

A Late Jurassic deep-bodied actinopterygian fish from Antarctica
SOLEDAD GOUIRIC-CAVALLI, ARI IGLESIAS, BÁRBARA CARIGLINO, and MARCELO A. REGUERO
Gouiric-Cavalli, S., Iglesias, A., Cariglino, B., and Reguero, M.A. 2024. A Late Jurassic deep-bodied actinopterygian fish from Antarctica. Acta Palaeontologica Polonica 69 (3): 467–483.
Mesozoic deep-bodied actinopterygians are of interest given, among others, the various modes of feeding exhibited by these fishes. Regrettably, most of their fossil record is restricted to a limited number of localities in Europe. During the Late Jurassic fragmentation of Pangaea, the exchange of fauna between the European Tethys and the paleo-Pacific (southwestern of the South American region) was possible via the marine pathways connecting these areas. This exchange led to the speciation of fish taxa, particularly in the Southern Hemisphere. Although new species are continuously being discovered, our understanding of the Late Jurassic marine ichthyofaunas of the Southern Hemisphere remains limited. The Mesozoic ichthyofaunas of the Antarctic seas are rich but relatively poorly known presenting a significant opportunity for further research. Noteworthy, previous reports have documented the presence of actinopterygians in the Late Jurassic of the Antarctic Peninsula. Since 2016, a team of researchers from Argentina has been exploring the Upper Jurassic–Lower Cretaceous Ameghino (= Nordenskjöld) Formation outcrops, resulting in the discovery of a large collection of actinopterygian fishes. Nevertheless, most of the material remains undescribed. In this article, we report the discovery of a deep-bodied actinopterygian found at the Longing Gap, the type locality of the Ameghino Formation in the Antarctic Peninsula. The study of these newly collected materials allows for their taxonomic assignment to Ameghinichthys antarcticus, a taxon previously described for the locality but based on isolated and fragmentary material. Additionally, this study confirms that A. antarcticus belongs to Dapediiformes. Ameghinichthys antarcticus is among the youngest records of Dapediiformes and represents the southernmost record of the group worldwide.
Key words: Actinopterygia, Dapediiformes, Dapediidae, ichthyofaunas, Mesozoic, Gondwana.
Soledad Gouiric-Cavalli [sgouiric@fcnym.unlp.edu.ar; ORCID: https://orcid.org/0000-0003-2026-5973 ], División Paleontología de Vertebrados, Museo de La Plata, Universidad Nacional de La Plata (UNLP). Paseo del Bosque s/n, 1900 La Plata, Buenos Aires, Argentina. Consejo Nacional de Investigaciones Científicas y Tecnológicas (CONICET), Godoy Cruz 2290, C1425FQB Ciudad Autónoma de Buenos Aires, Argentina.
Ari Iglesias [ari_iglesias@yahoo.com.ar; ORCID: https://orcid.org/0000-0002-9098-8758 ], Instituto de Investigaciones en Biodiversidad y Medioambiente (CONICET-UNCO). Pasaje Gutiérrez 1415, R8400GAO, San Carlos de Bariloche, Río Negro, Argentina.
Bárbara Cariglino[ barichi10@gmail.com; ORCID: https://orcid.org/0000-0002-4346-3502 ], División Paleobotánica y Paleopalinología, Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”, Av. Ángel Gallardo 470, C1405DJR, Ciudad Autónoma de Buenos Aires, Argentina.
Marcelo A. Reguero [regui@fcnym.unlp.edu.ar; ORCID: https://orcid.org/0000-0003-0875-8484 ], Instituto Antártico Argentino, Dirección Nacional del Antártico. Balcarce 295, C1064AAF Ciudad Autónoma de Buenos Aires, Argentina and División Paleontología de Vertebrados, Museo de La Plata, Universidad Nacional de La Plata (UNLP). Paseo del Bosque s/n, 1900 La Plata, Buenos Aires, Argentina. Consejo Nacional de Investigaciones Científicas y Tecnológicas (CONICET), Godoy Cruz 2290, C1425FQB Ciudad Autónoma de Buenos Aires, Argentina.
Received 27 March 2024, accepted 8 August 2024, published online 27 September 2024.
Copyright © 2024 S. Gouiric-Cavalli et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Dapediiformes and the sole family, Dapediidae, comprises deep-bodied neopterygians with rhomboid ganoid scales and predominantly marine taxa, ranging in age from the Late Triassic (Tintori 1983) to the Late Jurassic (Thies and Hauff 2011). Dapediiformes cluster fishes with a body deeply fusiform to nearly circular in outline and laterally compressed; circumorbital bones consisting of a series of infraorbitals and a series of suborbitals; vertical branch of preoperculum covered by suborbitals to a varying extent; and both dorsal and anal fins hem-like (Lehman 1966; Thies and Hauff 2011: 187). The geographic distribution of dapediiforms includes Europe (Wenz 1968; Tintori 1983; Lambers 1999; Thies and Hauff 2011), India (Jain 1973), Australia (Gibson 2016), and North America (Schaeffer 1967; Gibson 2016). Dapedium is the most speciose genus, with 15 recognized species, recorded from the Late Triassic to the Early Jurassic in Europe alone (Maxwell and López-Arbarello 2018).
The family Dapediidae was erected by Lehman (1966) base on a deep-bodied phenotype. Subsequently, Thies and Hauff (2011) provided a formal diagnosis of Dapediiformes challenging previous attempts to diagnose the family.
The family Dapediidae presently includes genera Aetheolepis, Dandya, Dapedium, Hemicalypterus, Heterostrophus, Paradapedium, Sargodon, Scopulipisic, and Tetragonolepis (Thies and Hauff 2011; Gibson 2016; Latimer and Giles 2018; Szabó and Pálfy 2020). However, Schaeffer’s (1967: 322) prior research regarded Hemicalypterus as a Semionotiformes and Aetheolepis as a Pholidophoriformes, two structurally analogous fishes linked by their squamation pattern. Schaeffer (1967) queried Lehman’s (1966) hypothesis that Dapediidae included Dapedium, Tetragonolepis, Heterostrophus, and Dandya. His research suggested that Hemicalypterus, Dapedium, and Tetragonolepis had evolved independently from a fusiform “Semionotus” ancestor.
The phylogenetic relationships of these deep-bodied actinopterygians have been controversial. Previous hypotheses suggested dapediids within Semionotiformes (Gardiner 1960), basal ginglymodians, or as a group outside the crown Holostei (López-Arbarello and Sferco 2018; Latimer and Giles 2018), or even as a sister taxon of Teleosteomorpha (e.g., Gardiner 1996; Arratia 1999). Thies and Waschkewitz (2016) conducted a review of the genus Dapedium and proposed that Dapediiformes consisted of a single family, Dapediidae. The study did not support a sister relationship between Dapedium and teleosts.
It is noteworthy that almost all published phylogenetic analyses that include dapediids lack representative taxon sampling including only one taxon, Dapedium, and few species (e.g., Olsen 1984; López-Arbarello 2012; López-Arbarello and Sferco 2018, but see Gibson 2016 and Latimer and Giles 2018). Recent phylogenetic analyses have recovered Dapediiformes as a sister taxon to Ginglymodi within Holostei (Gibson 2016) or stem Holostei (Latimer and Giles 2018). The monophyly of Dapediiformes among neopterygians has been relatively well-tested by phylogenetic analysis with a broad taxon sampling (Gibson 2016; Latimer and Giles 2018). However, the relationships among the putative members of the family are far from being understood (see Latimer and Giles 2018: fig. 10). To date, the only marine Late Jurassic record of Dapediiformes is represented by Heterostrophus latus; retrieved from the Solnhofen Lithographic Limestones of Germany.
The paleobiology and paleoecology of Dapediformes is of interest given the various modes of feeding the group exhibited (Bellwood and Hoey 2004; Clarke and Friedman 2018; Cawley et al. 2021). Some taxa have been considered facultative durophagous (e.g., Thies and Hauff 2011; Smithwick 2015; Tintori and Lombardo 2018), while others herbivorous or partially herbivorous (Gibson 2016). Scavenging has been also proposed for a Dapedium (Thies and Hauff 2011). Relative to where in the water column Dapediiformes lived and fed, some taxa are interpreted as probably demersal, living and feeding near the bottom (e.g., Sargodon, Hemicalypterus, Dandya). Whereas others, such as Dapedium and Heterostrophus have been hypothesized as not being demersal (e.g., Tintori and Lombardo 2018).
The objective of this study is to present new information on the diversity of Late Jurassic Antarctic ichthyofaunas. To achieve this objective, we conducted a comprehensive anatomic study of an almost complete deep-bodied actinopterygian, and several additional fish remains recovered from the Upper Jurassic–Lower Cretaceous Ameghino (= Nordenskjöld) Formation at Longing Gap, Antarctic Peninsula (Fig. 1). The newly collected specimens are compared with the holotype of Ameghinichthys antarcticus which was previously collected at the same locality earlier (Arratia et al. 2004) and has similar scale ornamentation. A comprehensive anatomical description of the specimens is provided, along with a discussion of their taxonomic assignment and paleobiogeographical significance in high-latitude seas.
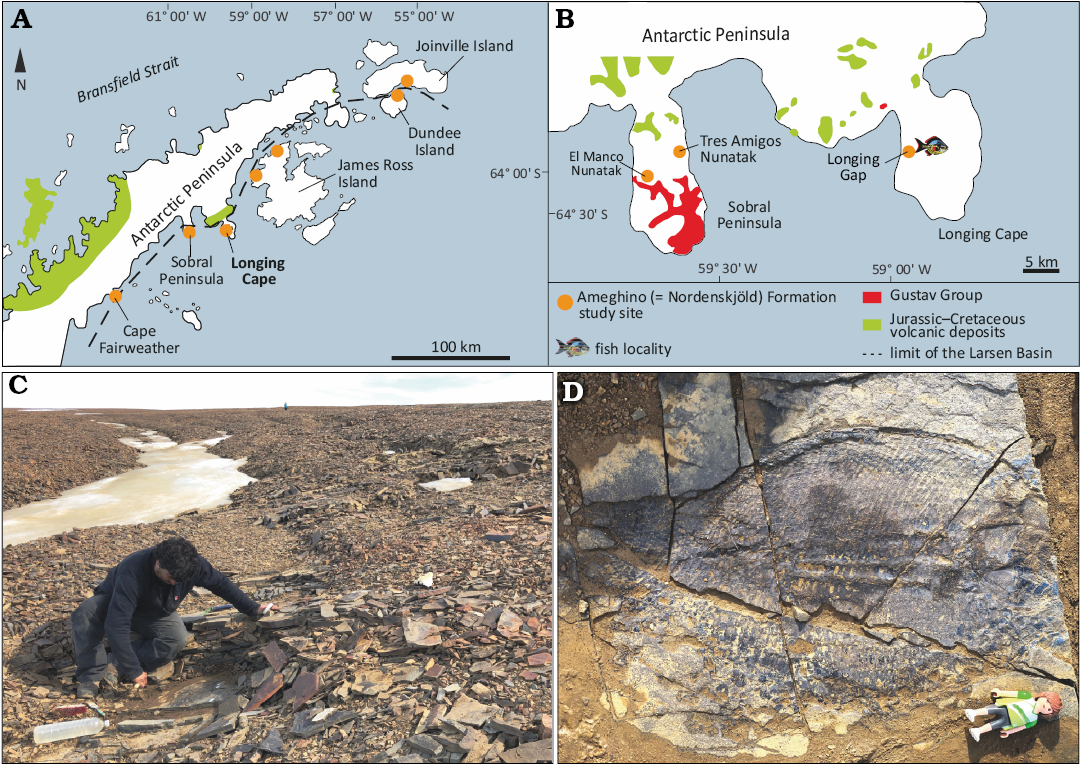
Fig. 1. Locality information for specimens of the dapediid fish Ameghinichthys antarcticus Arratia et al., 2004. A. Geological scheme of the Larsen Basin showing Jurassic outcrops of the Ameghino Formation and Jurassic–Cretaceous volcanic rocks. B. Geological sketch map of the Sobral Peninsula and the Longing Cape. The fish symbol indicates outcrops at the type locality of the Ameghino Formation from which the specimens studied here were recovered (modified from Elliot 1988 and Kietzmann and Scasso 2020). C. Outcrop from where Ameghinichthys antarcticus Arratia et al., 2004 (IAA-Pv 846) was recovered (author as scale). D. Ameghinichthys antarcticus Arratia et al., 2004 (IAA-Pv 846) in the field, before recovering (height of Playmobil® for scale equals 7.5 cm).
