceloid genus (Fig. 8).
A chronological calibration was attempted, taking into account the fossil record of genera that belong to studied families. For the Jurassic, based on the results of Figs. 1 and 2 we obtained the Fig. 9. For the Triassic, we combined the results of Fig. 1 and Fig. 7 to obtain the Fig. 10.
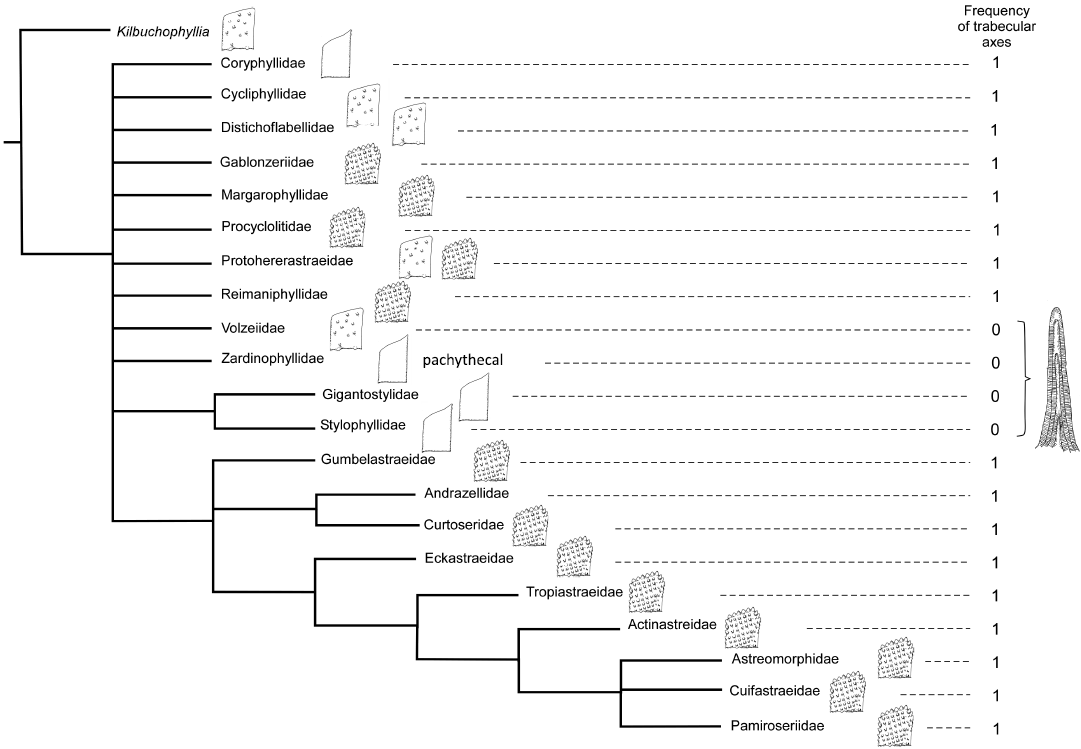
Fig. 6. Strict consensus of four maximum parsimony cladograms of 21 Triassic families inferred with 98 characters and Kilbuchophyllia as outgroup. Schematic septa (smooth, with non-aligned granules and with aligned granules) are presented. The drawing at right illustrates character state: septum dominated by thickening deposits.
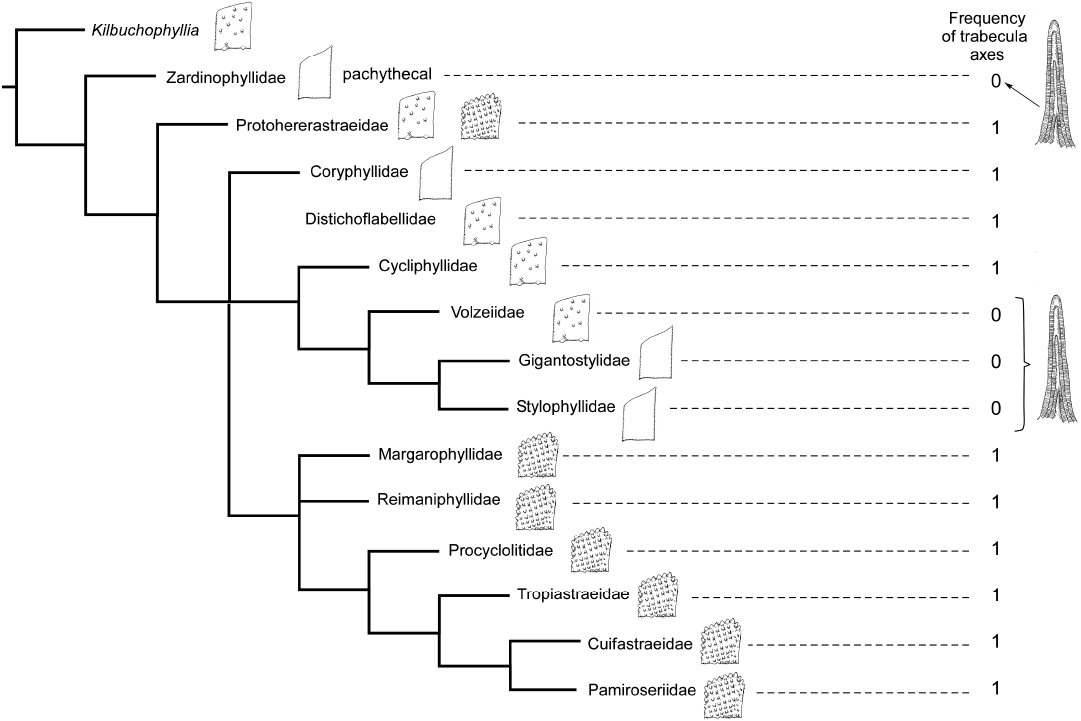
Fig. 7. Strict consensus of three maximum parsimony cladograms of 14 Triassic families represented by solitary or phaceloid genera, inferred with 75 characters and Kilbuchophyllia as an outgroup. Schematic septa (smooth, with non-aligned granules and with aligned granules) are presented. The drawings at right illustrate character state: septum dominated by thickening deposits.
