

New, large actinopterygian fishes from the upper Carboniferous of Nýřany, Czech Republic
PAVEL BARTÁK, MARTIN IVANOV, EVA TIHLAŘÍKOVÁ, MARTIN OLBERT, and VILÉM NEDĚLA
Barták, P., Ivanov, M., Tihlaříková, E., Olbert, M., and Neděla, V. 2024. New, large actinopterygian fishes from the upper Carboniferous of Nýřany, Czech Republic. Acta Palaeontologica Polonica 69 (3): 501–522.
The lacustrine coal deposits at Nýřany, Czech Republic, yielded a diversified vertebrate assemblage of the Middle Pennsylvanian (Moscovian) age, represented by the remains of early tetrapods, as well as numerous freshwater ichthyofauna, including xenacanthiform sharks, acanthodians, dipnoans, and ray-finned fishes. However, unlike some other upper Carboniferous localities, the actinopterygian diversity is limited to the three small-bodied species, most of them endemic to Nýřany locality and the equivalent strata elsewhere, indicating that the true taxonomic diversity of the group at the locality may be biased. Here we describe first skeletal remains of large actinopterygian fishes from the site, including a new genus and species, Stambergichthys macrodens gen. et sp. nov., which is represented by a well-preserved mandible with teeth. The micro-computed tomographic techniques revealed in the specimen a presence of a complex neurovascular system innerving the teeth and the jaw, and supplying both with blood vessels. The dentition consists of a single row of massive, homodont, conical teeth, which possess simplexodont plicidentine on their base, the characteristics supporting the predatory ecology of the new species. The isolated skeletal remains of large-bodied actinopterygians expand the knowledge on the diversity of the group in Nýřany, and their occurrence in coal deposits of relatively shallow lake indicates they represent allochthonous, poorly known aquatic vertebrate association, likely originating from the braided river system. These findings underline the importance of less complete skeletal materials occurring in well-known vertebrate assemblages of the upper Carboniferous coal-bearing localities.
Key words: Actinopterygii, dentition, continental basins, morphology, neurovascular system, palaeoenvironment, plicidentine, Palaeozoic.
Pavel Barták [bartak.pavel@mail.muni.cz; ORCID: https://orcid.org/0009-0009-3738-4849 ] and Martin Ivanov [mivanov@sci.muni.cz; ORCID: https://orcid.org/0000-0001-9108-9239 ], Department of Geological Sciences, Faculty of Science, Masaryk University, Kotlářská 267/2 611 37 Brno, Czech Republic.
Eva Tihlaříková [tihlarik@isibrno.cz; ORCID: https://orcid.org/0000-0002-7983-2971 ], Martin Olbert [olbert@isibrno.cz; ORCID: https://orcid.org/0000-0003-2280-3341 ], and Vilém Neděla [vilem@isibrno.cz; ORCID: https://orcid.org/ 0000-0001-6029-5435 ], Environmental Electron Microscopy Group, Institute of Scientific Instruments of the Czech Academy of Sciences, Královopolská 147, 612 00 Brno, Czech Republic.
Received 17 April 2024, accepted 25 July 2024, published online 30 September 2024.
Copyright © 2024 P. Barták et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The Nýřany locality is part of a coalfield situated southwest of Pilsen in the Czech Republic, which contains several upper Carboniferous (Middle Pennsylvanian) coal seams, developed within the grey-coloured sequence of fluviolacustrine sediments of the Pilsen Basin. Remnants of vertebrates come from several fossiliferous sapropelic horizons inside the Main Nýřany Coal Seam (Fritsch 1879; Milner 1980; Barták 2020) of the Nýřany Member (Kladno Formation), dated as the late Moscovian (Asturian) age (Opluštil et al. 2016). Their collection began in the second half of the 19th century (Fritsch 1870), with most important findings being derived from the Humboldt and Krimich mines in the vicinity of the Nýřany village. Over the past ca. 150 years, numerous vertebrate body fossils have been described from the site, representing one of the best known late Moscovian faunal assemblages worldwide. Much of the vertebrate skeletal remains are fairly complete, articulated specimens, indicating a rapid burial under the anoxic conditions with minimal effects of transport (Milner 1980). The renowned status of the Nýřany locality undoubtedly arises mainly from the highly diversified and especially well-preserved assemblage of early tetrapods, including baphetids (Beaumont 1977; Milner et al. 2009), embolomeres (Carroll 1970), various groups of temnospondyls and “lepospondyls” (for the recent overview, see Barták 2020), and the earliest amniotes (Carroll and Baird 1972; Reisz 1975). However, the ichthyofauna is also well-represented and relatively diverse, comprising acanthodians (Zajíc 1988), xenacanthiform sharks (Schneider and Zajíc 1994; Heidtke 1998), dipnoans (Fritsch 1888) and actinopterygian fishes (Štamberg 1978, 1991, 2013). In comparison to the other late Carboniferous localities such as Newsham (Northumberland, UK), Linton (Ohio, USA), Mazon Creek (Illinois, USA), Montceau-les-Mines (France), and Kounov (Czech Republic), the coal-bearing deposits of Nýřany have so far produced surprisingly few actinopterygian species. Although abundant in terms of specimen number, these are currently confined to only three small-bodied forms, the haplolepid Pyritocephalus sculptus and two species of the endemic genus Sceletophorus, i.e., S. biserialis and S. verrucosus, none of them exceeding the total length of 150 mm (Westoll 1944; Gardiner 1967; Štamberg 1978, 1991, 2013). These taxa have been perceived as inhabitants of small and shallow, poorly oxygenated freshwaters (Westoll 1944; Milner 1980), the environmental constraints of which would potentially prevented the settlement by a population of large-bodied actinopterygians known from elsewhere.
Here we describe the first skeletal remains of large actinopterygian fishes from the upper Carboniferous of Nýřany, consisting of isolated and fragmentary elements, of which a single lower jaw with well-preserved teeth is described as a new genus and species, Stambergichthys macrodens gen. et sp. nov. The micro-computed tomography of this specimen reveals the presence of a primitive internal organization of the tooth dentine, which is discussed in the context of recent findings of plicidentine in the Actinopterygii. Here described materials not only expand the knowledge on the diversity of the group at the locality, but also contribute significantly to the problematic of faunal associations and palaeoenvironment at the Nýřany locality, and expand the stratigraphic record of the large-bodied actinopterygians in the Central and West Bohemian basins of the Czech Republic.
Institutional abbreviations.—CGS, Czech Geological Survey, Prague, Czech Republic; NHMW, Naturhistorisches Museum Wien, Austria; NMP, National Museum, Prague, Czech Republic; ÚGV PAL, Palaeontological collection of the Department of Geological Sciences, Faculty of Science, Masaryk University, Brno, Czech Republic.