Institutional abbreviations.—ANU, Australian National University, Canberra, Australia; AMF, Australian Museum Fossil collection, Sydney, Australia; BGS, British Geological Survey, London, UK; CM, Carnegie Museum, Pittsburg, USA; CPBA-V, Colección de Paleontología Vertebrados de la Facultad de Ciencias Exactas Universidad de Buenos Aires, Argentina; GBIF, Global Biodiversity Information Facility; IAA-Pv, Repositorio de Colecciones Paleontológicas y Geológicas, Instituto Antártico Argentino, San Martín, Buenos Aires, Argentina; JME, Jura Museum Eichstätt, Eichstätt, Germany; OUM, Oxford University Museum, UK; SMNS, Staatliches Museum für Naturkunde, Stuttgart, Germany; NHMUK, Natural History Museum, London, UK, WARMS, Warwickshire Museum, Warwick, UK.
Geological setting
The Ameghino (= Nordenskjöld) Formation (Medina and Ramos 1981, 1983; Farquharson 1982, 1983) comprises a Kimmeridgian–Berriasian (Upper Jurassic–lowermost Cretaceous) sedimentary sequence that is scattered across the Antarctic Peninsula (Doyle and Whitham 1991; Del Valle et al. 1992; Kiessling et al. 1999; Fig. 1A). The Ameghino Formation consists of marine mudstone and tuff beds that are grouped into two members, from bottom to top: Longing and Ameghino (Whitham and Doyle 1989), which record a change from largely anoxic, basinal deposition, to largely dysoxic, slope sedimentation, respectively (Doyle and Whitham 1991; Whitham 1993; Hathway 2000; Scasso 2001). The sediments of the Ameghino Formation are inferred to be pelagic to hemipelagic, and possibly deposited near active volcanic arc (Doyle and Whitham 1991; Whitham 1993; Scasso 2001).
The fossil record of the Ameghino Formation is mostly characterized by invertebrates (e.g., ammonoids, nautiloids, bivalves, and radiolarians; Medina and Ramos 1983; Whitham and Doyle 1989; Kiessling and Scasso 1996; Kiessling et al. 1999). The vertebrate records in this sequence are represented mainly by actinopterygian fishes and vertebrate coprolites (Gouiric-Cavalli et al. 2017, 2019; Bigurrarena Ojeda et al. 2023 and references therein). Previous reports on actinopterygians from the Ameghino Formation consist of fish remains recovered mainly from scree (Arratia et al. 2004; Gouiric-Cavalli et al. 2017, 2019). Over the last eight years (2016–2023), the exhaustive work carried out by researchers from the Museo de La Plata led to the discovery of different and numerous actinopterygian-bearing horizons throughout the sequence, from which several complete specimens were recovered and are currently under study by a multidisciplinary project lead by the senior author.
The specimen IAA-Pv 846 studied here was found in situ in monoclinal strata of the Longing Member of the Ameghino Formation (Fig. 1B) at a site previously unexposed due to permanent ice cover, but now available. The strata are placed at the middle section of the unit and correlated to the uppermost lower Tithonian to lower upper Tithonian based on radiolarians and ammonites (Kiessling 1999). Other newly fragmentary material and the holotype of Ameghinichthys antarcticus (CPBA-V-14065) described here come from scree near the outcrop above.
Material and methods
The specimens studied herein were collected under the current legislation of the Antarctic Treaty by the Scientific Committee on Antarctic Research of the Dirección Nacional del Antártico and the Instituto Antártico Argentino. The specimen housed at CPBA-V was collected by Roberto Scasso and Wolfgang Kiessling (Arratia et al. 2004). Specimens housed at IAA-Pv were collected by Soledad Gouiric-Cavalli and Ari Iglesias.
Studied material.—Holotype (CPBA-V-14065) and 10 other specimens (all housed at IAA-Pv) preserved mainly as imprints, consisting of body squamation, fragmentary scale patches, fins, nearly complete specimen and a skull roof with associated jaw bones.
Comparative material.—High-resolution images of Dapedium (i.e., D. puncatum Agassiz, 1835; D. granulatum Agassiz, 1835; Dapedium sp.) specimens held at the NHMUK were made available on request via the Natural History Museum Data Portal (http://data.nhm.ac.uk.); high-resolution images of the holotypes of Heterostrophus latus Wagner, 1863, and H. phillipsi Woodward, 1929, held at the British Geological Survey and WARMS were accessed through the GB3D Type Fossils Online project (http://www.3d-fossils.ac.uk). Additional specimens of H. latus held at JME were accessed through the GBIF project (https://www.gbif.org/occurrence/2987987653). Specimens of Dapedium were also accessed through the GBIF project: D. colei Agassiz, 1835, https://www.gbif.org/species/4838422; D. granulatum, https://www.gbif.org/species/8637449; D. orbis Agassiz, 1836, https://www.gbif.org/species/8642556; D. pholidotum (Agassiz, 1832), https://www.gbif.org/species/4838424; D. politum Leach, 1822, https://www.gbif.org/species/8457261; D. punctatum Agassiz, 1835, https://www.gbif.org/species/8611055; D. radiatum (Agassiz, 1835), https://www.gbif.org/species/ 8815433; D. magnevillei (Agassiz, 1835), https://www.gbif.org/species/9147864; Hemicalypterus weiri Schaeffer, 1967, https://www.gbif.org/species/8647225. High-resolution images of Sargodon tomicus Plieninger, 1847; Aetholepis mirabilis Woodward, 1895, and Heterostrophus latus were provided by Andrea Tintori (Dipartimento di Scienze della Terra “Ardito Desio” Uiversità Degli Studi Di Milano, Italy), Lynne Bean (Research School of Earth Sciences, Australian National University, Australia), and Amy Henrici (Carnegie Museum of Natural History, USA), respectively.
We also examined the specimens listed below by direct observation, high-resolution photographs (indicated by °), and from the literature cited below.
Aetheolepis mirabilis: ANU 61121°, AMF 117883°, AMF 120501°, AMF 120502°. Dandya ovalis (Gorjanovich-Kramberger, 1905): Tintori (1983), Hornung et al. (2019). Ameghinichthys antarcticus Arratia et al., 2004: CPBA-V-14065. Dapedium angulifer (Agassiz, 1835): WARMS G1120°; Dapedium ballei Maxwell & López-Arbarello, 2018: SMNS 96990, Maxwell and López-Arbarello (2018); Dapedium caelatum Quenstedt, 1858: Thies and Waschkewitz (2016); Dapedium colei: NHMUK PV P 1561°, P 4431; Dapedium granulatum: NHMUK PV P 3538, OUM J. 03005; Dapedium magnevillei: Woodward (1895), Wenz (1968), Maxwell and López-Arbarello (2018), Thies (1988); Dapedium noricum Tintori, 1983: Tintori (1983); Dapedium orbis: NHMUK PV P 4221°, NHMUK PV P 29217°; Dapedium pholidotum: Thies (1988), Thies and Herzog (1999), Thies and Hauff (2011), Thies and Waschkewitz (2016); Dapedium politum: Wenz (1968), Thies (1988), Thies and Waschkewitz (2016); Dapedium punctatum: Wenz (1968), Thies (1988), Thies and Hauff (2011), Thies and Waschkewitz (2016); Dapedium radiatum: NHMUK PV P 1564°; Dapedium stollorum Thies & Hauff, 2011: Thies and Hauff (2011), Thies and Waschkewitz (2016). Hemicalypterus weiri Schaeffer, 1967: Gibson (2016). Heterostrophus phillipsi: BGS GSM113113°; Heterostrophus latus: CM 4762°, JME (SOS) 3576. Paradapedium egertoni Jain, 1973: Jain (1973). Sargodon tomicus: Tintori (1983).
Methods.—Antarctic specimens generally did not require extensive preparation but, when necessary, pneumatic tools, micro jacks, and sharpened tungsten carbide needles were used. A silicone peel of the skull and pectoral fin of IAA-Pv 846 was prepared for the examination of a positive silicone cast under binocular microscopy. Magnesium oxide powder was used for contrast enhancement. The sieving process was performed on an area of 4 × 4 m. The specimens were examined using Zeiss Stemi C-2000 stereomicroscopes with different resolutions. Each specimen was photographed under normal lighting conditions using a Canon digital SLR camera with a compact macro lens (EF 50mm f/2.5). Drawings of the specimens were made based on high-resolution photographs using a digital drawing tablet.
Anatomical terminology.—Cranial bones are named according to homology-based terminology (Westoll 1943; Schultze 2008). The traditional terminology is given in parentheses along the text. The postcranial descriptive terminology follows Arratia (2008a, 2009). Scale measurements and squamation areas follow Thies and Waschkewitz (2016).
Systematic palaeontology
Class Actinopterygii Cope, 1872
Subclass Neopterygii Regan, 1923
Order Dapediiformes Thies & Waschkewitz, 2016
Family Dapediidae Lehman, 1966 sensu Thies & Hauff, 2011
Genus Ameghinichthys Arratia et al., 2004
Type species: Ameghinichthys antarcticus Arratia et al., 2004; Uppermost lower Tithonian–lower upper Tithonian, Longing Gap, Ameghino Formation (Longing Member), Antarctic Peninsula.
Ameghinichthys antarcticus Arratia et al., 2004
Figs. 1D, 2–8, 9A.
2004 Ameghinichthys antarcticus; Arratia et al. 2004: 42, fig. 3.
2017 Ameghinichthys antarcticus; Gouiric-Cavalli et al. 2017: 18.
Type material: Holotype: CPBA-V-14065, piece of body squamation preserved mainly as an imprint (“The position of these scales relative to the lateral line is unknown” Arratia et al. 2004: 43, fig. 3; Fig. 2). Paratypes: IAA-Pv 317, fragmentary scale patch; IAA-PV 342, fragmentary body consisting of part of caudal peduncle with associated anal fin, IAA-Pv 425, fragmentary body consisting of patches of thick scales and lepidotrichia; IAA-Pv 429, patch of thick scales at the base of the caudal fin; IAA-Pv 431, fragments of fins and associated scales; IAA-Pv 431a, caudal peduncle scale patch? with basal fulcra; IAA-Pv 432, a nearly complete skull roof with associated but disarticulated jaw bones; IAA-Pv 433, a small patch of scales (3 mm long and 2 mm wide with six to eight longitudinal ridges) associated with 16 incomplete fin rays; IAA-Pv 441, a large dorsal scale patch preserved as an impression; IAA-Pv 846, a nearly complete fish body.
Type locality: Longing Cape, Longing Gap locality, Antarctic Peninsula.
Type horizon: Uppermost lower Tithonian–lower upper Tithonian, Longing Member, Ameghino Formation, Larsen Basin.
Material.—Type material (CPBA-V-14065) and paratypes (IAA-PV 317, IAA-PV 342, IAA-PV 425, IAA-PV 429, IAA-PV 431a, IAA-PV 431b, IAA-PV 432, IAA-PV433, IAA-PV 441, IAA-PV 486), from the type locality and horizon.
Emended diagnosis (after Arratia et al. 2004).—Ameghinichthys antarcticus is based on a unique combination of the following characters, please note that potential autapomorphies are indicated by an asterisk [*]: medium-sized (average size 400 mm standard length) hypsisomatic (= deep-bodied) fish; fusiform to almost circular and laterally compressed body; skull roof densely ornamented with small ganoine tubercles; cheekbones less ornate than skull roof bones; bones comprising the skull roof show a fusion of the postparietal (parietal) with the dermopterotic [*]; wedge-shaped premaxilla with small and styliform teeth; conical maxillary teeth; trapezoidal opercle with a convex to straight ventral contact with the subopercle and with a dorso-anterior projection [*]; rostral and ventral margin of the opercle forming a obtuse angle of about 113° [*]; opercle length/height ratio of ca. 1.4 [*]; at least five rectangular infraorbitals; opercle, subopercle, and the first branchiostegal ray vertically aligned; vertical arm of the preopercle relatively wide and mainly covered by suborbitals, ventral arm of the preopercle triangular or tongue-shaped and with several short secondary sensory canals; large and rounded presupracleithrum [*]; supracleithrum with a short postero-dorsal projection [*]; origin of the pectoral fin at level of opercle-subopercle contact [*]; pectoral fin with two basal fulcra and s-shaped fringing fulcra [*]; hem-like dorsal fin; a series of paired lanceolate basal fulcra preceding the dorsal fin; small, spiny ossified elements (= Pattern C) fringing fulcra lie on the marginal leading ray of the dorsal fin [*]; scales immediately below the dorsal fin are rectangular (higher than wide); thick (2–3 mm) rhomboid scales on the dorsal and ventral portion of the body; thinner rhomboid scales in the flank and caudal peduncle; smooth posterior margin of scales; length/height ratio of flank scales behind the operculum (= area 4) of 0.48; length/height ratio of caudal peduncle scales (= area 3) of 0.75; scales of the dorsal and ventral portion of the body with ornamentation consisting in longitudinal ganoin ridges that do not reach the posterior margin and are separated from each other; anteriormost scales above the lateral line with ganoin ridges running almost parallel to the dorsal scale margin and covering most of the scale surface; ventralmost body scales with fewer ganoin ridges running obliquely dorsoventrally from anterior part and scales considerably longer than deep; scales behind the opercular bones rectangular, higher than long, smooth, and arranged in at least six scale rows; dorsal body scales anterior to the origin of the dorsal fin heavily ornamented with ganoin ridges and tubercles; nine quadrangular scales in the dorsalmost part of transverse scale row immediately posterior to the skull.