Discussion
The first significant result is that well-resolved trees can be obtained from morphological data with a limited number of taxa (for instance Fig. 2). When the number of families increases, the number of polytomic nodes tends to increase as well (Fig. 8). It is suggested that a broad field of new investigations is open with this large set of characters and many new analyses can be done, but it is preferable to limit the number of taxa either by restricting the age of taxa or by selecting a part of the phylogeny that is supported by more data.
Importantly, the question of the legitimacy of taxonomic boundaries between families must be re-evaluated. Are families really built on synapomorphies? Are families too split or too diverse for their morphologies to be represented each by a single taxon? The latter question is especially relevant as each family was coded based on a single genus and, consequently the variability of the family is not taken into account. Are Thamnasteriidae and Pamiroseriidae distinct from each other, and from Recent Agariciidae? Is the Periseris Jurassic genus placed within Latomeandridae distinct from the Triassic Thamnasteriamorpha placed within Tropiastreidae? Did the practice of taxonomists specializing in different chronological time slices introduce some bias in the current taxonomic frame? Fig. 8 in which Jurassic and Triassic families alternate in the apical branch fuels this debate.
Another critical point concerns the definitions of morphological characters and their different states. Despite the careful attention to avoid ambiguity and false homologies, some subjectivity remains in the coding process especially when a transition can occur between two character states. For example, Stylophyllidae is considered having smooth septa, which is disputable according to the scale and also according to the choice of representatives (see for instance their septal spines with their “shaggy” ornamentation in Stolarski and Russo 2002). Another example is related to the difficulty of gathering microstructural details from the type material. For example, in Amphiastrea we lack microstructural data for the type species (Amphiastrea basaltiformis), and character coding was performed on Amphiastrea gracilis according to Eliášová (1975).
Interestingly, the relative stability of the large groups of families can be traced. The most stable group is the apical group characterised by regularly aligned granules. In this group, costae are systematically present, the mid-septal line disappears resulting in regularly spaced trabecular axes. Synapticulae and pennular septa also progressively appear. For this reason, we relate this stable group to the Complex clade of Recent corals as it is defined by the molecular phylogenetic approach (Romano and Palumbi 1996; Kitahara et al. 2016; Quattrini et al. 2020; Quek et al. 2023). This is also in accordance with the study by Cuif et al. (2003), which placed agariciids among complex corals based both molecular phylogeny and septal ornamentation. In the well-resolved Jurassic trees, the different datasets produce similar groupings (Figs. 2–4). In less resolved Triassic trees, the distribution is more unstable but the apical group remains rather coherent. Incorporating Jurassic and Triassic families (Fig. 8) does not perturb this apical group.