Nomenclatural acts.—This published work and the nomenclatural acts it contains have been registered in ZooBank: urn:lsid:zoobank.org:pub:24B92487-A962-474F-B9E2-D819D0BCB203
Geological setting
The Central and West Bohemian continental basins, extending in the western and central part of the Czech Republic, represent the late Carboniferous fluviolacustrine sedimentary infill deposited on the Neoproterozoic and early Palaeozoic basement, and subdivided into Pilsen, Manětín, Radnice, Žihle, Kladno-Rakovník and Mšeno-Roudnice basins, the lithostratigraphic subdivision of which follows the same general terminology (Fig. 1). In the Pilsen Basin, the oldest lithostratigraphic unit represents the Kladno Formation, subdivided into the Radnice and Nýřany members, between which the stratigraphic hiatus related to the Leonian Phase of the Variscan Orogeny, lasting about 3.6 Ma, has been documented (Opluštil et al. 2016; Martínek et al. 2017). The deposits of the Radnice Member are unconformably overlaid by the fluvial sedimentation of the Nýřany Member, consisting of basal conglomerates and dominated by the grey-coloured arcoses, mudstones and claystones, in the Pilsen Basin attaining a thickness of about 290 m (Pešek et al. 1998, 2001). In addition to these deposits, intercalated volcaniclastics and groups of coal seams occur across the Nýřany Member. The zircon samples obtained from the volcanogenic interlayers have been 206Pb/238U dated, providing the absolute age of the Nýřany Member of 308.3–305.9 Ma (Opluštil et al. 2016), corresponding to the late Moscovian (Asturian) to early Kasimovian (Cantabrian) stage, i.e., the boundary of the Middle/Upper Pennsylvanian. The numerous coal seams in the Nýřany Member, representing lacustrine deposits, are usually of relatively low thickness (up to 2.5 m), and subdivided into Touškov, Nýřany, Chotíkov and Nevřeň coal groups (Pešek 1994). The Nýřany group of coal seams consists of two, in some cases three, seams attaining a thickness of about 1 meter, of which the lower, i.e., the Main Nýřany Coal Seam, yielded abundant vertebrate body-fossils. These are derived from several sapropelic horizons at the base of the coal seam, and were historically documented in some coal mines (e.g., Humboldt, Krimich) in the Nýřany and Třemošná coalfields (Fritsch 1879). The sapropelic horizons developed within the Main Nýřany Coal Seam, which produced all vertebrate remains thus far documented at the Nýřany locality, including the material described herein, indicate anoxic conditions in stagnant waters of a relatively shallow freshwater lake (Milner 1980).
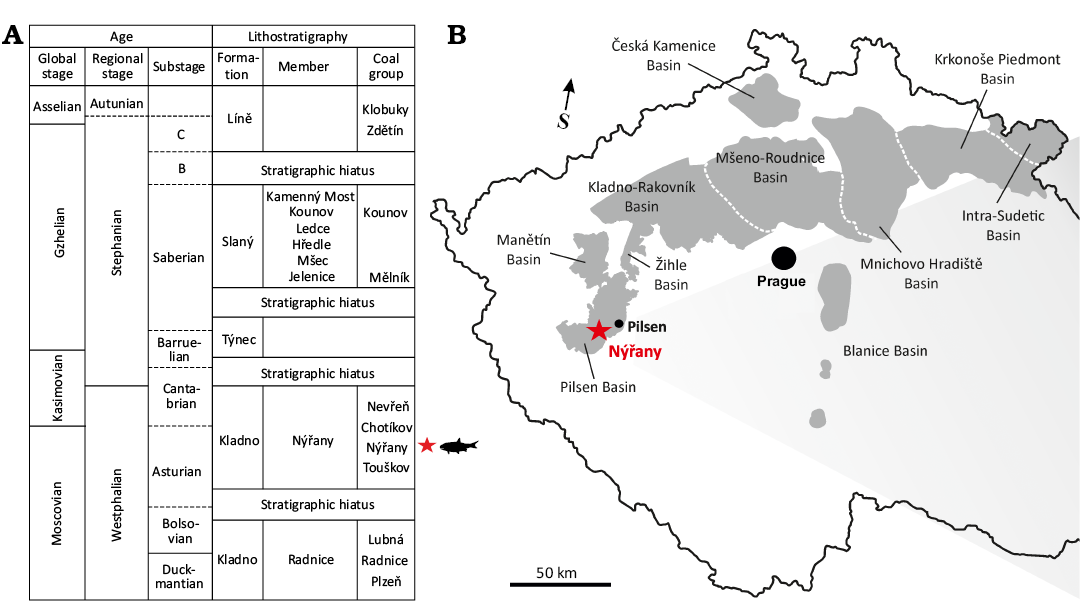
Fig. 1. A. The stratigraphic position of the actinopterygian material described in present study. The stratigraphic scheme is modified from Martínek et al. (2017). B. Permo-Carboniferous continental basins of the Czech Republic with marked location of Nýřany locality.
Material and methods
The holotype of Stambergichthys macrodens gen. et sp. nov. (ÚGV PAL00174) is housed in the Palaeontological collections of the Department of Geological Sciences, Masaryk University, Brno (Czech Republic), and consists of an isolated right mandible with teeth in medial view. The isolated and fragmentary elements of two other specimens from the palaeontological collections of the National Museum, Prague, Czech Republic, and the Naturhistorisches Museum Wien, Austria, are referred to indeterminate large actinopterygian fishes. These are represented by the following material: NMP M546, right maxilla with teeth from lateral view and NHMW-Geo-2023/0311/0001, left cleithrum from medial view. All specimens are preserved on a slab of dark coal shale coming from the upper Carboniferous deposits of Nýřany, Czech Republic. In most cases, the material consists of the mineralized bone tissue of a light brown colour, which represents a common type of preservation for the vertebrate body-fossils at the locality. The holotype of S. macrodens gen. et sp. nov. was scanned with the high-resolution, micro-computed tomography device, GE phoenix v|tome|x L240, at the Central European Institute of Technology (CEITEC) in Brno (Czech Republic). The following parameters were used in the course of the specimen scanning: the accelerating voltage of 140 kV, the current of 220 µA, the voxel size of 27 µm, 0.2 mm copper beam filter. The resulting µCT slices were manually segmented in a software ITK-SNAP v. 3.8.0 (Yushkevich et al. 2006) in order to obtain the 3D surface model. The surface morphology of the teeth was imaged using laser scanning confocal microscope Keyence VK-X 1100 with the magnification of 5× and the field of view of 2730 µm; full-ring light mode and laser confocal mode were used. For imaging of micro-morphological details of uncoated tooth samples with a large field of view and depth of field, the Advanced environmental scanning electron microscopy (A-ESEM) was used (Neděla et al. 2020; Stelate et al. 2021). All A-ESEM experiments were performed at the Institute of Scientific Instruments of the Czech Academy of Sciences using modified microscope QUANTA 650FEG (Thermo Fisher Scientific). The observation conditions were as follows: water vapour pressure of 300 Pa, beam accelerating voltage of 20 kV, a probe current of 80 pA, and a working distance of 12 mm. Micrographs were recorded using wide field aperture detector WFAD for A-ESEM (Bačovský et al. 2022). Macrographic/large field images were made by composing micrographs using Maps software (Thermo Fisher Scientific). The additional morphological details of the specimen were imaged with Leica DMC5400 (20 mpx) digital camera connected to the Leica MZ-16 stereomicroscope.