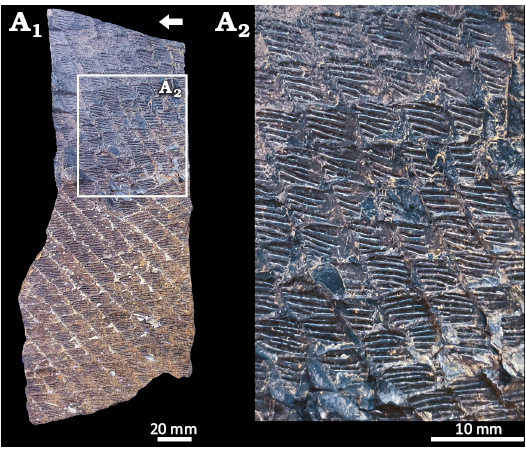
Fig. 2. Dapediid fish Ameghinichthys antarcticus Arratia et al., 2004 (holotype CPBA-V-14065) from the Tithonian, Upper Jurassic of Ameghino (= Nordenskjöld) Formation, Longing Cape, Antarctic Peninsula. Overview (A1), close-up of CPBA-V-14065 showing the scales (A2). Arrow points anteriad.
Description.—The holotype CPBA-V-14065 is an incomplete piece of body squamation preserved as an imprint (Fig. 2). The most informative specimen, IAA-Pv 846, is a nearly complete body fish preserved in the right lateral view (Figs. 1D, 3, 9A). Four fractures running down the trunk of the specimen IAA-Pv 846 (Fig. 3). The main fracture runs from the ventral part of the trunk to the dorsal part of the caudal peduncle, three major fractures running perpendicular to the body axis of the fish (Fig. 3). IAA-Pv 846 has most of the bones and scales preserved in as imprint (= negative form; Fig. 3). IAA-Pv 846 is interpreted as a medium-sized fish with an average standard length of 400 mm. The posterior part of the head is about 120 mm in height. The depth of the body is about 240 mm, but an exact measurement is not possible because the ventral edge of the fish is distorted. The head is incomplete, with only the postorbital region preserved (Figs. 3, 4). The body is slightly post-mortem distorted (Fig. 3).
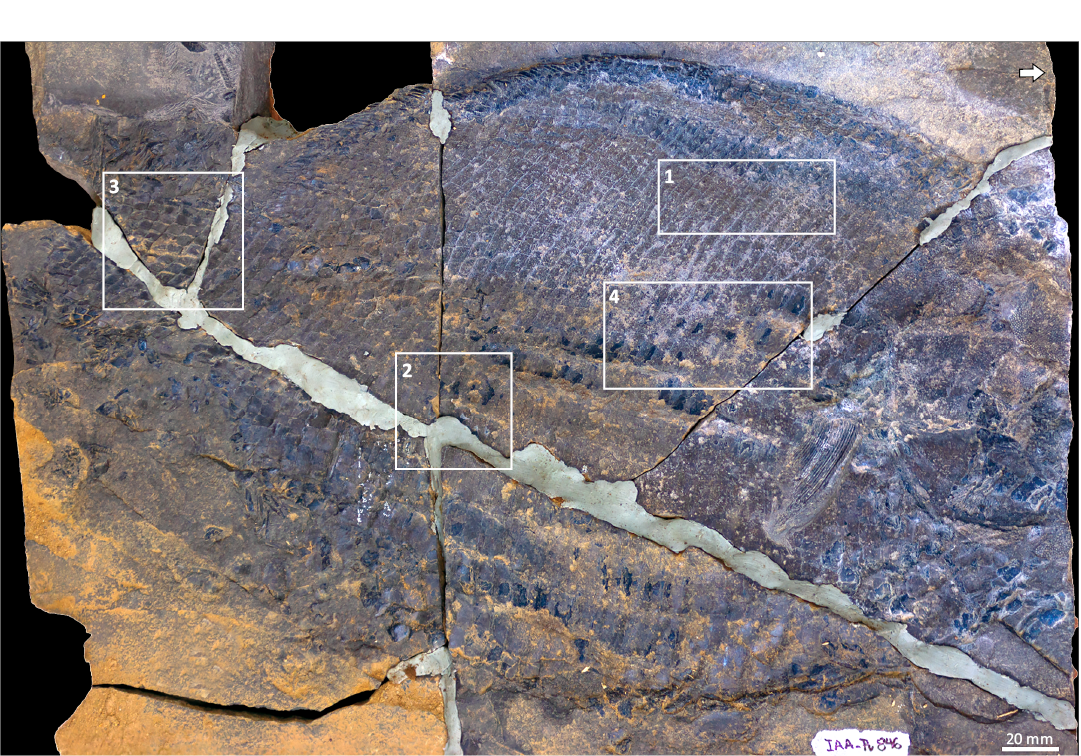
Fig. 3. Dapediid fish Ameghinichthys antarcticus Arratia et al., 2004, from the Tithonian, Upper Jurassic of Ameghino (= Nordenskjöld) Formation, Longing Cape, Antarctic Peninsula. Overview of the IAA-Pv 846, the rectangles 1–4, indicate the areas of the squamation enclosing the selected scales that were measured. Arrow points anteriad.
The dermal bones of the skull roof are densely ornamented with relatively small tubercles (Fig. 4); this ornamentation makes it difficult to identify sutures between bones. However, some sutures can be well recognized when the skull is coated with magnesium oxide powder.
Another relevant specimen is IAA-Pv 432, this specimen preserves a dentigerous bone, interpreted here as a ?dermopalatine bone (Fig. 5), the teeth on this bone are located only in the anterior portion and are poorly preserved (with only small tooth bases that seem to be grouped in a patch or pavement). Upper jaw bones of IAA-Pv 432 bear conical and small (ca. 3 mm) teeth.
Braincase: Almost all bones of the endochondral neurocranium are covered by dermal bones. In the occipital region of IAA-Pv 846 and IAA-Pv 432, behind and below the postparietals, there are two bones interpreted here as the exoccipitals (Figs. 4A2, 5A2).
Skull roof bones: The bones comprising the skull roof in Dapediiformes show either a coossification or fusion of the parietal (frontal), postparietal (parietal), and dermopterotic (DPF) or independent parietal, postparietal and dermopterotic bones (e.g., Thies and Waschkewitz 2016).
In Ameghinichthys antarcticus the pattern of coosification or fusion in IAA-Pv 846 shows the posterior portion of both postparietals (parietal) separated medially. However, each postparietal is fused with the dermopterotic (Fig. 4A2). The parietal (frontal) and postparietal (parietal) are in contact through a faint suture (Fig. 5). IAA-Pv 432 (Fig. 5) shows a fusion of the dermopterotic with part of the parietal (frontal) and postparietal (parietal). The total skull length of IAA-Pv 432 is ca. 35 mm. While the skull of IAA-Pv 846 is incomplete, the preserved section of the postparietals + dermopterotics is roughly 25 mm in length. Also, the skull roof of this specimen preserves the extrascapulars which are independent bones (Fig. 4A2).
Postparietals (parietal): Each postparietal is fused with the dermopterotic forming a complex bone (Fig. 4A2). Each coosification of postparietals + dermopterotics is a rectangular complex bone, larger than wide contacting midline by a dentata suture independent (Figs. 4, 5). In IAA-Pv 846 right and left coosifications reach 30 mm wide and bear a middle pit-line (Fig. 4). Postparietals + dermopterotic are densely ornamented with small and closely spaced ganoine tubercles and few rugae.
Extrascapulars: The extrascapulars are in a series posterior to the postparietal (parietal). Each extrascapular is a plate-like mostly rectangular bone (Fig. 4). The extrascapular series of IAA-Pv 846 have at least three extrascapulars. Each extrascapular is densely ornamented with ganoine tubercles and short rugae. The supratemporal commissure is located near the caudal margin of the extrascapulars (Fig. 4A2).
Dermopterotic: Because the dermopterotic is fused with the postparietal (parietal), the sutures that would provide information on the shape and size of the dermopterotic cannot be identified (Figs. 4, 5). The zone is heavily ornamented with ganoine rugae and tubercles. It remains unclear whether the rugae and tubercles radiate from ossification centres.
Snout region: IAA-Pv 432 has preserved the right nasal bone, which is roughly triangular. The sutures between the nasal and parietal are indistinct (Fig. 5). It is ambiguous whether the rostral region in IAA-Pv 432 includes the rostral and/or part of the ethmoid region of the neurocranium (Fig. 5A2).
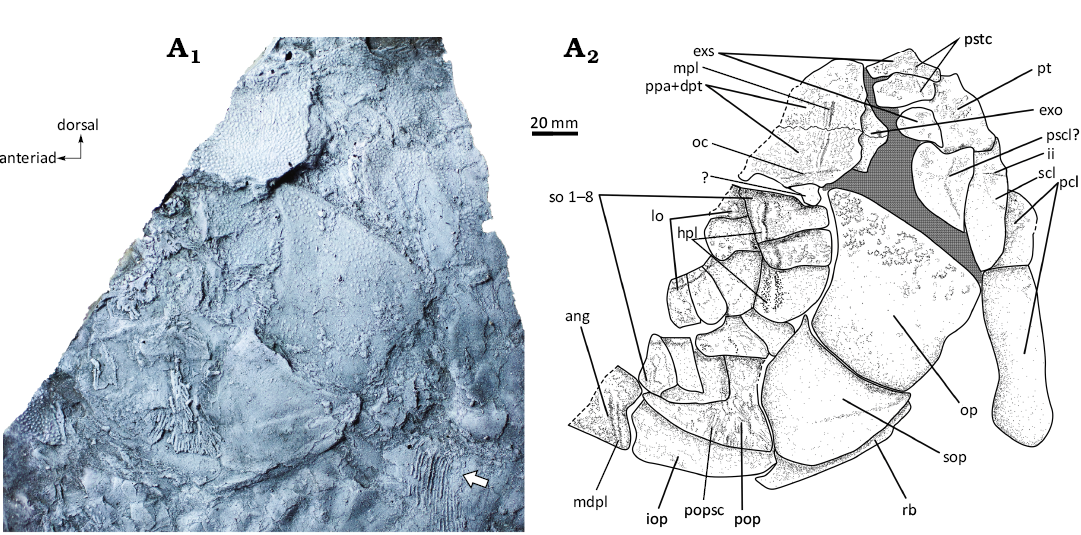
Fig. 4. Dapediid fish Ameghinichthys antarcticus Arratia et al., 2004 (IAA-Pv 846) from the Tithonian, Upper Jurassic of Ameghino (= Nordenskjöld) Formation, Longing Cape, Antarctic Peninsula. Silicone peel of the anterior portion (A1) showing the incompletely preserved head and pectoral fin (arrow). Explanatory drawing of the skull (A2). Abbreviations: ang, angular; exo, exoccipital; exs, extrascapular; hpl, horizontal pit line of the cheek; ii, supracleithrum pit line; io, infraorbital; iop, interoperculum; mdpl, mandibular pit line; mpl, middle pit line; oc, otic sensory canal; op, operculum; pcl, postcleithrum; pop, preoperculum; popsc, preopercular sensory canal; pscl?, presupracleithrum?; ppa + dpt, postparietal (frontal) + dermopterotic; pstc, pores of the supratemporal commissure; pt, posttemporal; rb, branchiostegal ray; scl, supracleithrum; so, suborbital; sop, suboperculum.