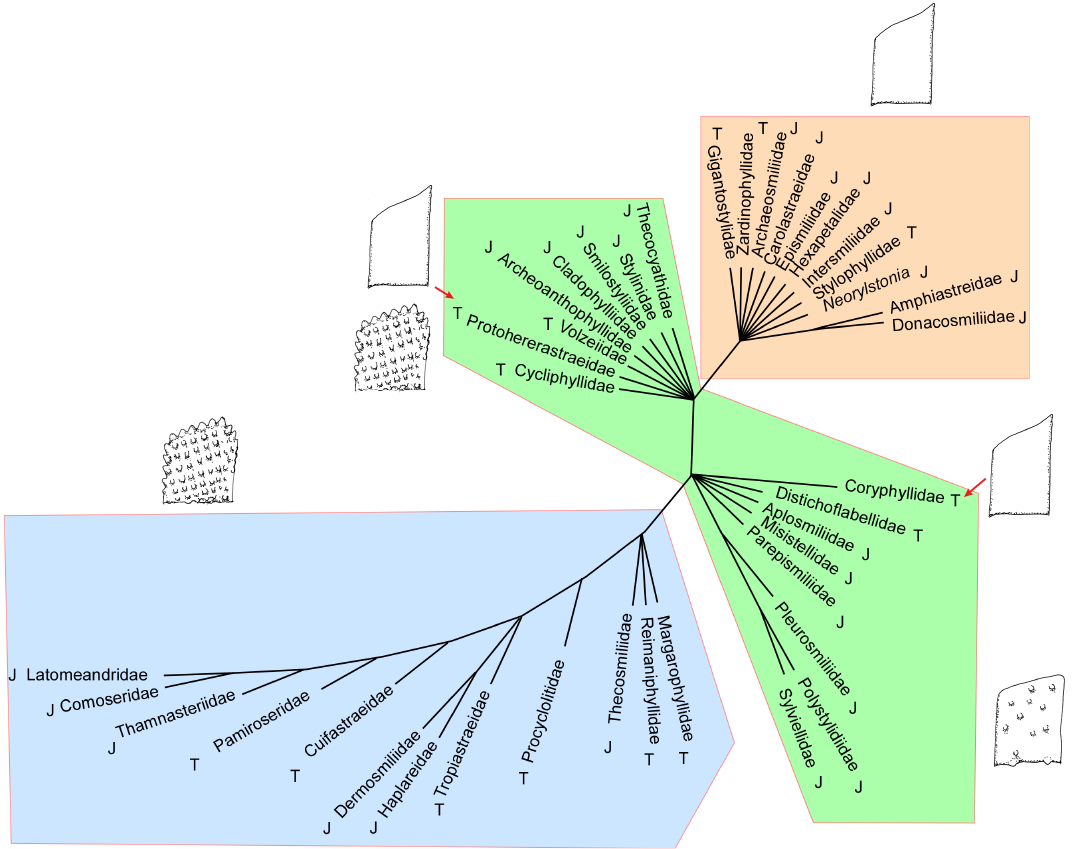
Fig. 8. Strict consensus of 48 maximum parsimony cladograms of 39 Triassic (T) and Jurassic (J) families represented by solitary or phaceloid genera, inferred with 75 characters and without outgroup. Schematic septa (smooth, with non-aligned granules and with aligned granules) are presented; exceptions indicated by red arrows.
Tracing boundaries between the Basal clade (as defined in Kitahara et al. 2016) and the Robust clade is more difficult. This boundary also appears to be tentative in Recent phylogenomic studies (Quattrini et al. 2020; Quek et al. 2023), which placed Gardineriidae and Micrabaciidae (represented by Rhombopsammia, Letepsammia, and Stephanophyllia) separately at the base of the Robust clade rather than sister to the entire Scleractinia. The Jurassic trees produce two separate groups at the bottom of the tree. One is characterised by smooth septa, the other one by scattered granules on the septa (Figs. 3, 4). This distinction is not sensitive to the change in initial data (98 characters versus 75, see Fig. 2, 3 and Fig. 4). One exception worth noting is the placement of Archeoanthophyllidae (a badly known and poorly represented family) in the scattered granules group.
For the earlier families of the Triassic, the distinction of the groups is much more difficult or even impossible (Figs. 5–7). In the most comprehensive tree (Fig. 8), the distinction between smooth septa corals and scattered granules corals is again significant even if the intermediate group cannot be fully explained by this single character. The general structure of the latter tree calculated without outgroup, allows the recognition of a global picture driven by a gradient in morphological complexity. One tip of the tree links the simplest shapes (for instance Archeosmiliidae), and the opposite tip shows highly ornamented septa (typically Comoseridae, with trabeculae, pennulae, synapticulae and pores). Unfortunately, it is not known if this general structure illustrates the history of the evolution of corals or if it traces the way that evolution has followed in several convergent episodes. Cryptosepta, an extremely simplified zardinophyllid coral (nearly only a wall with very rudimentary septa) was described in a post-extinction Triassic–Jurassic context (Gretz et al. 2015), suggesting that evolutionary reversions (reinitialization of evolutionary clocks, according to the terminology of Guex 2006) can happen.