Systematic palaeontology
Gnathostomata Gegenbaur, 1874
Osteichthyes Huxley, 1880
Actinopterygii Cope, 1887 sensu Goodrich, 1930
Genus Stambergichthys nov.
ZooBankLSID: urn:lsid:zoobank.org:act:07AE2773-07C0-45AA-BE3B- 44FFB4C2DE0A
Etymology: In honour of Stanislav Štamberg (Museum of Eastern Bohemia, Hradec Králové, Czech Republic) for his long-term research on the Permo-Carboniferous actinopterygians from the European basins. Gender is masculine.
Type species: Stambergichthys macrodens gen. et sp. nov., by monotypy; see below.
Diagnosis.—As for type and only known species.
Stratigraphic and geographic range.—Moscovian (Middle Pennsylvanian) of Nýřany, near Pilsen, Czech Republic.
Stambergichthys macrodens sp. nov.
Figs. 2–5, 6A.
ZooBankLSID: urn:lsid:zoobank.org:act:9068DCBE-3FC8-4507-A250- 85EFEC9E7591
Etymology: From Greek μακρóς (makrós), meaning “long” or “large”, and Latin dens, i.e., “tooth”; in reference to the conspicuous teeth of the holotype.
Holotype: ÚGV PAL00174, an isolated right mandible with teeth exposed in medial view (Fig. 2A1).
Type locality: Nýřany, coal mine 13 km southwest of Pilsen, Czech Republic.
Type horizon: Main Nýřany Coal Seam, Nýřany Member, Kladno Formation, Pilsen Basin. A volcanic ash bed located ca. 85 m above the base of the Nýřany Member has been 206Pb/238U dated to 307.05 Ma ± 0.16 Ma, corresponding to the latest Moscovian stage (Asturian substage) of the Middle Pennsylvanian (Opluštil et al. 2016).
Diagnosis.—A large actinopterygian fish (estimated total length 600–700 mm) distinguished from all other early actinopterygians by the following unique combination of characters: well-developed posterodorsal process of mandible; external dermal sculpture consists of anastomosing ridges and grooves; single row of large, smooth, homodont, cone-like teeth with bulbous bases; monocuspid marginal tooth apices lateromedially compressed and distinctly curved medially; subtle mesial cutting edge without serration restricted to apical portion of teeth.
Stambergichthys macrodens gen. et sp. nov. differs from Acrolepis sedgwicki Agassiz, 1833, in more densely arranged teeth larger in size, and from Acropholis stensioei Aldinger, 1937, and Plegmolepis kochi Aldinger, 1937, by larger and more robust teeth fewer in number. Stambergichthys macrodens gen. et sp. nov. differs from Acrolepis gigas (Frič, 1877) by more widely rounded posteroventral part of the mandible, deeper middle portion of the mandible, angular not extending far anteriorly, and by the presence of anastomosing ridges and grooves on the dentary external surface. Stambergichthys macrodens gen. et sp. nov. differs from Brazilichthys macrognathus Cox & Hutchinson, 1991, Progyrolepis speciosus (Frič, 1875), Progyrolepis heyleri Poplin, 1999, Usclasichthys macrodens Heyler, 1977, Zaborichthys fragmentalis Štamberg, 1989, and NMMNH P-77557 (the unnamed large actinopterygian from Tinajas Member, New Mexico; Harris and Lucas 2017) by homodont dentition arranged in a single row close together, with slightly bulbous bases and medially recurved, flattened tooth tips. It further differs from Progyrolepis speciosus, Progyrolepis heyleri, and U. macrodens by smooth surface of marginal teeth, and from Z. fragmentalis by well-developed posterodorsal process of mandible. Stambergichthys macrodens gen. et sp. nov. differs from Brachydegma caelatum Dunkle, 1939, by more closely spaced teeth with bulbous bases, which extend far posteriorly to the base of the posterodorsal process.
Description.—Mandible: The holotype of Stambergichthys macrodens gen. et sp. nov. consists of the isolated right mandible with teeth, exposed in the rock matrix in medial view (Fig. 2A1). The preserved portion of the mandibular ramus measures 52 mm and it is incomplete anteriorly. Most of its posterior part is present in the form of mineralized bone tissue, although the small portion corresponding to the posterodorsal process is preserved as an imprint of the lateral surface (Fig. 2A1). The mandibular ramus is disrupted by the numerous cracks, some of which are obliquely directed and resulted in both the dorsoventral and mediolateral displacement within the jaw fragment. Similarly, the bone medial surface of the mandible is damaged in the anterior region and along the ventral margin of most of the teeth, resulting in the exposure of the internal structures of the dentary (Fig. 2A1, A3). The general form of the mandible is boomerang-like, with the posteroventral border of the jaw smoothly curved dorsally to form a distinct posterodorsal process of quadrangular shape, which is elevated above the marginal dentition of the mandible. The preserved portion of the mandible is markedly deep in the central part, and gradually tapers in both the anterior and posterior directions.