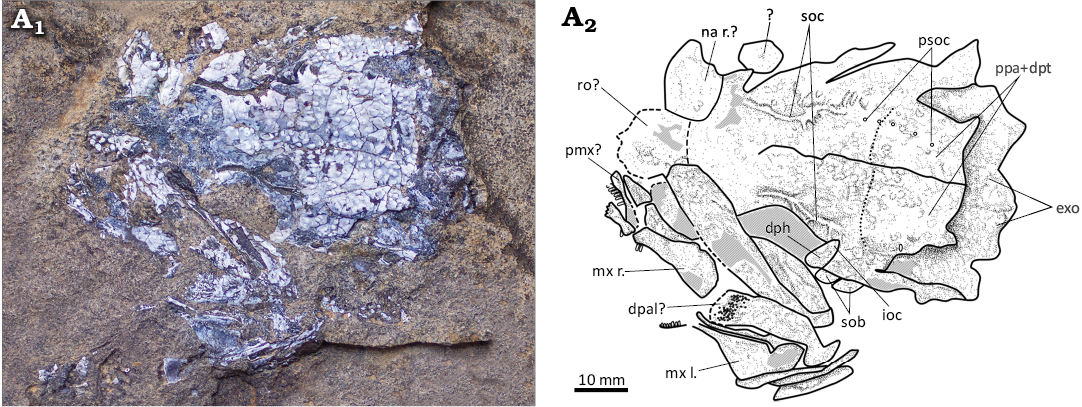
Fig. 5. Dapediid fish Ameghinichthys antarcticus Arratia et al., 2004 (IAA-Pv 432) from the Tithonian, Upper Jurassic of Ameghino (= Nordenskjöld) Formation, Longing Cape, Antarctic Peninsula. Skull roof and left lateral bones of the head (A1). Explanatory drawing of the skull (A2). Abbreviations: dph, dermosphenotic; exo, exoccipital; ioc, infraorbital sensory canal; mx l./r., maxilla left/right; na r?, right nasal?; pmx?, premaxilla?; ppa + dpt, postparietal (frontal) + dermopterotic; psoc, pores of the supraorbital canal; ro?, rostral?; sob, suborbital; soc, supraorbital canal.
Upper jaw: The upper jaw is incompletely preserved in IAA-Pv 432 being represented by the right and left maxilla and the right premaxilla (Fig. 5A2). The dentition is also poorly preserved.
Premaxilla: The premaxilla forms the tip of the snout. IAA-Pv 432 preserved the right premaxilla, which has four to five conical teeth (Fig. 5A2). It is uncertain whether the bone is median or external in view. No ascending process is recognized.
Maxilla: Both maxillae are preserved in IAA-Pv 432 (Fig. 5). Each maxilla is a wedge-shaped bony plate reaching its maximum heigh caudad and tapering anteriorly. The maxilla bears at least three styliform or conical teeth in the anteriormost portion. The maxilla outer surface is smooth.
Lower jaw: The available material does not include a complete lower jaw. Figure 4A2 shows the angular bone, which represents the posteriormost part of the lower jaw. The region bears the mandibular pit-line of the head lateral line system (Fig. 4A2).
Circumorbital series: Some of the circumorbital bones surrounding the orbit dorsally (i.e., supraorbitals) are not preserved. The dermosphenotic is preserved in IAA-Pv 432 (Fig. 5). The bones surrounding the orbit posteriorly and ventrally (i.e., infraorbitals), as well as the suborbitals are well-preserved.
Infraorbitals: The infraorbital series is incompletely preserved but still exhibits at least five plate-like posterodorsal infraorbital bones. The plate in the ventrocaudal portion of the orbit is the largest circumorbital (Fig. 4). Infraorbitals are ornamented with faint tubercles. Infraorbital sensory canal is barely visible and seems to be present rostrad. Fusion between infraorbitals and suborbitals is not clearly visible but suborbitals bear a sensory pit line that could represent the fusion between infra and suborbitals (Fig. 4A2).
Suborbitals: It is not clear if the suborbital series is incompletely preserved but IAA-Pv 846 has at least eight plate-like rectangular bones (Fig. 4). The suborbitals increase in width caudad (Fig. 4A2). Ventral suborbitals overlap the dorsal arm of the preoperculum. Ornamentation of suborbital consists of tubercles which are well developed in the dorsal elements. Ventral suborbitals are smooth. Few suborbitals have remnants of the horizontal pit line.
Opercular series: The opercular series is completely preserved in IAA-Pv 846. The dorsal arm of the preopercle is covered by suborbitals, thus, only the ventral arm of the preopercle is visible (Fig. 4).
Opercle: The opercle is trapezoidal with an anterior well-developed dorsal process (Fig. 4). Ornamentation in the form of ganoine tubercles is present only in the dorsalmost portion of the bone. The height of the opercle is ca. 330 mm, and its width is ca. 350 mm.
Subopercle: The subopercle is triangular, with a concave dorsal margin and convex anterior, posterior, and ventral margins. Posteriorly the subopercle overlaps a branchiostegal ray (Fig. 4A2). The external surface of the subopercle is smooth. The rostrad portion of the subopercle is in contact with the caudad part of the preopercle and at least with two suborbitals (Fig. 4).
Interopercle: The interopercle is roughly rectangular or blade-shaped with barely convex dorsal and ventral margins (Fig. 4). The external surface of the interopercle is smooth. The dorsal part of the interopercle is in contact with the ventral margin of the preopercle, and the rostral tip of the interopercle reaches the caudal portion of the lower jaw (Fig. 4A2). The interopercle in dapediids has a stable position between the branchial chamber and the lower jaw (e.g., Thies and Hauff 2008). The lateral surface of the bone seems to be smooth.
Preopercle: The dorsal arm of the preopercle in IAA-Pv 846 is not visible due to suborbitals covering the bone. The preopercle’s ventral arm is slender and elongated and bears the preopercular sensory canal, which has a single anteriad canal and bears branches in the postero-caudal region. Medially, the ventral portion of the preopercle articulates with the interopercle. The preopercle surface seems smooth, while the vertical grooves on the ventral arm result from preopercular sensory canal ramifications (Fig. 4).
Head lateral-line system: In dapediids the identification of cephalic sensory canals and/or pores related to the head lateral-line can be challenging because of ornamentation and dense bone ossification (Thies and Hauff 2008, 2011; Thies and Waschkewitz 2016).
The supraorbital sensory canal in IAA-Pv 432 is not concealed by bone (Fig. 5A1). It remains unclear whether the supraorbital sensory canal extends to the nasal bone. The otic canal is present in the dermopterotic portion (Fig. 4A2). Postparietals (parietals) bear the middle pit-line (Fig. 4). The infraorbital bones have alienated pores of the infraorbital sensory canal (Fig. 4A2). Segments of the horizontal pit-line of the cheek run on suborbitals 1–3 (Fig. 4A2). Mandibular pit line is on the posteriormost part of the lower jaw (Fig. 4A2). No further canals or pit-lines are observed in the available material.
Pectoral girdle and fin: Endochondral components of the pectoral girdle are not visible in the available material. Dermal components of the pectoral girdle of IAA-Pv 846 include the posttemporal, presupracleithrum?, supracleithrum, and two postcleithra (Fig. 4). Cleithrum is not visible in the available material.
Posttemporal: The posttemporal in IAA-Pv 846 is a rectangular and plate-like bone (Fig. 4) that connects the pectoral girdle to the head. The anterior margin of the posttemporal contacts the extrascapulars (Fig. 4A2). Ventrally, the posttemporal is in contact with the presupracleithrum? and supracleithrum (Fig. 4A2). The posttemporal is strongly ornamented with ganoine tubercles.
Supracleithrum: The triangular, plate-like supracleithrum in specimen IAA-Pv 846 is heavily ornamented with ganoine tubercles (Fig. 4). Its anterior margin is concave, while the posterior margin is convex (Fig. 4). The supracleithrum bears a short posteroventral projection, which is significantly smaller than the projection observed in other dapediids, such as Heterostrophus latus and H. phillipsi (Wagner 1863), Dapedium caelatum (Thies and Hauff 2008: 34, fig. 2), Dapedium ballei (Maxwell and López-Arbarello 2018: fig. 3), and Hemicalypterus weiri (Gibson 2016: fig. 3). The main lateral line’s cephalic portion traverses the supracleithrum of IAA-Pv 846 (Fig. 4), like in other Dapedium caelatum, D. stollorum species (Thies and Hauff 2008, 2011; Thies and Waschkewitz 2016). Additionally, the supracleithrum bears a short and curved pit-line (Fig. 4) resembling D. caelatum (Thies and Hauff 2008: 34).
Presupracleithrum: The bone tentatively identified as presupracleithrum is a large (being this a different condition from other dapediids) and rounded bone. It is located between the posttemporal, opercle, and supracleithrum (Fig. 4A2). Ornamentation consists of ganoine tubercles located mainly in the dorso-posterior portion of the bone.
Postcleithra: IAA-Pv 846 has two rectangular postcleithra differing from the scales in size and ornamentation pattern. The dorsal postcleithrum is ornamented with ganoin tubercles, and the ventral one is smooth. Postcleithra are higher than the scales (Figs. 3, 4).
Pectoral fin: The right pectoral fin is located high in the body (Fig. 6). Its insertion is at the level of the opercle-subopercle union (Fig. 6). At least 18 lepidotrichia are preserved in IAA-Pv 846, they are coated by enameloid. One large basal fulcrum precedes the pectoral fin (Fig. 6A2). Fringing fulcra is well-preserved; at least 26 fringing fulcra are observed (Fig. 6A2). Each fringing fulcrum is elongated, s-shaped, and almost completely covers the next one. The fringing fulcrum near the proximal region is larger than the distal fringing fulcra. The distal fulcrum seems to be wider than the proximal one. The main portion of the lepidotrichium is unsegmented; however, distal segmentation and bifurcation are evident. The pectoral fin is triangular, 30 mm wide and 50 mm deep.
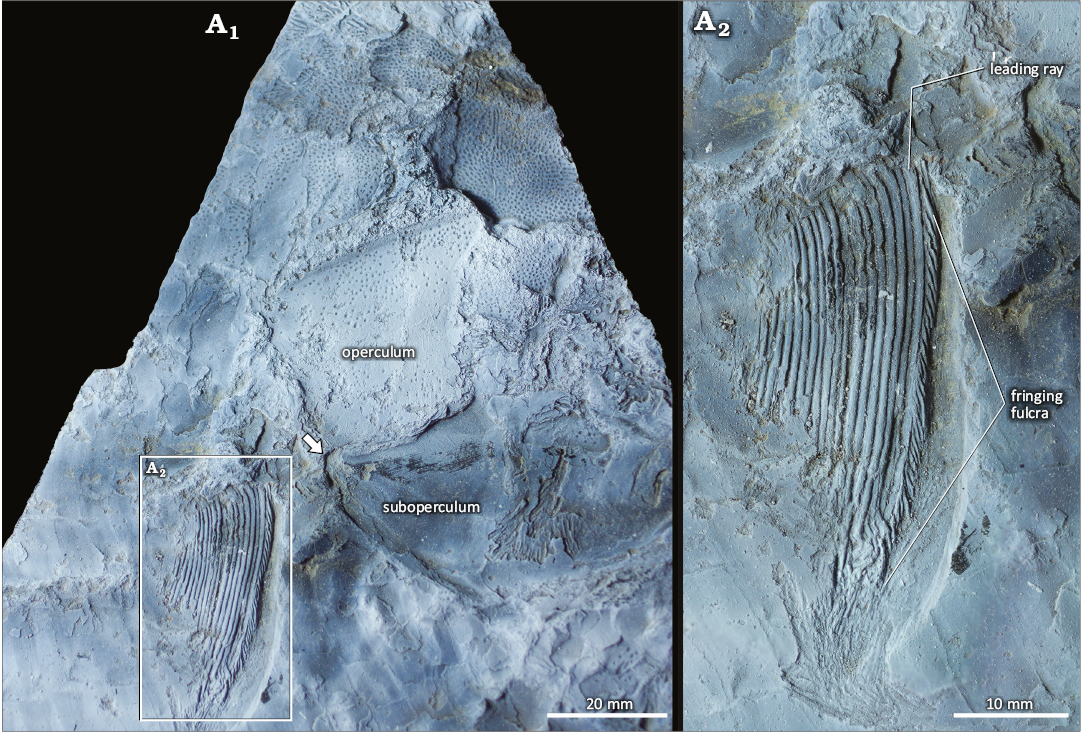
Fig. 6. Dapediid fish Ameghinichthys antarcticus Arratia et al., 2004 (IAA-Pv 846) from the Tithonian, Upper Jurassic of Ameghino (= Nordenskjöld) Formation, Longing Cape, Antarctic Peninsula. General view (A1) showing the pectoral fin insertion (arrow). Magnification of the pectoral fin (A2) showing a detail of the fringing fulcra and leading ray. The specimen was coated with magnesium oxide powder.