Pachythecal corals, including Zardinophyllidae and Amphiastreidae, are worth mentioning because the position of the group, inside or outside Scleractinia, is a matter of debate (Geistova and Kołodziej 2023). Koby (1888) placed the Amphiastreidae among Rugosa and subsequent authors made a special order for these corals (Hexanthiniaria; Montanaro-Gallitelli 1975) and some others explicitly associate this group with rugosan ancestors (Melnikova and Roniewicz 1976). Some others include them in the Scleractinia (Roniewicz and Stolarski 1999; Stolarski and Russo 2001) and proposed a phyletic history. In our cladograms, all involved families are not far from the base of the tree. In Triassic trees, Zardinophyllidae is always located nearest to the outgroup, regardless of the outgroup (Scleractinian or Rugosan). In Jurassic trees, pachythecal families (Amphiastreidae, Donacosmiliidae, Intersmiliidae, Hexapetalidae, Carolastraeidae) are often mixed with classical Scleractinia like Epismiliidae or Stylophyllidae; even when the outgroup is a Rugosan (Numidiaphyllum), the Amphiastreidae are separated from the outgroup by a clear scleractinian coral. In summary, our cladogram suggests an inclusion of pachythecal corals within Scleractinia but does not provide a very strong argument for this inclusion. The phylogenetic history of the group still needs more work to document possible Paleozoic ancestors as well as possible records of the lineage between the Liassic zardinophyllids and the Late Jurassic radiation of the group (Fig. 1).
The distribution of characters that structured the most inclusive cladogram (Fig. 8) calls for some remarks about the Cenozoic family Fungiidae. In molecular trees, Fungiidae appear as a recently derived branch within the Robust clade. The family shows morphological characters very close to Complex corals such as dentition of the septal distal edge, regularly spaced trabecular axes, and well-organized lateral ornamentation of septa. Even some species such as Cycloseris chinensis have produced pennulae. All these characters are likely to have arisen independently in different lineages. However, the fulturae of Fungiidae constitute a distinctive synapomorphic character that should not be considered as homologous in a manner similar to the synapticulae developed in the Complex group. The case of Fungiidae is interesting because it demonstrates that morphological evolutionary canalizations and convergent evolution exist and these issues are challenging for a cladistics approach based on morphological characters. However, as Fungiidae appear lately derived in molecular trees, it shows also that one solution is to limit the analysis to a smaller subset of taxa based on a time slice or a more restricted clade.
Taxonomic question.—The new cladograms of early Mesozoic corals suggest that early Mesozoic corals could be separated into two or three taxonomic groups. This partition could be related to the two (or three clades): (Basal?), Robust and Complex groups based on molecular studies. This taxonomic partition conflicts with all traditional classifications (Alloiteau 1952; Wells 1956; Beauvais 1981; Beauvais in Chevalier 1987; Veron 1995) that divided the order Scleractinia in various numbers of suborders that are generally incompatible with the divisions of Basal, Robust and Complex corals. Consequently, we do not recommend the use of these suborders with their varied conceptions. Our cladograms suggest that only the complex group is holophyletic and many extinct branches occur along the evolutionary path from the first Scleractinia to the emergence of the complex group. We agree with the use of paraphyly to designate these early groups characterised both by synapomorphies and symplesiomorphies (Hörland and Stuessy 2010).
Nomenclatural question.—What names should be applied to these groups? We note that Löser (2016) decided to apply the uncommonly used rank of superfamilies to group families independently of explicit phylogenetic hypotheses. We note also that Okubo (2016) has proposed two groups Refertina and Vacatina embryologically defined by the shape of the blastocoele that could respectively correspond to “Complex” and “Robust” clades. The Code on Zoological Nomenclature regulates taxonomic ranks up to the family group (Article 1). However, in the same article, it states that some articles regulate names of taxa at ranks above the family group. Among these articles, there is no mention of Article 23, which regulates the principle of priority and validity of taxa and no mention of Article 61 about the principle and use of type bearing names, so Refertina and Vacatina should be recognized as valid taxonomic units. However, as the shape of the blastocoele is not accessible for fossil corals, and as the relationship between embryology and topology of trees based on molecular cladistics is not very unambiguously established, we retain the practical naming of Basal, Robust and Complex groups as informal taxonomic categories to distribute fossil coral families in place of traditional suborders. The use of superfamilies (the highest rank in the family group), which are regulated by the code for their availability and validity does not seem necessary in our present state of knowledge on the phylogeny of corals.