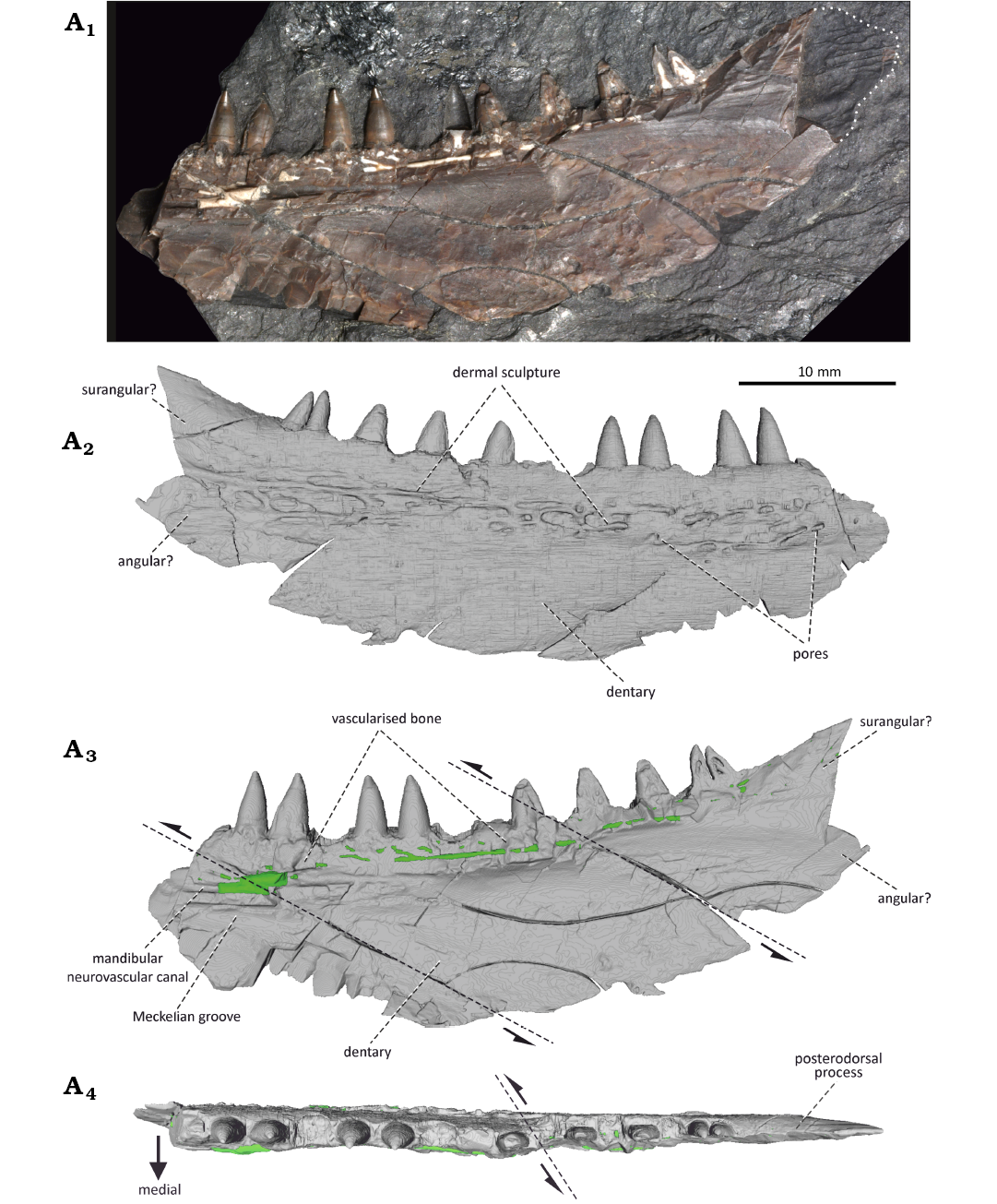
Fig. 2. The actinopterygian fish Stambergichthys macrodens gen. et sp. nov. from Moscovian, Middle Pennsylvanian, Nýřany Member, Kladno Formation, Nýřany, Czech Republic. The right mandible of the holotype, ÚGV PAL00174, as preserved in medial view (A1). µCT surface model of the mandible in lateral (A2), medial (A3), and dorsal (A4) views. The green colour in A3 and A4 represents the sedimentary infill of neurovascular mandibular canal. Dashed lines with arrows indicate fractures where displacement has occurred.
The majority of the lower jaw is formed by the dentary. It forms the entire anterior region of the jaw laterally and extends posteriorly slightly behind the level of the marginal teeth, where it contacts the angular and surangular, although the sutures cannot be clearly observed (Figs. 2A1–A3, 6A). The lateral surface of the dentary, imaged through the µCT, reveals the presence of a sculpture consisting of a system of elongate, anastomosing ridges and grooves (Fig. 2A2). The ornamentation extends along the entire length of the dentary and is confined to its central part, being absent in the ventral, as well as dorsal regions. It is currently unclear, to what degree this feature is affected by the taphonomy and the µCT processing, or whether the sculpture distribution represents a natural condition. The medial surface of the dentary is deeply excavated and smooth for most of its extent (Fig. 2A1, A3). It has a prominent medial wall just ventral to the tooth row, of which surface is damaged and exposes the internal structures of the dentary, including the vascularised bone tissue (bone of attachment), as well as a set of thin canals partly filled with a white aluminosilicate secondary mineralization. These canals are interpreted here as a part of the mandibular neurovascular system innerving the teeth and the mandible (see below). The narrow Meckelian groove is partly preserved in the anteroventral portion of the dentary, extending more posteriorly to form a large Meckelian fossa bordered dorsally by a prominent medial wall of the bone. The angular forms the caudal border of the mandibular posterodorsal process, and constricts the lateral extent of the dentary, although the exact boundary between the two bones is unclear (Figs. 2A2, A3, 6A). The lateral extent of the angular on the posterodorsal process of the mandible is apparent from the imprint of its lateral dermal sculpture, consisting of thin and relatively densely arranged ridges (Figs. 2A1). The angular is confined to the posterior border of the process and is extended anteriorly in the dorsal region, while ventrally it has a subquadrangular shape (Figs. 2A2, 6A). The smooth lateral surface anterior to the angular in posterodorsal part of the bone probably corresponds to the surangular, and represents the area of overlap with the posterior region of the maxillary postorbital plate (Figs. 2A1, A2, 6A).
Dentition: The marginal dentition is implanted in shallow depressions of the acrodont form and consists of a single row of large, conical teeth (Fig. 2A4). There is no evidence on the presence of a set of smaller lateral teeth common in many other early actinopterygians (Poplin and Heyler 1993). In addition to the nine more or less complete marginal teeth, seven more tooth positions are present in the preserved portion of the mandibular ramus, making the total count no less than sixteen teeth in the lower jaw of S. macrodens gen. et sp. nov. The teeth are homodont and set close together; the distance between the two adjacent tooth positions is only 0.8 mm. The largest teeth are 3.5 mm high and 2 mm long on the base. The dimensions of the marginal dentition are relatively constant throughout the length of the jaw, and only minor size decreasing is perceptible in the posterior region of the tooth row. The proportions of the teeth are relatively large in comparison to the dorsoventral depth of the jaw ramus, which reaches about 2.5 to 3.5 times of their apico-basal height. The general form of the teeth is cone-like and resembles the large laniary teeth of other Palaeozoic predatory actinopterygians (Fig. 3A1–A13; Poplin 1999; Štamberg 2018, 2020). The teeth are set perpendicular to the mandibular ramus and are upright along their entire height. The base is suboval in the cross section and slightly bulbous both mediolaterally and mesiodistally. A subtle mesial cutting edge with no serration is confined to the medially curved upper-most part of the tooth crown as a result of the lateral tooth compression (Fig. 3A3–A5, A12, A13). The surface of the teeth is smooth throughout their height and shows no presence of ridges or microsculpture in a form of tubercles and protuberances seen in some other actinopterygian forms (Richter 1983; Poplin 1999; Štamberg 2018, 2020). The smooth enameloid layer is present in the specimen and occupies about 40% of the tooth height in the apical part (Fig. 3A1, A2, A6–A11). Its bright surface forms a sharp transition between it and the collar enamel of the tooth shaft, and can be seen as a very thin, superficial layer in the µCT transversal and axial slices of the teeth (Figs. 3A15, 4A6; Germain et al. 2016). The internal structure of the marginal dentition, uncovered by the µCT, shows the presence of a large pulp cavity surrounded by a moderately thick layer of the dentine (Figs. 3A15, 4A1–A5). In the cross section, this dentine layer displays the presence of a simple form of plicidentine, which is restricted to the very base of the teeth and cannot be observed in any form (e.g., grooves and ridges) on the external surface of the implanted teeth. The individual dentine folds are not ramified and do not extend to the centre of the large pulp cavity of the teeth. This simple form of the dentine infolding corresponds well to the simplexodont type of plicidentine reported recently in some actinopterygian and sarcopterygian fishes (Meunier et al. 2015a, b).