Pelvic girdle and fin: The pelvic girdle and fin are obscured and poorly preserved in IAA-Pv 846 (Fig. 3).
Unpaired fins: The endoskeleton of the unpaired fins is covered by the squamation.
Dorsal fin: The dorsal fin of IAA-Pv 846 (Fig. 7A1) is incomplete and poorly preserved. Moreover, the lepidotrichia were distorted post-mortem (Fig. 7A2). The dorsal fin seems to initiate around the 24th complete scale row and is preceded by a series of paired lanceolate basal fulcra (Fig. 7A3), which resembles those described as pattern II by Arratia (2009). The leading ray of the dorsal fin carries the fringing fulcra pattern C (Fig. 7A3). Below the dorsal fin, scales are rectangular, higher than wide, and covered with ganoine (Fig. 7). The precise number of fin rays cannot be determined due to preservation issues. Nevertheless, each lepidotrichia comprises a basal unsegmented portion followed by several short distally branched segments (Fig. 7A2).

Fig. 7. Dapediid fish Ameghinichthys antarcticus Arratia et al., 2004 (IAA-Pv 846) from the Tithonian, Upper Jurassic of Ameghino (= Nordenskjöld) Formation, Longing Cape, Antarctic Peninsula. General view of dorsal fin (A1). Close-up of the dorsal fin in section b (A2), to show the lepidotrichia and rectangular scales. Close-up of in section c (A3), to show the fulcra (the specimen was coated with magnesium oxide powder). Arrows point rostrad. Abbreviations: bf, basal fulcra; d.fr, dorsal fin rays; ff, fringing fulcra; sc, scales.
Anal fin: The anal fin is badly preserved in both, IAA-Pv 846 and IAA-Pv 342. Lepidotrichia (ca. 23 lepidotrichia preserved in IAA-Pv 342) basal portion is hourglass-shaped and measures 10 mm in length.
Caudal fin: The caudal fin is not entirely preserved in any available specimens. IAA-Pv 431a, b comprises several lepidotrichia patches that appear to be those of the caudal fin. All lepidotrichia are segmented into short, quadrangular segments and dichotomized at least twice. Additionally, some patches are associated with teardrop-shaped scales and one with basal fulcra. Nonetheless, the precise number of fin rays, basal, and fringing fulcra remains unknown.
Squamation: The IAA-Pv 846 has 20 to 22 predorsal scales. It has 28 scales per transversal scale row are preserved at the middle portion of the fish, anterior to the dorsal fin insertion. The height of these scales increases ventrally while the ornamentation decreases from the dorsal to the ventral area.
Along the longitudinal midline of the body, IAA-Pv 846 has nearly 60 scales. Identification of lateral line scales is challenging due to preservation. Nevertheless, based on silicone peel observations, there are a minimum of four lateral line scales detected posterior to the cleithrum. Among the scales, the lateral line canal is positioned at the midline (Fig. 3). Rectangular scales are located towards the posterior of the opercle (Fig. 4A2). Furthermore, the posterior margin of the scales is smooth and straight (Figs. 3, 7, 8). Flank scales in IAA-Pv 846 are articulated, thus the peg-and-socket articulation and/or the scale processes are not visible. A detached scale of IAA-Pv 846 shows the peg-and-socket articulation and a short anterodorsal process (Fig. 8A). Scales of the caudal peduncle are diamond-shaped, changing to teardrop-shaped near the ventral part of the body. No ganoine was preserved in the caudal peduncle scales of IAA-Pv 846. On those scales that retained ganoine, the ornamentation of the scales is characterized by ganoine ridges, few rugae, and tubercles (Fig. 8B, C). The ganoine ridges do not extend to the back edge of the scale (Fig. 8B, C). In IAA-PV 433 each ganoine ridge is spoon-shaped, with the wider end pointing caudad (Fig. 8B). In IAA-PV 846 the scales of the uppermost section of the body, just behind the skull and anterior to the dorsal fin, are quadrangular and heavily ornamented (Fig. 8C), almost all these scales are preserved as impressions (Fig. 3). Most of the scales in IAA-Pv 846 are preserved as impressions or in mesial view whenever the ornamentation cannot be observed. However, in the regions where scale ornamentation is preserved, it decreases caudad and ventrad (Fig. 3). The number, size, and shape of ganoine ridges varies between specimens (Fig. 8B, C).
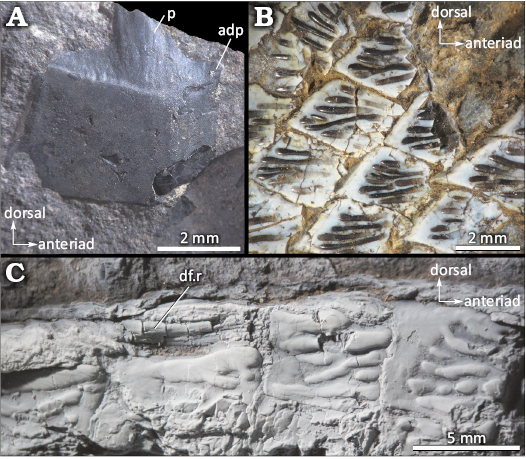
Fig. 8. Dapediid fish Ameghinichthys antarcticus Arratia et al., 2004, from the Tithonian, Upper Jurassic of Ameghino (= Nordenskjöld) Formation, Longing Cape, Antarctic Peninsula. A. IAA-Pv 846, detached quadrangular scale. B. IAA-Pv 433, body scales showing the spoon shaped ganoine ridges. C. IAA-Pv 846, predorsal scales showing scale ornamentation (the specimen was coated with magnesium oxide powder). Abbreviations: adp, anterior dorsal process; df.r, dorsal fin ray; p, peg of the peg-and-socket articulation.
The scale ornamentation of Ameghinichthys antarcticus bears some resemblance to that of all species of Ptycholepis, a “palaeonisciform” actinopterygian known from the Middle Triassic to the Lower Jurassic (Wenz 1968; Bürgin 1992) with a putative record from the Upper Jurassic of Thailand (Cavin et al. 2009). However, the scales of the species of the genus Ptycholepis (e.g., P. priscus Bürgin, 1992; P. bollensis Agassiz, 1832) are morphologically different from those of A. antarcticus, being shallower, broader than deep, and often with a posterior serrated margin (Wenz 1968; Bürgin 1992).
Remarks.—Ameghinichthys antarcticus shares plesiomorphic characters (i.e., body shape, dermal skull roof and cheek bones, and the smooth caudal margin of the scales) with other Dapediiformes. Ameghinichthys antarcticus is represented by a large specimen (more than 30 cm of total length) and considerably smaller specimens as well. The discovery of a nearly complete specimen in 2020 allows the attribution of both the isolated and fragmentary material recovered during previous field trips, to the species Ameghinichthys antarcticus originally figured and succinctly described by Arratia et al. (2004). Although the ornamented scales in Arratia et al. (2004) appear to be higher in number per scale row, their scale size is similar.
Stratigraphic and geographic range.—Uppermost lower Tithionian–lower upper Tithonian, Longing Member, Ameghino Formation, Larsen Basin; Longing Cape, Longing Gap locality, Antarctic Peninsula.
Discussion
Ameghinichthys as a Dapediiformes.—The study of Ameghinichthys antarcticus led us to the recognition of the species as a member of Dapediiformes since it has unquestionable similarities to the Dapediidae family: a deep disc-shaped and compressed body; hem-like dorsal and anal fins; dorsal fins with more than 20 rays, and vertical branch of the preoperculum covered by suborbitals (e.g., Thies and Hauff 2011; Gibson 2016).
It is noteworthy that the phylogenetic hypothesis for Dapediiformes presented elsewhere shows several unresolved (i.e., Gibson 2016: fig. 9, left) or completely unresolved (Latimer and Giles 2018: fig. 10) relationships among members of Dapediidae. Thus, we propose the phylogeny of Dapediiformes needs to be explored in the framework of a cladistic analysis with the addition of Ameghinichthys antarcticus, as it is beyond the scope of the present contribution. We assume that the inclusion of A. antarcticus morphological characters in the data matrices provided for Dapediiformes (i.e., Gibson 2016; Latimer and Giles 2018) will lead to unresolved relationships among dapediiform taxa, as the Antarctic specimen does not exhibit most of the characters used in these phylogenies. Furthermore, the published matrices, based on the data matrix of López-Arbarello (2012), do not appear to be useful for resolving phylogenetic relationships among Dapediiformes, because that matrix was built to resolve the relationships among ginglymodian fishes, with the addition of few dapediiform characters. We believe that a new data matrix needs to be built when trying to solve the phylogenetic position of Dapediiformes among neopterygians, and also among the members within Dapediiformes, so a careful study of morphological characters must be done.
Relative to the preservation of Ameghinichthys antarcticus, we emphasize that comparisons with other dapediiforms are rather difficult. Based on the combination of diagnostic morphological characters, five appear to be unique to Ameghinichthys among Dapediiformes: (i) complex skull roof bone composed by the fusion of the postparietals with the dermopterotic; (ii) trapezoidal opercle with a convex to straight ventral contact with the subopercle and with a dorso-anterior projection; (iii) opercle, subopercle, and interopercle vertically aligned; (iv) subopercle, interopercle, and first branchiostegal ray meet at a point posteriorly just below the posterior margin of the opercle; (v) origin of the pectoral fin at the level of the opercle-subopercle contact. The other morphological characters are not apomorphic for the genus when considered individually. In combination, they are unique and useful for the diagnosis of Ameghinichthys.
Comparisons with morphologically similar Dapediiformes.—Following, we compare Ameghinichthys with other Dapediiformes—mainly with those recorded in Jurassic beds.
The Early Jurassic dapediiform, Paradapedium, from the continental Kota Formation of India (Jain 1973) has a small head in relation to the body, and the body height is taller than that of Ameghinichthys, therefore giving this fish a completely different body-shape than that of Ameghinichthys. Also, Paradapedium has independent dermal skull roof bones, in contrast with Ameghinichthys, in which the dermopterotic is fused with the postparietals. Paradapedium has dorsal and ventral scale ridges with pectinate scales, and dorsoventrally elongated flank and abdominal scales; Ameghinichthys does not have pectinated scales. The continental dapediiform Aetheolepis, found in the Upper Jurassic Talbrargar Fossil Fish Bed in Australia (Gibson 2016; Szabó and Pálfy 2020) and considered the sister taxon of Heterostrophus (Gibson 2016), does not resemble Ameghinichthys.
To date, the only marine Late Jurassic dapediiform known is Heterostrophus latus from the Kimmeridgian–Tithonian of the Tethys Sea within the Solnhofen Archipelago (Wagner 1863; Lambers 1999). Ameghiinchthys antarcticus (Fig. 9A) and Heterostrophus latus (Fig. 9B) exhibit comparable standard length (ca. 450 mm and 420 mm, respectively), yet have evident differences in their body shape: A. antacticus is more disc-shaped with a height of ca. 240 mm in comparison with H. latus, which has a height of ca. 150 mm the flank scales of the lateral line and midabdominal region that are significantly larger in A. antarcticus (ca. 12–20 mm) than in H. latus (ca. 6.4–7.0 mm). Heterostrophus latus has 29–35 predorsal scales, 62–65 scales are along the longitudinal midline of the body, and 45–53 scales in the transversal scale row at the level of the insertion of the dorsal fin. Ameghinichthys antarcticus has 20–22 predorsal scales; ca. 60 scales along the longitudinal midline of the body, and 28 scales per transversal scale row anterior to the dorsal fin insertion. The height of these scales increases ventrally while the ornamentation decreases from the dorsal to the ventral area. Scales above the lateral line are smaller and more quadrangular in H. latus than those present in A. antarcticus. Along the longitudinal midline of the body, IAA-Pv 846 has nearly 60 scales. In addition, the scales of A. antarcticus are heavily ornamented with ganoine rugae whereas those of H. latus bear faint ganoine ridges. Moreover, H. latus lacks the ganoine tubercle ornamentation of the dermal skull roof (Wagner 1963: 615; Maxwell and López-Arbarello 2018). According to Wagner (1963: 616), the pectoral fin rays of H. latus are bifurcated, while those in A. antarcticus lack bifurcation.