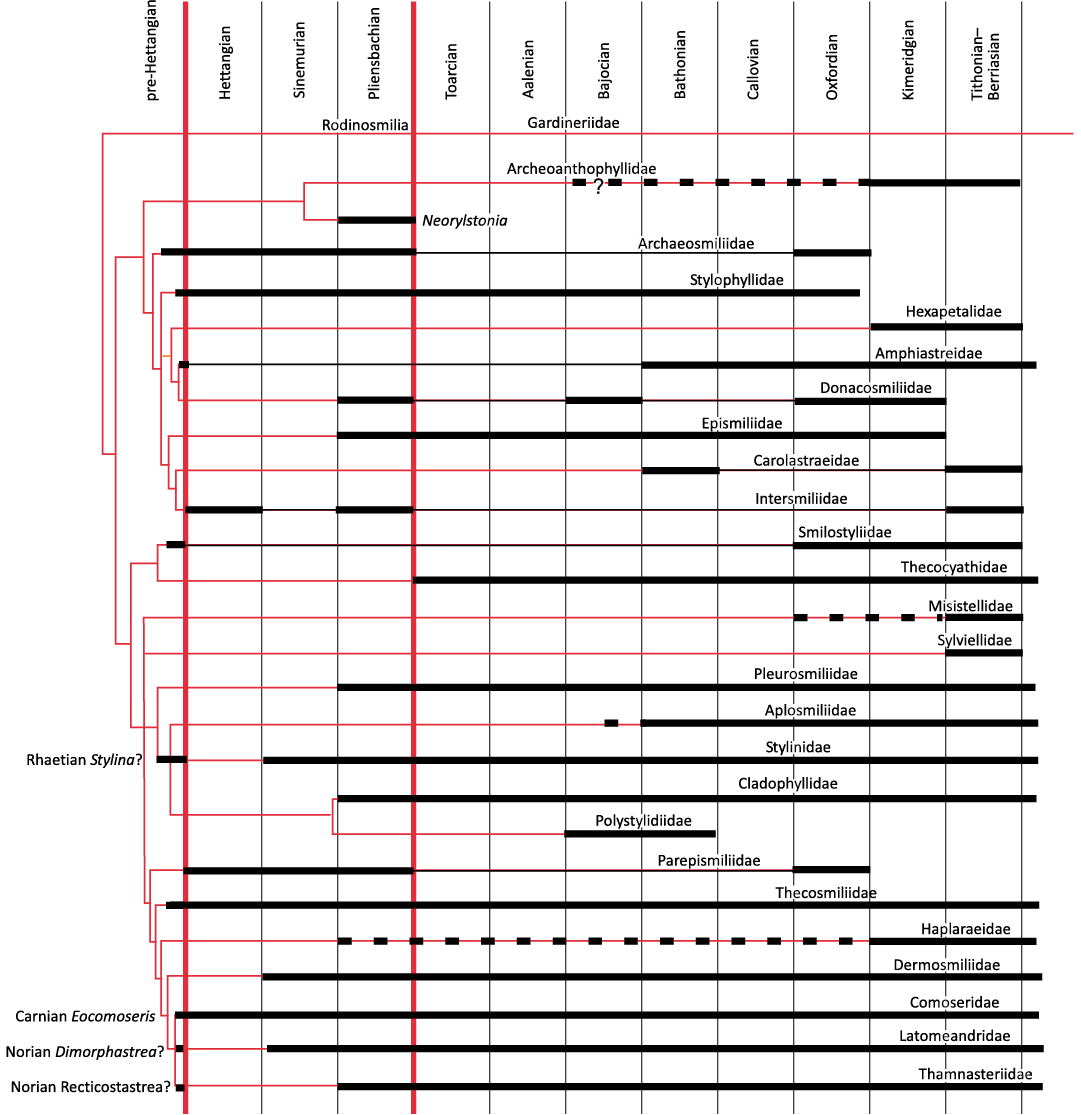
Fig. 9. Cladogram of Fig. 2 with constraining chronological data of the fossil record. Red lines represent the assumed phyletic relationships. Bold horizontal lines represent the distribution of fossils, and dashed lines uncertainties. Vertical red lines represent mass extinctions.
Chronological calibration.—The chronological calibration of cladograms (Figs. 9, 10) demonstrates that the disparity of corals was already very high at the beginning of their appearance in the Mesozoic fossil record as early as the Anisian (Middle Triassic) . This very early stage of disparity suggests that the evolution of Scleractinia is probably more ancient than previously considered in hypotheses that considered corals absent in the Early Triassic for evolutionary reasons and not for taphonomical reasons. Evaluations made on molecular grounds suggest that the origin of the clade Scleractinia goes back around 400 million years ago and this seems compatible with the above statement. Finding scleractinian corals in the Late Paleozoic appears to be necessary to corroborate this view.
The diversification of families during the Late Triassic as it was proposed in Wells (1956) is no longer supported. Furthermore, the diversification of families in proportions proposed by Krasnov (1970) is not backed by the fossil record. However, there are potential misinterpretations of Figs. 9 and 10 that could excessively reduce the number of Jurassic emergences. When two families are related by a cladogenesis, if a fossil occurrence is ancient for only one of the families, the family that appears later could have derived from the older one that becomes paraphyletic. For instance, it is plausible that Aplosmilidae was derived from Stylinidae during the Bathonian (Middle Jurassic), or Gigantostylidae from Stylophyllidae during the Norian (Late Triassic).