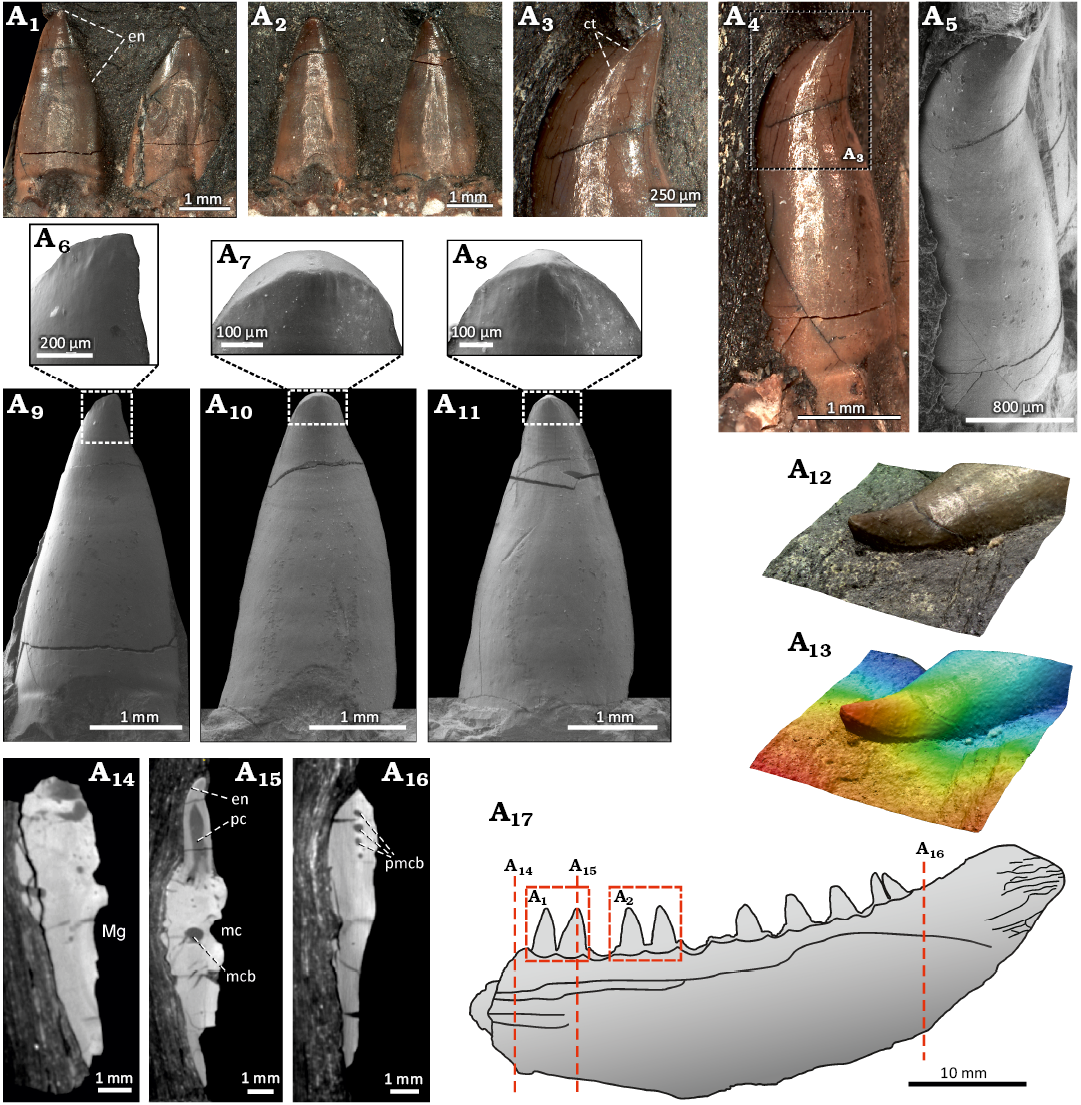
Fig. 3. The actinopterygian fish Stambergichthys macrodens gen. et sp. nov. from Moscovian, Middle Pennsylvanian, Nýřany Member, Kladno Formation, Nýřany, Czech Republic. Marginal dentition and transversal sections of the right mandible of the holotype, ÚGV PAL00174. Photographs of the marginal teeth in medial view (A1, A2). Anterior-most preserved tooth showing apically restricted cutting edge in mesial view (A3, A4, A5). Close up images from A-ESEM showing apical portion of the marginal teeth in medial view (A6, A7, A8). Morphology of the well-preserved anterior marginal teeth from A-ESEM in medial view (A9, A10, A11). 3D model of the third anterior-most preserved tooth displayed through the confocal microscopy showing mesial cutting edge (A12, A13). Transversal sections through the mandibular ramus (A14, A15, A16). Interpretative drawing of the mandible showing the course of slices and details of the teeth depicted in other figures (A17). Abbreviations: ct, cutting edge; en, enameloid; mc, mandibular neurovascular canal; mcb, mandibular neurovascular canal branch; Mg, Meckelian groove; pc, pulp cavity; pmcb, posterior mandibular neurovascular canal branches. Scale bars for A12 and A13 not available.
Neurovascular system: The X-ray µCT imaging techniques revealed inside the mandibular ramus of S. macrodens gen. et sp. nov. the presence of a slender bony canal, located immediately below the marginal dentition, which is interpreted here as a passage for the mandibular branch of the trigeminal nerve (V, ramus mandibularis trigemini; Figs. 2A1, A3, 3A14–A16, 5). The continuity of the mandibular canal has been disrupted by the numerous fractures and short displacements that occur in the dentary of the specimen. The descending branch of the mandibular nerve, coming from the trigeminal ganglion, probably entered the mandibular ramus of S. macrodens gen. et sp. nov. in its posterior region, as reported in some extant lower actinopterygians (Allis 1922; Piotrowski and Northcutt 1996). The posterior-most portion of the preserved part of the mandibular canal splits into four or five subparallel branches which more anteriorly, at the level of the posterior-most teeth, pass into the lateral ramus of the mandibular canal. The lateral ramus alters in morphology from nodular to rod-like along its course, and is accompanied by the subparallel medial ramus in the posterior part of the mandible, which retains the form of a simple bar throughout its length. Both rami merge at the level of the tenth posterior-most tooth position to form a single main ramus that passes into the more anterior portion of the mandible. Its form is well exposed in the longitudinal section at the medial surface of the dentary due to the breakage of the bone (Figs. 2A1, A3, 5A4). Both rami of the mandibular canal posteriorly, as well as a single main ramus anteriorly, split into several branches towards the dentary teeth to form a dental plexus, through which the nerves and blood vessels passed, supplying the pulp cavities. In the anterior portion of the jaw fragment, a robust branch of the mandibular neurovascular canal extends posterolaterally from its main trunk and terminates in several smaller branches leading to the pores on the lateral surface of the dentary which might have innerved the external surface of the mandible. No mandibular sensory canal of the lateral line system (Leuzinger et al. 2020) could be clearly observed in the dentary of S. macrodens gen. et sp. nov., although a few narrow, elongate, parallel canaliculi present in the more ventral position of the jaw ramus might be related to the cephalic sensory system.