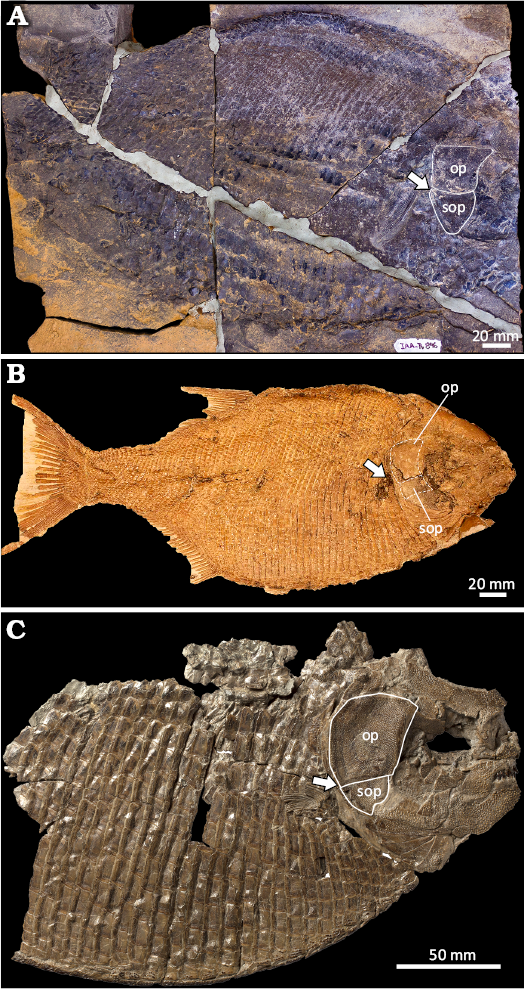
Fig. 9. Comparative general body shape and pectoral fin position in Jurassic dapediids. A. Ameghinichthys antarcticus Arratia et al., 2004 from the Tithonian, Upper Jurassic of Ameghino (= Nordenskjöld) Formation, Longing Gap, Antarctic Peninsula (IAA-Pv 846). B. Heterostrophus latus Wagner, 1863 from the lower Tithonian, Upper Jurassic of Solnhofen, Germany (holotype CM 4762, photograph courtesy of A. Henrici). C. Heterostrophus phillipsi Woodward, 1929, from the Callovian, Middle Jurassic of England (holotype BGS GSM113113 modified from http://www.3d-fossils.ac.uk, used under http://creativecommons.org/licenses/bync-sa/3.0). Position and shape of the operculum and suboperculum in B are illustrated in dotted lines because limits are not clear due to preservation. Arrows indicate the insertion of the pectoral fin. Abbreviations: op, operculum; sop, suboperculum.
Ameghinichthys antarcticus shares similarities in the body shape and pectoral fin position with Heterostrophus phillipsi from the Callovian, Middle Jurassic of Peterborough Member of the Oxford Clay Formation, in the Southeastern UK (Woodward 1929; Fig. 9C). The pectoral fin in both species is located at the articulation between the opercle and subopercle (Fig. 9). However, the opercle, subopercle, and preopercle in A. antarcticus are proportionally smaller than those of H. phillipsi. Comparisons of the dorsal, anal, and caudal fins of Ameghinichthys antarcticus, Heterostrophus latus, H. phillipsi, and other dapediiforms are precluded by differential preservation. Lastly, the skull dermal bones of H. phillipsi show denser ornamentation when compared with A. antarcticus.
The squamation of Dapediiformes is characterized by rhomboid ganoid scales that vary in shape, ornamentation patterns, size, and distribution over the body. While thick rhomboid scales are present only in the anterior portion of the body region of the Triassic genera, Hemicalypterus, Dandya, and Sargodon (e.g., Gibson 2016; Tintori 1983), the Jurassic dapediiforms have the whole body covered by scales.
Conversely, Aetheolepis has rhomboid scales of amioid type in the posterior portion of the body. Rhomboid scales extend across the entire body in the Jurassic genera Dapedium, Tetragonolepis, Paradapedium, Heterostrophus, and Ameghinichthys.
Flank scales, consisting of the lateral line and midabdominal scales, are rectangular, with a greater width/height ratio. The scales behind the operculum of Ameghinichthys antarcticus have a scale length/height ratio of 0.48, like that of D. caelatum (Thies and Waschkewitz 2016: 21) and are among the larger scales within dapediiforms. Meanwhile, the scales located at the dorsal and ventral midline are diamond-shaped. The precaudal and caudal fin base scales are teardrop-shaped with a length greater than their height. Among Dapediiformes the posterior scale margin can be serrated (e.g., Dapedium politum, D. radiatum, D. punctatum) or smooth (e.g., D. ballei, Heterostrophus latus, H. phillipsi, Ameghinichthys antarcticus). Scale surface ornamentation in dapediiforms varies among species and includes a pitted surface (e.g., Dapedium ballei), heavily tuberculate surface (e.g., D. magnevillei, D. granulatum), delicate rugae (e.g., Heterostrophus latus), and heavily rugae (e.g., Ameghinichthys antarcticus, H. phillipsi). The scales of the dorsal and ventral midline of Ameghinichthys antarcticus resemble those of the ventral midline of H. phillipsi.
Ameghinichthys antarcticus shares several morphological characters with for instance Dapedium stollorum, D. punctatum, D. pholidotum, and D. ballei, such as the presence of a large interopercle that can reach the lower jaw articulation (e.g., Thies and Hauff 2011; Thies and Waschkewitz 2016); interopercle and ventral arm of the preopercle of similar size (e.g., Thies and Waschkewitz 2016; Maxwell and López-Arbarello 2018); conical and unicuspid teeth on the premaxilla. It is worth noting that Ameghinichthys antarcticus has few conical teeth on the maxilla, and teeth of the premaxilla and maxilla are relatively fine and small compared to the above mentioned species of Dapedium. The preopercle of Ameghinichthys antarcticus is strongly covered by the interopercle as in D. noricum. The supraorbital canals of Ameghinichthys antarcticus are well developed and not concealed by bone (Fig. 5A). The interopercle position in Ameghinichthys antarcticus resembles that observed in Dapedium stollorum (Thies and Hauff 2011; Thies and Waschkewitz 2016). Ameghinichthys antarcticus does not have a serrated posterior margin of its scales as in D. noricum (Tintori 1983), which is also a smaller fish with a total length of about 100 mm. Ameghinichthys antarcticus differs significantly from the Triassic genera Dandya ovalis, which lacks ornamentation on dermal bones and has different squamation patterns (Tintori 1983; Hornung et al. 2019). It also differs from Hemicalypterus weiri and Sargodon tomicus due to dentition, squamation patterns, and interopercle size and shape (Tintori 1983; Gibson 2016).
Body size, ontogeny, and diet of Ameghinichthys.—Dapediiforms range in size from ca. 100–1000 mm (Tintori 1983, 1998; Tintori and Lombardo 2018). Most Dapedium species have a standard length of ca. 80–500 mm (Maxwell and López-Arbarello 2018). We estimate the body size of Ameghinichthys antarcticus (IAA-Pv 846) to be ca. 450 mm. The skull length represents between 25–30% of the total length in Sargodon tomicus (Tintori 1998) and Hemicalypterus weiri (Gibson 2016) but represents about 18% of the total length in species of the genus Dapedium.
In extant non-teleostean actinopterygian fishes, the lateral line system of the skull is initially opened in superficial grooves (Maxwell and López-Arbarello 2018). We interpret the small, nearly complete skull roof of specimen IAA-Pv 432 as belonging to a juvenile (due to the open sensory canal systems) or subadult (due to the open sensory canal systems and the heavily ornamentation in the skull roof bones) ontogenetic state.
Based on their dentition, it has been inferred that the Dapediiformes diets were varied; some taxa were herbivorous like Hemycalipterus weiri (Gibson 2016) and probably Dandya ovalis (Tintori and Lombardo 2018), others were true durophagous like Sargodon tomicus (Tintori and Lombardo 2018) and others were generalist durophagous such as Dapedium granulatus and Scopulipiscis saxciput (Smithwick 2015; Latimer and Giles 2018). Diet likely changed with ontogeny, and that dapediiforms primarily had a generalistic diet. Currently, there is limited information available regarding the dentition of Ameghinichthys antarcticus. However, like other dapediiforms, it may have been a generalist durophagous, consuming hard-shelled prey such as ammonites and bivalves, which are abundantly found in the Ameghino Formation. The Ameghino Formation hosts a wide array of hard-shelled invertebrates (belemnites, ammonites, bivalves, and crustaceans) and vertebrates (mostly comprising small to medium-sized actinopterygian fishes). This allows for the thriving of both generalist and durophagous species. To date, the only known putative durophagous candidates of the ichthyofauna in the Ameghino Formation are Ameghinichthys antarcticus and indeterminate Ginglymodi (SGC personal observation) actinopterygians.
The first and last occurrences of dapediids are associated with fossil Lagerstätten, being the first undoubted occurrence in the Norian (Upper Triassic) Zorzino Limestones and the late occurrence in the Kimmeridgian–Tithonian (Upper Jurassic) Solnhofen Lithographic Limestones (Szabó and Pálfy 2020). In this context, the report of Ameghinichthys antarcticus in Tithonian of the Ameghino Formation contributes new insights regarding the ichthyofauna diversity in the Late Jurassic, and at the beginning of the Gondwana break-up in southern high latitudes. Ameghinichthys antarcticus is an endemic Antarctic Dapediiformes. Like other dapediforms, Ameghinichthys inhabited an epeiric sea, living near the bottom, in well-oxygenated waters. This epeiric sea was associated with the Mesozoic rifting at high latitudes. We postulate that following death, the specimen floated in the water column for a brief period before sinking and being subsequently buried in calm, oxygen-deficient, and stratified bottom waters (e.g., Kietzmann et al. 2009).
Basin evolution, paleobiogeography, and faunal exchange.—Dapediiforms were widespread in the Northern Hemisphere. Their fossil record includes the Upper Triassic of Italy, Austria, Switzerland, UK, Saudi Arabia, and the USA; the Lower Jurassic of UK, France, Germany, and India; and the Upper Jurassic of UK, Germany, and Australia. Dapediiformes comprises both freshwater and marine species inhabiting lacustrine, fluvial to deltaic environments and moderately shallow marine environments (Gibson 2016; Smithwick 2015, and references therein). The environments in which these fishes lived were typically well oxygenated (Tintori and Lombardo 2018). However, the bottom waters, in which the specimens fell, were eventually buried, and fossilized, were anoxic, therefore favouring their preservation (e.g., Röhl and Schmid-Röhl 2005; Tintori and Lombardo 2018).
The Antarctic Larsen Basin developed in the Jurassic as a result of a continental rifting during the early stages of the Gondwanan supercontinent break-up. West (South America and Africa) and East Gondwana (Antarctica, Australia, India, and New Zealand) landmasses subjected to transtension during the Jurassic (Fitzgerald 2002). A transgressive post-rift megasequence was deposited during the Kimmeridgian–early Berriasian (Macdonald et al. 1988; Hathway 2000). Sedimentation during the Kimmeridgian–early Berriasian is characterized by a paralic succession (i.e., clastic depositional environments developed along or near coastlines, including deltas, shoreline-shelf systems and estuaries; Hampson et al. 2017).
The deposition of the Ameghino Formation is hypothesized to have occurred within an epeiric or epicontinental sea (= shallow seas that developed over large areas when cratonic interiors flooded during periods of high sea-level) in a period of thermal subsidence phase and with a maximum eustatic sea level during the Jurassic, which served to maximize sediment starvation (e.g., Farquharson 1983; Farquharson et al. 1984; Doyle and Whitham 1991; Pirrie and Crame 1995; Hathway 2000). The uppermost Tithonian black shales of the Ameghino Formation recorded the peak transgression and relative sediment starvation on the outer shelf. Volcanism regularly contributed air fall tufts to the marine succession, although the absence of epiclastic detritus suggests that the magmatic arc lacked significant subaerial relief at this time (Farquharson et al. 1984). Nevertheless, the emerging arc could have acted as a barrier to the free circulation of waters (Hathaway 2000).