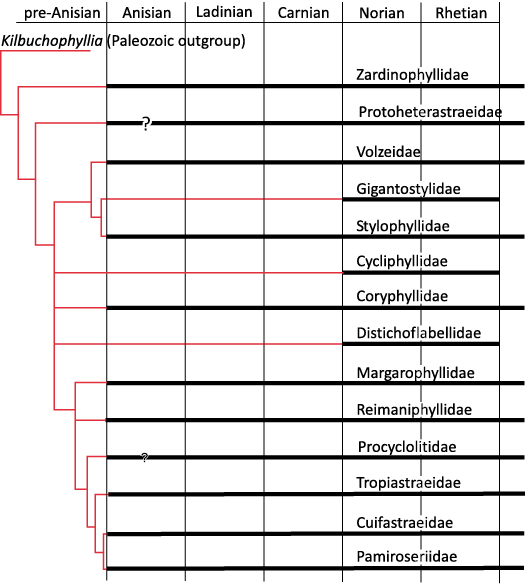
Fig. 10. Cladogram of Fig. 7 with constraining chronological data of the fossil record. Red lines represent the assumed phyletic relationships. Bold horizontal lines represent the distribution of fossils. Vertical bold line represents a mass extinction.
Conclusions
A significant assemblage of morphological characters can be used to perform cladistic analyses on fossil corals. These characters are not numerous enough to study all families of post-Paleozoic scleractinian corals. However, regions of the tree, for example of Jurassic families, can be studied separately with success. In the obtained cladograms, the most apical group (the furthest from the base) is a potential candidate for the ancestry of Complex corals defined from the molecular trees. The numerous extinct branches that punctuate the history of corals from their beginning to the emergence of Complex corals may be associated with the paraphyletic group(s) of Robust (or Robust + Basal) corals.
The distribution of significant characters in the better-resolved cladograms of Triassic and Jurassic coral families reveals a structure of the trees that can be interpreted in terms of growing morphological complexity. Initially in its history, corals may have smooth septa, without distal teeth, dominated by thickening deposits. Corals subsequently evolved ornamented septa, teeth, synapticulae and even pores, microstructure with regularly organised trabecular axes. The chronological calibration based on a detailed biostratigraphic chart of genera demonstrates that the disparity among Scleractinia (in the sense of diversity of organisation plans) is already evident as early as the Anisian stage, lending support to the hypothesis of a deep Paleozoic origin of the group.
Corallospere Group Members
(in alphabetical order)
Rosemarie Baron-Szabo (Smithsonian Institution, Washington DC, USA), Ann Budd (University of Iowa, Iowa City, USA), Stephen Cairns (National Museum of Natural History, Smithsonian Institution, Washington DC, USA), Jill Darrell (Natural History Museum, London, UK), Danwei Huang (Lee Kong Chian Natural History Museum, National University of Singapore, Singapore), Kenneth Johnson (Natural History Museum, London, UK), Marcelo Kitahara (Departamento de Ciências do Mar, Universidade Federal de São Paulo, Brazil), Bernard Lathuilière (Laboratoire GéoRessources, Université de Lorraine, France), Ewa Roniewicz (Institute of Paleobiology PAS, Warsaw, Poland), Brian Rosen (Natural History Museum, London, UK), Nadia Santodomingo (Department of Earth Sciences, University of Oxford, UK), Jarosław Stolarski (Institute of Paleobiology PAS, Warsaw, Poland)
Acknowledgements
We are thankful to colleagues of the Corallosphere for the numerous discussions about the morphological characters, especially to Nancy Budd, for leading the Corallosphere group and helping us in reviewing the English text of the manuscript. Jarek Stolarski and an anonymous referee are thanked for peer reviews.
References
Alloiteau, J. 1952. Embranchement des coelentérés. In: J. Piveteau (ed.), Traité de Paléontologie, 376–684. Masson & Cie, Paris.
Beauvais, L. 1981. Sur la taxinomie des Madréporaires mésozoïques. Acta Palaeontologica Polonica 25 (for 1980): 345–360.
Boivin, S., Vasseur, R., Lathuilière, B., Lazar, I., Durlet, C., Martindale, R.C., El Hmidi, K., and Martini, R. 2019. A little walk between Early Jurassic sponges and corals: a confusing morphological convergence. Geobios 57: 1–24. Crossref
Budd, A.F. and Bosellini F.R. 2016. Revision of Oligocene Mediterranean meandroid corals in the scleractinian families Mussidae, Merulinidae, and Lobophylliidae. Journal of Systematic Palaeontology 14: 771–798. Crossref
Budd, A.F., Woodell, J.D., Huang, D., and Klaus,
J.S. 2019. Evolution of the Caribbean subfamily Mussinae (Anthozoa:
Scleractinia: Faviidae): transitions between solitary and colonial
forms. Journal of Systematic Palaeontology
17: 1581–1616. Crossref
Cairns, S.D. 1984. An application of phylogenetic analysis to the Scleractinia: family Fungiidae. Palaeontographica Americana 54: 49–57.