Remarks.—The fragmentary nature of the type specimen of S. macrodens gen. et sp. nov. merits detailed account of its actinopterygian characteristics before the taxonomic assignment at the specific level and comparison to other taxa can be addressed. The thin enameloid layer confined to the apical portion of the teeth represents the only actinopterygian synapomorphy present in the holotype of S. macrodens gen. et sp. nov. (Ørvig 1978b; Patterson 1982). Although a number of other characteristics of the jaw fragment have a wider distribution among the early osteichthyians, all of these features occur to varying degrees in early actinopterygians, further supporting its attribution to this group. These include (i) simplexodont plicidentine at the tooth base, (ii) mandibular ramus dorsoventrally deep in posterior part, (iii) smoothly curved posteroventral margin of mandible, (iv) prominent posterodorsal process of quadrangular shape, (v) laterally flattened mandibular ramus, (vi) external dermal sculpture formed by elongated ridges and grooves.
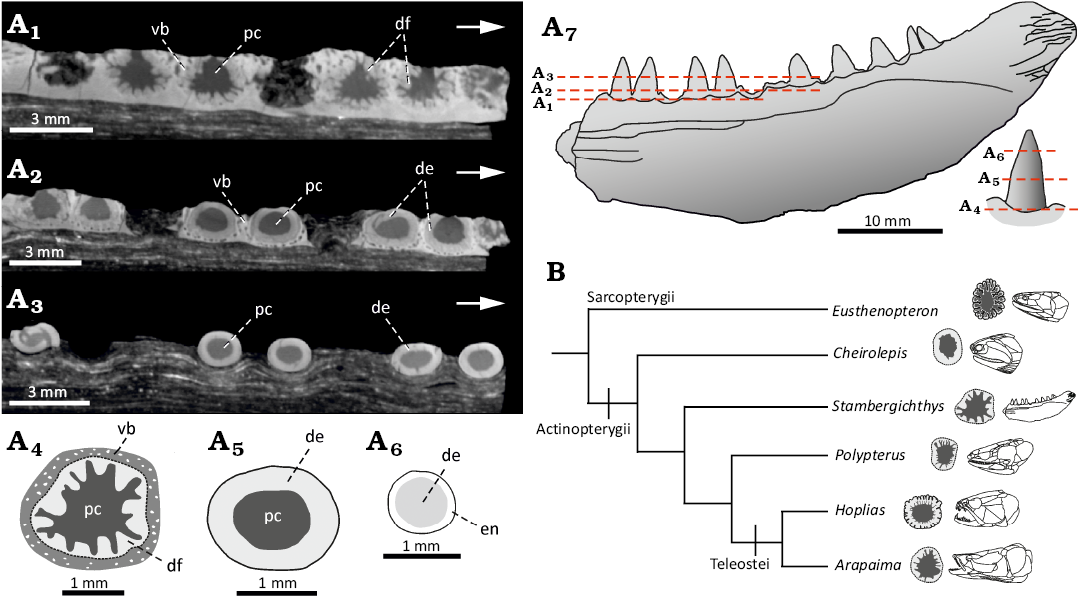
Fig. 4. The actinopterygian fish Stambergichthys macrodens gen. et sp. nov. from Moscovian, Middle Pennsylvanian, Nýřany Member, Kladno Formation, Nýřany, Czech Republic. Cross section through marginal dentition of the holotype, ÚGV PAL00174, showing the morphology and distribution of plicidentine. A. µCT axial slices at different levels of marginal teeth (A1, A2, A3). Interpretative drawings of cross sections at the base (A4), in the middle (A5) and close to apical tip (A6) of a single tooth. Interpretative drawing of the specimen showing the course of slices in other figures (A7). B. Phylogenetic diagram showing distribution and morphology of plicidentine in marginal teeth of selected extinct and extant actinopterygian fishes. Abbreviations: de, dentine; df, dentine fold; en, enameloid; pc, pulp cavity; vb, vascularised bone (bone of attachment).
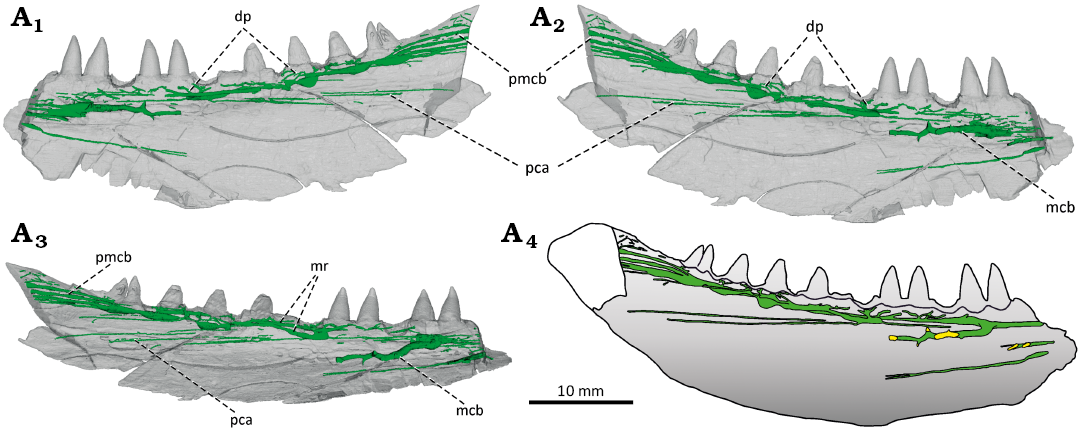
Fig. 5. The actinopterygian fish Stambergichthys macrodens gen. et sp. nov. from Moscovian, Middle Pennsylvanian, Nýřany Member, Kladno Formation, Nýřany, Czech Republic. Neurovascular system in the right mandible of the holotype, ÚGV PAL00174, displayed through the µCT. The mandible in medial (A1), lateral (A2), and dorsolateral (A3) views. Reconstruction of the mandibular neurovascular system in lateral view (A4). Green colour represents the canals of mandibular neurovascular system, yellow colour marks the position of lateral pores. Abbreviations: dp, dental plexus; mcb, mandibular neurovascular canal branch; mr, mandibular canal ramus; pca, parallel canaliculi; pmcb, posterior mandibular neurovascular canal branches.