The anoxia observed in the lower member of the Ameghino Formation is widespread in the Weddell Sea and the South Atlantic (Whitham and Doyle 1989; Farquharson 1983). The anoxia is associated with the upwelling of paleo-Pacific currents near the outer margin of the regional basin, resulting in an extended oxygen minimum zone and the presence of sills in areas further from the Pacific margin (Hathaway 2000). The record of plant debris, large wood, and terrigenous clastic content in the Ameghino Formation, evidence landmasses existed in the region (Hathaway 2000). However, the connection between landmasses and the open sea remains unclear.
During the Jurassic the paleogeography is characterized by the opening of marine corridors that separated Laurasia and Gondwana. The principal corridors included the Hispanic (= Caribbean) Corridor in Central America, and the Mozambique (= Trans-Gondwana, Trans-Erythraean or South African Seaway) Corridor in the south, which separated Africa from the Madagascar-India-Antarctica block. The presence of these marine pathways resulted in climatic and palaeoceanographic conditions that allowed the exchange of faunas between Laurasia and Gondwana (e.g., Damborenea 2017; Gouiric-Cavalli et al. 2019).
The paleogeographic relationships of Gondwana seas in South America and Antarctica with the Tethys have been the subject of considerable research, recently with a particular focus on actinopterygian fishes (e.g., Arratia 2008b; Arratia et al. 2004; Gouiric-Cavalli et al. 2019; Gouiric-Cavalli and Arratia 2022) and marine reptiles (e.g., Gasparini and Iturralde-Vinent 2006; Campos et al. 2021). The fossil record of Dapediiformes, which includes the oldest marine records of the group in various European localities (see above), suggests that a Dapediiformes group migrated and/or dispersed to the southernmost seas through the available marine pathways (i.e., Hispanic and/or Mozambique corridors) to the Larsen Basin in the Antarctic Peninsula, where a subsequent speciation occurred in the waters surrounding Antarctica. The group’s plasticity would have enabled some taxa to invade freshwater environments in the dapediiform evolutionary history.
Conclusions
The ichthyofauna of the Ameghino Formation (Antarctic Peninsula) is ecologically and taxonomically diverse (e.g., Arratia et al. 2004; Gouiric-Cavalli et al. 2017; Gouiric-Cavalli et al. 2019). This ichthyofauna includes suspension-feeders (Gouiric-Cavalli et al. 2019), ichthyophagous (Arratia et al. 2004; Gouiric-Cavalli et al. 2017) and durophagous (Gouiric-Cavalli et al. 2017; SGC personal observation, this contribution) actinopterygians.
Ameghinichthys antarcticus was initially erected from a single fragment of flank squamation preserved as an imprint (Arratia et al. 2004). Here we provide a new and comprehensive diagnosis for the species based on numerous new and better-preserved materials. Ameghinichthys antarcticus is assigned to the order Dapediiformes based on: (i) a deep disc-shaped and compressed body; (ii) hem-like dorsal and anal fins; (iii) dorsal fins with more than 20 rays, (iv) vertical branch of the preoperculum covered by suborbitals; (v) the position of the pectoral fin placed high in the body, (vi) scale morphology, and (vii) scale and skull roof bone ornamentation. Our study also provides novel information on the structures associated with the dorsal fin (basal fulcra resembling type I and fringing fulcra of pattern C) and the sensory canals of the head lateral-line system. We propose that Ameghinichthys is closely related to Dapedium based on the general morphology of the body. The Antarctic genus represents both the southernmost worldwide record and the second marine Dapediiformes for the Late Jurassic. It also represents, together with Heterostrophus latus from the Solnhofen Lithographic Limestones, the latest known fossil record for Dapediiformes.
Our finding highlights the importance of the ongoing explorations in the Antarctic Mesozoic fishes, adding to the knowledge of the evolutionary history of Southern Hemisphere marine ichthyofaunas. Further research on dapediiforms should encompass a comprehensive anatomical study and a phylogenetic hypothesis exploring not only relationships between different species but also testing the position of dapediiforms among neopterygian actinopterygians.
Acknowledgments
We thank Carlos Gómez (Comando Conjunto Antártico, Ejército Argentino), Mauricio Bigurrarena Ojeda, José O’Gorman, Leonel Acosta Burlaille, Juan José Moly (all División Paleontología Vertebrados, Facultad de Ciencias Naturales y Museo, La Plata, Argentina) for their efforts in fieldwork and specimen collection. Logistic support in Antarctica was provided by the Comando Conjunto Antártico and the Dirección Nacional del Antártico from Argentina. Special thanks to Leonel Acosta Burlaille (División Paleontología Vertebrados, Facultad de Ciencias Naturales y Museo, La Plata, Argentina) for the detailed and careful fossil preparation and making the silicone casts of the specimen IAA-Pv 846. SGC appreciates information and insightful discussions with Andrea Tintori (Dipartimento di Scienze della Terra “Ardito Desio” Uiversità Degli Studi Di Milano, Italy), Detlev Thies (Leibniz Universität Hannover, Germany) and Lynne Bean (Research School of Earth Sciences, Australian National University, Australia). Marian Tanuz (CPBA-V), Cecilia Amenábar and Agustín Cuparo are responsible for loaning and providing access to fossil specimens from the Repositorio de Colecciones Paleontológicas y Geológicas of the Instituto Antártico Argentino, Cuidad Autónoma de Buenos Aires, Argentina. We thank the Universidad Nacional de La Plata and Museo de La Plata, División Paleontología Vertebrados for their support of this research. We thank the Agencia Nacional de Promoción Científica y Tecnológica for financial support through PICT 2017-0607 (to Marcelo Reguero), PICT 2019-02419 (to Soledad Gouiric Cavalli), and PICTO Malvinas-2021-00004 (to Florencia Milanese and SGC). We thank the reviewers Andrea Tintori (Dipartimento di Scienze della Terra “Ardito Desio” Uiversità Degli Studi Di Milano, Italy) and Gloria Arratia (Biodivestity Institute and Natural History Museum, Kansas, USA), for their valuable comments that improved the quality of the manuscript.
Authors’ contributions
SGC: conceptualization, data curation, formal analysis, investigation, visualization, methodology, writing-original draft; SGC, AI, BC: writing-review and editing; SGC, MR: funding acquisition.
References
Agassiz, L. 1832–1843. Recherches sur les Poissons Fossiles. II. Contenant l’Histoire de l’Ordre des Ganoïdes. 336 pp. Petitpierre, Neuchatel. Crossref
Arratia, G. 1999. The monophyly of Teleostei and stem-group teleosts. Consensus and disagreements. In: G. Arratia and H.-P Schultze (eds.), Mesozoic Fishes 2: Systematics and Fossil Record, 265–334. Verlag Dr. Friedrich Pfeil, Munchen.
Arratia, G. 2008a. Actinopterygian postcranial skeleton with special reference to the diversity of fin ray elements, and the problem of identifying homologies. In: G. Arratia, H.-P. Schultze, and M.V.H. Wilson (eds.), Mesozoic Fishes 4: Homology and Phylogeny, 49–101. Verlag Dr. Friedrich Pfeil, München.
Arratia, G. 2008b. The varasichthyid and other crossognathiform fishes, and the break-up of Pangaea. In: L. Cavin, A. Longbottom, and M. Richter (eds.), Fishes and the Breack-up of Pangaea. Geological Society of London Special Publication 295: 71–92. Crossref
Arratia, G. 2009. Identifying patterns of diversity of the actinopterygian fulcra. Acta Zoologica 90: 220–235. Crossref
Arratia, G., Scasso, R.A., and Kiessling, W. 2004. Late Jurassic fishes from Longing Gap, Antarctic Peninsula. Journal of Vertebrate Paleontology 24: 41–55. Crossref
Bellwood D. and Hoey A. 2004. Feeding in Mesozoic fishes: a functional perspective. In: G. Arratia and A. Tintori (eds.), Mesozoic Fishes 3: Systematics, Palaeoenvironment and Biodiversity, 639–649. Verlag Dr. Friedrich Pfeil, München.
Bigurrarena Ojeda, M., Gouiric-Cavalli, S., Pérez, L.M., and Reguero, M.A. 2023. Bromalites from the Ameghino (= Nordensköld) Formation Upper Jurassic of Antarctic Peninsula. Publicación Electrónica de la Asociación Paleontológica Argentina 23 (2): 45–64.
Bürgin, T. 1992. Basal ray-finned fishes (Osteichthyes; Actinopterygii) from the Middle Triassic of Monte San Giorgio (Canton Tessin, Switzerland). Mémoires Suisses de Paléontologie 114: 1–164.
Campos, L., Fernández, M., Herrera, Y., Talevi, M., Concheyro, A., Gouiric-Cavalli, S., O’Gorman, J., Santillana, S.N., Acosta Burlaille, L., Moly, J.J., and Reguero, M. 2021. Bridging the southern gap: First definitive evidence of Late Jurassic ichthyosaurs from Antarctica and their dispersion routes. Journal of South American Earth Sciencies 109: 103259. Crossref
Cavin, L., Deesri, U., and Suteethorn, V. 2009. The Jurassic and Cretaceous bony fish record (Actinopterygii, Dipnoi) from Thailand. Geological Society, London, Special Publications 315: 125–139. Crossref
Cawley, J.J., Marramá, G., Carnevale, G., Villafaña, J.A., López-Romero, F.A., and Kriwet, J. 2021. Rise and fall of †Pycnodontiformes: Diversity, competition and extinction of a successful fish clade. Ecology and Evolution 11: 1769–1796. Crossref
Clarke, J.T. and Friedman, M. 2018. Body-shape diversity in Triassic–Early Cretaceous neopterygian fishes: sustained holostean disparity and predominantly gradual increases in teleost phenotypic variety. Paleobiology 44: 402–433. Crossref
Cope, E.D. 1872. Observations on the systematic relations of the fishes. Proceedings of The American Association for the Advancement of Science 20: 317–347.
Damborenea, S.E. 2017. Revisión de los biocoremas marinos globales del Jurásico según la distribución de los moluscos bivalvos. Publicacion Electrónica de La Asociación Paleontológica Argentina 17: 31–49. Crossref
Del Valle, R.A., Elliot, D.H., and Mcdonald, D.I.M. 1992. Sedimentary basins on the east flank of the Antarctic Peninsula: proposed nomenclature. Antarctic Science 4: 477–478. Crossref
Doyle, P. and Whitham, A.G. 1991. Palaeoenvironments of the Nordenskjöld Formation: an Antarctic Late Jurassic–Early Cretaceous black shale-tuff sequence. In: R.V. Tyson and T.H. Pearson (eds.), Modern and Ancient Continental Shelf Anoxia, 397–414. The Geological Society, London. Crossref
Elliot, D.H. 1988. Tectonic setting and evolution of the James Ross Basin, northern Antarctic Peninsula. Geological Society of America Memoir 169: 541–555. Crossref
Farquharson, G.W. 1982. Late Mesozoic sedimentation in the northern Antarctic Peninsula and its relationship to the southern Andes. Journal of the Geological Society of London 139: 721–727. Crossref
Farquharson, G.W. 1983. The Nordenskjöld Formation of the Northern Antarctic Peninsula: an Upper Jurassic radiolarian mudstone and tuff sequence. British Antarctic Survey Bulletin 60: 1–22.
Farquharson, G.W., Hamer, R.D., and Ineson, J.R. 1984. Proximal volcaniclastic sedimentation in a Cretaceous back-arc basin, northern Antarctic Peninsula. Geological Society, London, Special Publications 16: 219–229. Crossref
Fitzgerald, P. 2002. Tectonics and landscape evolution of the Antarctic plate since the breakup of Gondwana, with an emphasis on the West Antarctic Rift System and the Transantarctic Mountains. Royal Society of the New Zealand Bulletin 35: 453–469.
Gardiner, B.G. 1960. A revision of certain actinopterygian and coelacanth fishes, chiefly from the Lower Lias. Bulletin of the British Museum (Natural History) 4: 239–384. Crossref
Gardiner, B.G. 1996. Interrelationships of basal neopterygians. In: M.L.J. Stiassny L.R. Parenti, and G.D. Johnson (eds.), Interrelationships of Fishes, 117–146 Academic Press, San Diego. Crossref
Gasparini, Z. and Iturralde-Vinent, M. 2006. The Cuban Oxfordian herpetofauna in the Caribbean seaway. Neues Jahrbuch für Geologie und Paläontologie, Abhandlugen 240: 343–371. Crossref
Gorjanovic-Kramemberg, K. 1905. Die Obertriadische Fischfauna von Hallein in Salzburg. Beiträge zur Paläontologie und Geologie Österreich-Ungarns un des Orients-Mitteilungen des Geologishchen und Paläntologischen institutes der Universität Wien 18: 193–224.