Chevalier, J.P. 1987. Ordre des scléractiniaires. In: P.P. Grassé (ed.), Traité de Zoologie, Vol. 3, fasc. 3, 403–764. Masson, Paris.
Cuif, J.P. 1977. Arguments pour une relation phylétique entre les madréporaires paléozoïques et ceux du Trias. Implications systématiques de l’analyse microstructurale des madréporaires triasiques. Annales des Sciences naturelles 3ième Série 56: 1–54.
Cuif, J.-P., Lecointre, G., Perrin, C., Tillier, A., and Tillier, S. 2003. Patterns of septal biomineralization in Scleractinia compared with their 28S rRNA phylogeny: a dual approach for a new taxonomic framework. Zoologica Scripta 32: 459–473. Crossref
Eliášová, H. 1975. Sous ordre Amphiastraeina Alloiteau, 1952 (Hexacorallia) des calcaires de Stramberk (Tithonien, Tchécoslovaquie). Casopis pro Mineralogii a Geologii 30: 1–23.
Geistova, Z. and Kołodziej, B. 2023. Prolific development of Amphiastreids and other pachythecal corals (Hexanthiniaria?, Scleractinia?) in reefs on the Stramberk carbonate platform (Tithonian–Berriasian). 14th Symposium of the International Fossil Coral and Reef Society, 56. Institute of Paleobiology of the Polish Academy of Sciences, Warsaw.
Goloboff, P.A. 1999. Analyzing large data sets in reasonable times: solutions for composite optima. Cladistics 15: 415–428. Crossref
Goloboff, P.A., Farris, J.S., and Nixon, K.C. 2008. TNT, a free program for phylogenetic analysis. Cladistics 24: 774–786. Crossref
Gretz, M., Lathuilière, B., and Martini, R. 2015. A new coral with simplified morphology from the oldest known Hettangian (Early Jurassic) reef in southern France. Acta Palaeontologica Polonica 60: 277–286. Crossref
Guex, J. 2006. Reinitialization of evolutionary clocks during sublethal environmental stress in some invertebrates. Earth and Planetary Science Letters 242: 240. Crossref
Hörandl, E. and Stuessy, T.F. 2010. Paraphyletic groups as natural units of biological classification. Taxon 59: 1641–1653. Crossref
Huang, D., Benzoni, F., Fukami, H., Knowlton, N., Smith, N.D., and Budd, A. F. 2014. Taxonomic classification of the reef coral families Merulinidae, Montastraeidae, and Diploastraeidae (Cnidaria: Anthozoa: Scleractinia). Zoological Journal of the Linnean Society 171: 277–355. Crossref
Huang, D., Arrigoni, R., Benzoni, F., Fukami, H., Knowlton, N., Smith, N. D., Stolarski, J., Chou, L.M., and Budd, A.F. 2016. Taxonomic classification of the reef coral family Lobophylliidae (Cnidaria: Anthozoa: Scleractinia). Zoological Journal of the Linnean Society 178: 436–481. Crossref
Kitahara, M., Fukami, H., Benzoni, F., and Huang, D. 2016. The new systematics of Scleractinia: integrating molecular and morphological evidence. In: S. Goffredo and Z. Dubinsky (eds.), The Cnidaria, Past, Present and Future. 41–59. Springer, Cham. Crossref
Koby, F. 1888. Monographie des polypiers jurassiques de la Suisse. Mémoires de la Société Paléontologique Suisse 15: 401–456.
Krasnov, E.V. 1970. Phylogeny and the problem of unity of Scleractinian groups [in Russian]. Izvestiya visshikh uchebnykh zavedeniy, Geologija i razvedka 7: 15–40.
Melnikova, G. and Roniewicz, E. 1976. Contribution to the systematics and phylogeny of Amphiastraeina (Scleractinia). Acta Palaeontologica Polonica 21: 97–114.
Lathuilière, B. 1996a. Itinéraires astogéniques chez des coraux simples et coloniaux Montlivaltiides du Bajocien de France. Geobios 29: 577–603. Crossref
Lathuilière, B. 1996b. Is morphology a good way to understand the evolution of corals? Paleontological Society Papers 1: 81–105. Crossref
Löser, H. 2016. Catalogue of Cretaceous corals. Vol. 4 Systematic Part. 710 pp. CPress, Dresden.
Maddison, W.P. and Maddison, D.R. 2021. Mesquite: a modular system for evolutionary analysis. Version 3.70 [available online, http://www.mesquiteproject.org].