A thin enameloid layer is clearly present in the dentition of S. macrodens gen. et sp. nov. (Figs. 3A1, A2, A15, 4A6) and, unlike chondrichthyans where the enameloid covers most of the tooth shaft (e.g., Jambura et al. 2019), it is confined to the apical portion of the teeth, forming the characteristic acrodin caps of actinopterygians. Furthermore, the simplexodont form of plicidentine is present at the base of the marginal teeth, which is now known to be widely distributed among the various actinopterygian forms (see Viviani et al. 2022 for the recent summary), although not necessarily confined to them (Meunier et al. 2015b). The marginal teeth arranged in two series have been interpreted to represent a plesiomorphic condition for the actinopterygians, with the large, cone-like teeth placed medially in respect to the diminutive lateral dentition (Poplin and Heyler 1993; Lund and Poplin 1997). Although this characteristic indeed frequently occurs in the Palaeozoic forms, the presence of a single marginal tooth row present in S. macrodens gen. et sp. nov. does not preclude its attribution to Actinopterygii, as it is currently known from many other early actinopterygian genera (e.g., Dunkle 1939; Lund 2000; Poplin and Lund 2000; Hamel 2005; Štamberg 2016; Stack et al. 2021; Argyriou et al. 2022), and is considered to represent an apomorphic state of this group (Lund and Poplin 1997). The mandible of S. macrodens gen. et sp. nov. is deepest in its posterior part in front of the posterodorsal process, and it gradually narrows both anteriorly and posterodorsally along the smoothly curved ventral margin of the bone (Figs. 2, 6A). The same general form of the lower jaw can be seen in many Palaeozoic actinopterygians, and the resemblance is especially evident in taxa such as Paratarrasius hibbardi Lund & Melton, 1982, “Elonichthys” hypsilepis Hay, 1900, Progyrolepis heyleri, Eurynotus crenatus Agassiz, 1835, and Brachydegma caelatum, in which the pronounced posterodorsal process is well exposed (Lund and Melton 1982; Schultze and Bardack 1987; Poplin 1999; Štamberg 2018; Friedman et al. 2019; Argyriou et al. 2022). Moreover, the mandible is markedly flattened mediolaterally, as present in early osteichthyians (Botella et al. 2007; Zhu et al. 2009), as well as sarcopterygian (e.g., Jeffery 2002; Porro et al. 2015a) and actinopterygian fishes (e.g., Arratia and Cloutier 1996; Giles et al. 2015; Figueroa et al. 2019), but absent in early tetrapods (Ahlberg and Clack 1998; Porro et al. 2015b). In early actinopterygians, the form of dermal sculpture on the external surface of the jaws and skull roof varies most commonly from the granular to ridged, both capped with a ganoine layer (Ørvig 1978a, b). The ridges may be relatively coarse and pronounced (e.g., Lund 2000; Mickle et al. 2009), or rather fine and vermicular, as in Progyrolepis (Štamberg 1991, 2018; Poplin 1999). Although the presence of the ganoine cannot be readily confirmed in the mandible of S. macrodens gen. et sp. nov., the dermal sculpture on the dentary conforms to the first pattern, whereas the second condition is present on the external surface of the angular (Fig. 2A1). All the characteristics discussed above therefore indicate that the holotype of S. macrodens gen. et sp. nov. represents an actinopterygian fish.
The mandible of S. macrodens gen. et sp. nov. is in general form similar to that of other large-bodied predatory genera like Brazilichthys macrognathus (Cox and Hutchinson 1991; Figueroa et al. 2019; Figueroa and Andrews 2023), Brachydegma caelatum (Dunkle 1939; Argyriou et al. 2022), Rastrolepis riojaensis López-Arbarello et al., 2006, and acrolepids (Aldinger 1937; Gardiner 1963; Štamberg 1991, 2018; Poplin 1999; López-Arbarello et al. 2010), having elongated mandibular ramus tapering anteriorly, and the pronounced posterior articulating region elevated above the marginal tooth row. The well-preserved mandibles from the lower Permian of France and Brazil reveal that the prominent posterodorsal region of the lower jaw in Progyrolepis heyleri and Brazilichthys macrognathus consists of both angular and surangular, whereas the dentary is posteriorly rounded and is not involved in its construction (Štamberg 2018; Figueroa and Andrews 2023). Although the sutures are difficult to be traced in S. macrodens gen. et sp. nov., the congruent arrangement of these elements can also be assumed for this region (Fig. 6A).
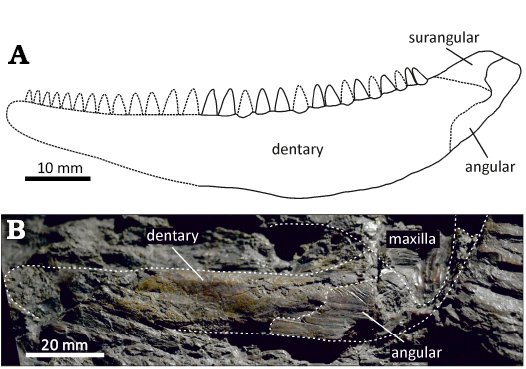
Fig. 6. The comparison of the mandible of actinopterygian fishes (A) Stambergichthys macrodens gen. et sp. nov. (Moscovian, Middle Pennsylvanian, Nýřany Member, Kladno Formation, Nýřany, Czech Republic) and (B) Acrolepis gigas (Frič, 1877) (Gzhelian, Upper Pennsylvanian, Mšec Member, Slaný Formation, Žilov, Czech Republic). A. ÚGV PAL00174, holotype, the reconstruction of the right mandible (reversed) in lateral view. B. NMP M125, lectotype, the photography of the left mandible in lateral view.