Gibson, S.Z. 2016. Redescription and Phylogenetic Placement of †Hemicalypterus weiri Schaeffer, 1967 (Actinopterygii, Neopterygii) from the Triassic Chinle Formation, Southwestern United States: New Insights into Morphology, Ecological Niche, and Phylogeny. PLOS ONE 11: e0163657. Crossref
Gouiric-Cavalli, S. and Arratia, G. 2022. A new †Pachycormiformes (Actinopterygii) from the Upper Jurassic of Gondwana sheds light on the evolutionary history of the group. Journal of Systematics Palaeontology 19: 1517–1550.
Gouiric-Cavalli, S. Acosta Burlaille, L., Iglesias, A., Moly, J.J., O’Gorman, J.P., Reguero, M.A., Santillana, S., Talevi, M., Vieytes, C., Bigurrarena Ojeda, M., and Lusky, J. 2017. Late Mesozoic marine Antarctic fishes: future perspectives based on the newly collections recovered in the Ameghino and López de Bertodano Formations. Research and Knowledge 3: 16–21.
Gouiric-Cavalli, S., Rasia, L.L., Márquez, G.J., Rosato, V., Scasso, R.A., and Reguero, M.A. 2019. First pachycormiform (Actinopterygii, Pachycormiformes) remains from the Late Jurassic of the Antarctic Peninsula and remarks on bone alteration by recent bioeroders. Journal of Vertebrate Paleontology 35: 1–10. Crossref
Hampson, G.J, Reynolds, A.D, Kostic, B., and Wells, M.R, 2017. Introduction to the sedimentology of paralic reservoirs: recent advances. Geological Society, London, Special Publications 444: 1–6. Crossref
Hathaway, B. 2000. Continental rift to back-arc basin: Jurassic–Cretaceous stratigraphical and structural evolution of the Larsen Basin, Antarctic Peninsula. Journal of the Geological Society, London 157: 417–432. Crossref
Hornung, T., Kogan, I., Moosleitner, G., Wolf, G., and Van der Vielen, J. 2019. The Norian fish deposits of Wiestal (“Seefeld Member”, Northern Calcareous Alps, Salzburg, Austria)—taxonomy and palaeoenvironmental implications. Austrian Journal of Earth Sciences 121: 125–165. Crossref
Jain, S.L. 1973. New specimens of Lower Jurassic holostean fishes from India. Palaeontology 16: 149–177.
Kiessling, W. 1999. Late Jurassic radiolarians from the Antarctic Peninsula. Micropaleongology 45: 1–96. Crossref
Kiessling, W. and Scasso, R.A. 1996. Ecological perspectives of Late Jurassic radiolarian faunas from the Antarctic Peninsula. In: A.C. Riccardi (ed.), Advances in Jurassic Research, 317–326. Transtech publications, Zûrich.
Kiessling, W., Scasso, R.A., Zeiss, A., Riccardi, A., and Medina, F. 1999. Facies and depositional processes in an Upper Jurassic to Lower Cretaceous pelagic sedimentary sequence. Geodiversitas 21: 687–713.
Kietzmann, D.A., Cuitiño, J.I., Medina, R.A., and Scasso, R.A. 2009. Análisis de las cadenas de Markov y series de Fourir en una secuencia hemipelágica del Jurásico Superior de la Península Antártica. Latin American Journal of Sedimentology and Basin Analysis 16: 45–56.
Kietzmann, D.A. and Scasso, R.A. 2020. Jurassic to Cretaceous (upper Kimmeridgian–?lower Berriasian) calcispheres from high palaeolatitudes on the Antarctic Peninsula: Local stratigraphic significance and correlations across Southern Gondwana margin and the Tethyan realm. Palaeogeography, Palaeoecology, Palaeoecology 537: 109–419. Crossref
Lambers, P.H. 1999. The actinopterygian fish fauna of the Late Kimmeridgian and Early Tithonian “Plattenkalke” near Solnhofen (Bavaria, Germany): state of the art. Geologie en Mijnbouw 78: 215–229. Crossref
Latimer, A. and Giles, S. 2018. A giant dapediid from the Late Triassic of Switzerland and insights into neopterygian phylogeny. Royal Society Open Science 5 (8) [available online, http://dx.doi.org/10.1098/rsos.180497]. Crossref
Leach, W.E. 1822. Dapedium politum. In: H.T. de la Beche (ed.), Remarks on the Geology of the South Coast of England, from Bridport Harbour, Dorset, to Babbacombe Bay, Devon. Transactions of the Geological Society of London (Series 2) 1: 45. Crossref
Lehman, J.P. 1966. Actinopterygii. In: J. Piveteau (ed.), Traité de Paléontologie IV (3), 1–242. Masson et Cie., Paris.
López-Arbarello, A. 2012. Phylogenetic interrelationships of ginglymodian fishes (Actinopterygii: Neopterygii). PLoS ONE 7: e39370. Crossref
López-Arbarello, A. and Sferco, M.E. 2018. Neopterygian phylogeny: the merger assay. Royal Society Open Science 5 (3) [available online, https://doi.org/10.1098/rsos.172337]. Crossref
Maxwell, E. and López-Arbarello, A. 2018. A new species of the deep-bodied actinopterygian Dapedium from the Middle Jurassic (Aalenian) of southwestern Germany. PeerJ: 10.7717/peerj.5033. Crossref
Macdonald, D.I.M, Barker, P.F., Garrett, S.W., Ineson, J.R., Pirrie, D., Storey, B.C., Whitham, A.G., Kinghorn, R.R.F., and Marshall, J.E.A. 1988. A preliminary assessment of the hydrocarbon potential of the Larsen Basin, Antarctica. Marine and Petroleum Geology 5: 34–53. Crossref
Medina, F.A. and Ramos, V.A. 1981. Geología de las inmediaciones del Refugio Ameghino Tierra de San Martín, Península Antártica. Actas VIII Congreso Geológico Argentino 2: 871–882.
Medina, F.A. and Ramos, V.A. 1983. Geología de las inmedicaciones del Refugio Ameghino (64o 26’S; 58o 59’W Tierra de San Martin, Península Antártica). Dirección Nacional Del Antártico, Instituto Antártico Argentino 229: 1–14.
Olsen, P.E. 1984. The skull and pectoral girdle of the parasemoinotid fish Watsonulus eugnathoides from the Early Triassic Sakamena Group of Madagascar, with comments on the relationships of the holostean fishes. Journal of Vertebrate Paleontology 4: 481–499. Crossref
Pirrie, D. and Crame, J.A. 1995. Late Jurassic palaeogeography and anaerobic-dysaerobic sedimentation in the northern Antarctic Peninsula region. Journal of the Geological Society, London 152: 469–480. Crossref
Plieninger, T. 1847. Abbildunge von Zahnen aus der oberer Grenzbreccie des Keupers bei Degerloch und Steinenbronn. Jahreshefte des Vereins für vaterläudische Naturkunde in Württemberg 3: 164–167.
Quenstedt, F.A. 1858. Der Jura. 842 pp. Laupp and Siebeck, Tübingen.
Regan, C.T. 1923. The skeleton of Lepidosteus, with remarks on the origin and evolution of the lower neopterygian fishes. Proceedings of the Zoological Society of London 1923: 445–461. Crossref
Röhl, H.-J. and Schmid-Röhl, A. 2005. Lower Toarcian (Upper Liassic) black shales of the central european epicontinental basin: a sequence stratigraphic case study from the SW German Posidonia shale. SEPM Special Publication 82: 165–189. Crossref
Scasso, R.A. 2001. High frequency of explosive volcanic eruptions in a Late Jurassic volcanic arc: the Ameghino Formation on the Antarctic Peninsula. Journal of Sedimentary Research 71: 101–106. Crossref
Schaeffer, B. 1967. Late Triassic fishes from the western United States. Bulletin of the American Museum of Natural History 135: 285–342.
Schultze, H.-P. 2008. Nomenclature and homologization of cranial bones in actinopterygians. In: G. Arratia H.-P. Schultze, and M.V.H. Wilson (eds.), Mesozoic Fishes 4: Homology and Phylogeny, 23–48. Verlag Dr. Friedrich Pfeil, Munchen.
Smithwick, F.M. 2015. Feeding ecology of the deep-bodied fish Dapedium (Actinopterygii, Neopterygii) from the Sinemurian of Dorset, England. Palaeontology 58: 293–311. Crossref
Szabó, M. and Pálfy, J. 2020. Dapedium sp. from the Toarcian (Lower Jurassic) Úrkút Manganese Ore Formation (Bakony Mts., Hungary) and an overview of diversity of dapediiform fishes. Palaeobiodiversity and Palaeoenvironments 100: 179–195. Crossref
Thies, D. 1988. Dapedium pholidotum (Agassiz, 1832)? (Pisces’ Actinopterygii) aus dem Unter-Toarcium NW-Deutschlands. Geologica et Paleontologica 22: 89–121.
Thies, D. and Hauff, R.B. 2008. A neotype for Dapedium caelatum Quenstedt, 1858 (Actinopterygii, Neopterygii, Semionotiformes) from the Early Jurassic (Early Toarcian) of South Germany. Geologica et Palaeontologica 42: 23–38.
Thies, D. and Hauff, R.B. 2011. A new species of Dapedium Leach, 1822 (Actinopterygii, Neopterygii, Semionotiformes) from the Early Jurassic of South Germany. Palaeodiversity 4: 185–221.
Thies, D. and Herzog, A. 1999. New information on †Dapedium Leach 1822 (Actinopterygii, †Semionotiformes). In: G. Arratia and H.-P. Schultze (eds.), Mesozoic Fishes 2: Systematics and Fossil Record, 143–152. Verlag Dr. Friedrich Pfeil, München.
Thies, D. and Waschkewitz, J. 2016. Redescription of Dapedium pholidotum (Agassiz, 1832) (Actinopterygii, Neopterygii) from the Lower Jurassic Posidonia Shale, with comments on the phylogenetic position of Dapedium Leach, 1822. Journal of Systematic Palaeontology 14: 339–364. Crossref
Tintori, A. 1983. Hypsisomatic Semionotidae (Pisces, Actinopterygii) from the Upper Triassic of Lombardy (N. Italy). Rivista Italiana di Paleontologia e Stratigrafia 88: 417–442.
Tintori, A. 1998. Fish biodiversity in the marine Norian (Late Triassic) of northern Italy: The first Neopterygian radiation. Italian Journal of Zoology 65: 193–198. Crossref
Tintori, A. and Lombardo, C. 2018. The Zorzino Limestone Actinopterygian Fauna from the Late Triassic (Norian) of the Southern Alps. In: L.H. Tanner (ed.), The Late Triassic World: Earth in a Time of Transition. Topics in Geobiology 46: 315–350. Crossref
Wagner A. 1863. Monographie der fossilen Fische aus den lithographischen Schiefern Bayern’s. Abhandlungen der königlich bayerischen Akademie der Wissenschaften II 9: 613–748.
Wenz, S. 1968. Complements a l’étude des poissons actinopterygiens du Jurassic Français. Cahiers du Paleontologie 6: 1–276, CNRS, Paris.
Westoll, T.S. 1943. The origin of the tetrapods. Biological Reviews 18: 78–98. Crossref
Whitham, A.G. 1993. Facies and depositional processes in an Upper Jurassic to Lower Cretaceous pelagic sedimentary sequence. Antarctica Sedimentology 40: 331–349. Crossref
Whitham, A.G. and Doyle, J.A. 1989. Stratigraphy of the Upper Jurassic–Lower Cretaceous Nordenskjöld Formation of Graham Land. Antarctica. Journal of South American Earth Sciences 2: 371–384. Crossref
Woodward, A.S. 1895. Catalogue of the Fossil Fishes in the British Museum (Natural History). Part III. 544 pp. London, Longmans & Co.
Woodward, A.S. 1929. The Upper Jurassic fish Heterostrophus. Proceedings of the Zoological Society of London 1929: 561–566. Crossref
Acta Palaeontol. Pol. 69 (3): 467–483, 2024
https://doi.org/10.4202/app.01158.2024