Medina, M, Collins, AG, Takaoka, TL, Kuehl, J.V., and Boore, J.L. 2006. Naked corals: skeleton loss in Scleractinia. Proceedings of the National Academy of Sciences of the United States of America 103: 9096–9100. Crossref
Montanaro-Gallitelli, E. 1975. Hexanthiniaria, a new ordo of Zoantharia (Anthozoa, Coelenterata). Bollettino della Societa Paleontologica Italiana 14: 21–25.
Nixon, K.C. 1999. The Parsimony Ratchet, a new method for rapid parsimony analysis. Cladistics 15: 407–414. Crossref
Okubo, N. 2016. Restructuring the traditional suborders in the order Scleractinia based on embryogenetic morphological characteristics. Zoological Science 33: 116–123. Crossref
Oliver, W.A. 1980a. On the relationship between Rugosa and Scleractinia (summary). Acta Palaeontologica Polonica 25 (for 1979): 395–402.
Oliver, W.A. 1980b. The relationship of the scleractinian corals to the rugose corals. Paleobiology 6: 146–160. Crossref
Quattrini, A.M., Rodríguez, E., Faircloth, B.C., Cowman, P.F., Brugler, M.R., Farfan, G.A., Hellberg, M.E., Kitahara, M.V., Morrison, C.L., Paz-Garcia, D.A., Reimer, J.D., and McFadden, C.S. 2020. Palaeoclimate ocean conditions shaped the evolution of corals and their skeletons through deep time. Nature Ecology & Evolution 4: 1531–1538. Crossref
Quek, Z.B.R., Jain, S.S., Richards, Z.T., Arrigoni, R., Benzoni, F., Hoeksema, B.W., Carvajal, J.I., Wilson, N.G., Baird, A.H., Kitahara, M.V., Seiblitz, I.G.L., Vaga, C.F., and Huang, D. 2023. A hybrid-capture approach to reconstruct the phylogeny of Scleractinia (Cnidaria: Hexacorallia). Molecular Phylogenetics and Evolution 186: 107867. Crossref
Romano, S.L. and Palumbi, S. 1996. Evolution of Scleractinian corals inferred from molecular systematics. Science 271: 640–642. Crossref
Roniewicz, E. and Stolarski, J. 1999. Evolutionary trends in the epithecate scleractinian corals. Acta Palaeontologica Polonica 44: 131–166.
Schindewolf, O.H. 1942. Zur Kenntniss der Polycoelien und Plerophyllen. eine Studie über den Bau der “Tetrakorallen” und ihre Beziehungen zu den Madreporarien Abhandlungen des Reichsamts für Bodenforschung 204: 1–324.
Scrutton, C.T. and Clarkson, E.N.K. 1991. A new scleractinian-like coral from the Ordovician of the Southern Uplands, Scotland. Palaeontology 34: 179–194.
Stanley, G.D.J. and Fautin, D.G. 2001. The origins of modern corals. Science 291: 1913–1914. Crossref
Stanley, G.D., Jr. and Schootbrugge, B.V. de 2009. The evolution of the coral-algal symbiosis. In: M.J.H. van Oppen and J.M. Lough (eds.), Coral Bleaching. Ecological Studies 205: 7–19. Crossref
Stolarski, J., Roniewicz, E., and Grycuk, T. 2004. A model for furcate septal increase in a Triassic scleractiniamorph. Acta Palaeontologica Polonica 49: 529–542.
Stolarski, J. and Russo, A. 2001. Evolution of the post Triassic pachytecaliine corals. Bulletin of the Biological Society of Washington 10: 242–256.
Stolarski, J. and Russo, A. 2002. Microstructural diversity of the stylophyllid (Scleractinia) skeleton. Acta Palaeontologica Polonica 47: 651–666
Stolarski, J., Meibom, A., Przeniosło, R., and Mazur, M. 2007. A Cretaceous Scleractinian coral with a calcitic skeleton. Science 318: 92–94. Crossref
Stolarski, J., Kitahara, M., Miller, D.J., Cairns, S.D., Mazur, M., and Meibom, A. 2011. The ancient evolutionary origins of Scleractinia revealed by azooxanthellate corals. BMC Evolutionary Biology 11: art. 316. Crossref
Volz, W. 1896. Die Korallen der Trias. Monographisch bearbeitet. II. In: F. Frech and W. Volz (eds.), Die Korallen der Schichten von St Cassian in Süd-Tirol. Palaeontographica 43: 1–127.
Veron, J.E.N. 1995. Corals in Space and Time: The Biogeography and Evolution of the Scleractinia. 321 pp. Comstock/Cornell, Ithaca.
Wells, J.W. 1956. Scleractinia. In: R.C. Moore (ed.), Treatise on Invertebrate Paleontology, Part F, F328–F444. The University of Kansas Press, Lawrence, Kansas.
Acta Palaeontol. Pol. 69 (2): 249–262, 2024
https://doi.org/10.4202/app.01136.2024