The preserved portion of the jaw fragment of S. macrodens gen. et sp. nov. measures 52 mm. However, the mandible is clearly incomplete anteriorly, and we assume that about one-quarter to one-third of the lower jaw might be missing (Fig. 6A). If this estimation is correct, then the complete mandible of the specimen could have reached about 70–80 mm in length. Based on the similarly sized lower jaws, Štamberg (1991) and Poplin (1999) estimated the total body length of Progyrolepis at about 600–700 mm. Although no information on body proportions, such as the ratio of skull length to total body length, are available in S. macrodens gen. et sp. nov., we assume the estimated length of the specimen could be roughly similar to that of Progyrolepis speciosus and Progyrolepis heyleri. In the Permo-Carboniferous continental basins of the Czech Republic, several actinopterygian species reaching a size comparable to S. macrodens gen. et sp. nov. are known, including Acrolepis gigas, Progyrolepis speciosus, and Zaborichthys fragmentalis, the material all of which was first hand re-examined for comparison purposes. The lectotype of Acrolepis gigas is an almost complete specimen preserved as part and counterpart in a large siderite concretion, which includes, among others, the skull and mandible, and measures 1250 mm in total length (Štamberg 1991). The mandible of A. gigas shows the general plesiomorphic morphology shared with similar actinopterygians of reasonable size, but is much larger compared to that of S. macrodens gen. et sp. nov., reaching a total length of at least 120 mm (Fig. 6B; Štamberg 2006). The mandibular ramus is very slender and straight anterior to the posterodorsal process in this species, whereas in S. macrodens gen. et sp. nov. it is deep and has broadly rounded dorsal and ventral margins. Unlike the massive, far anteriorly projecting angular of A. gigas, the bone appears to be relatively narrow and restricted to the posterior border of the posterodorsal process in S. macrodens gen. et sp. nov., with no evidence on its anterior extension in a manner seen in the former. Moreover, the external dermal sculpture is only apparent on the angular of A. gigas, where it is formed by the elongate parallel ridges, whereas the dentary seems to lack the well-developed anastomosing ridges and grooves present in S. macrodens gen. et sp. nov., although this may partly result from its poor preservation. Fritsch’s (1895: pl. 130) illustration of the specimen shows details of the lower jaw, as well as dentition, which resembles in general morphology to S. macrodens gen. et sp. nov. An inspection of the specimen by one of the authors (PB) showed that the structures formerly interpreted as teeth are formed by the coarse depressions of irregular shape and distribution near the dorsal margin of the mandible, and these are only present on the counterpart slab. We consider these structures to be of inorganic origin as irregularities on the surface of the matrix, and agree with Štamberg (1991) that teeth are not preserved. In Progyrolepis speciosus, the morphology of the jaw and especially the form and arrangement of the dentition are very distinct from S. macrodens gen. et sp. nov., consisting about 11–12 well spaced, large laniaries with abruptly tapering apical parts and no medial curvature, accompanied by the series of much smaller and more numerous lateral teeth (Štamberg 1991, 2020). The type specimen of Zaborichthys fragmentalis is represented by the scattered skeletal remains of a moderately large individual, the mandible of which would have probably not exceeded a total length of 50 mm (Štamberg 1991). It differs from S. macrodens gen. et sp. nov. in morphology of the posterior part of the mandible, which is much more robust and lacks a well-developed posterodorsal process. Štamberg (1991, 2013) referred to Z. fragmentalis an isolated right maxilla with teeth (CGS YA1355; erroneously designated as lower jaw). The well-preserved marginal dentition of this specimen consists of two types of teeth arranged in two rows, thus differing from a single row of stout and homodont teeth of S. macrodens gen. et sp. nov.
The exceptionally well-preserved marginal dentition of the newly described species enables a detailed comparison of its morphology and arrangement with other early actinopterygians possessing a single row of teeth in the jaws. The homodont conical teeth arranged in a row close to each other and having bulbous bases, present in S. macrodens gen. et sp. nov., exhibit a resemblance with the dentition of Acrolepis sedgwicki, as well as some other acrolepid specimens described by Schaumberg (1996) from the upper Permian marine deposits of Germany (Kupferschiefer) and England (Marl-Slate). Since the mandibular dentition has not yet been described in the literature for Acrolepis sedgwicki, the comparison with this species is limited here to the upper jaw dentition, despite minor differences between the teeth of the maxilla and mandible might have existed. The reported marginal teeth of Acrolepis sedgwicki have conical to bulbous shape and are arranged in a row close to each other, similar to S. macrodens gen. et sp. nov. However, the teeth present in Acrolepis sedgwicki appear to be smaller relative to the depth of the jaw and have different proportions. The tooth height/longitudinal diameter ratio in the largest teeth of Acrolepis sedgwicki reaches 2.2–2.3 (based on Schaumberg 1996) while in S. macrodens gen. et sp. nov. the same parameter ranges from 1.6 to 1.8. Similarly, the ratio corresponding to the distance between adjacent tooth positions/tooth longitudinal diameter varies from 0.6 to 1.3 in Acrolepis sedgwicki, indicating that the tooth spacing is greater relative to S. macrodens gen. et sp. nov., in which the ratio is only 0.2–0.3. The indeterminate acrolepid mandibles described by Schaumberg (1996: figs. 3, 8) can also be readily distinguished from S. macrodens gen. et sp. nov. based on their diminutive dentition relative to the mandibular depth and the higher tooth number in the lower jaw. The knowledge on the morphology and arrangement of marginal teeth in other acrolepids is limited. López-Arbarello et al. (2010) demonstrated that family Acrolepidae as established by Aldinger (1937) is doubtful, and restricted the content of the group to only a few genera, among which a considerable dental variability appears to be present. For instance, the dentition of Acropholis stensioei and Plegmolepis kochi is very small relative to the depth of the jaw (Aldinger 1937), contrasting with the large marginal teeth of S. macrodens gen. et sp. nov., although details of the dentition cannot be compared until a redescription of these taxa is made. On the other hand, Challaia elongata (Cabrera, 1944), Watsonichthys pectinatus (Traquair, 1877), Progyrolepis speciosus, and Progyrolepis heyleri display very large conical laniaries accompanied by teeth of much smaller size (Gardiner 1963; Štamberg 1991, 2018; López-Arbarello et al. 2006, 2010), which are clearly distinct from the homodont dentition of S. macrodens gen. et sp. nov. Apart from the acrolepids, the dentition of S. macrodens gen. et sp. nov. resembles that of Brachydegma caelatum, in which the large conical teeth of equal size and shape show medially recurved apical tips (Dunkle 1939; Argyriou et al. 2022). However, the teeth of the latter are much higher relative to the mandibular depth and are more widely spaced. Indeed, the tooth height/mandibular height ratio at the jaw mid-length corresponds to 0.6 in Brachydegma caelatum, whereas in S. macrodens gen. et sp. nov. it reaches only about 0.3. Similarly, the ratio of the distance between adjacent tooth positions and the tooth longitudinal diameter on the base is much higher (0.6) in Brachydegma caelatum compared to S. macrodens gen. et sp. nov. (0.2–0.3). Moreover, the dentition of Brachydegma caelatum is confined to anterior two-thirds of the lower jaw (Dunkle 1939) whereas in S. macrodens gen. et sp. nov. the tooth row extends far posteriorly to the base of the posterodorsal process, and the teeth in the former lack a bulbous base. Although much smaller in body length, the dentition similar to S. macrodens gen. et sp. nov. can also be found in Igornichthys bohemicus Štamberg, 2016, from the lower Permian of the Czech Republic, in which a single row of straight, homodont, conical teeth with smooth shaft is present (Štamberg 2016, 2020). However, the well-preserved teeth of this species are markedly pointed apically and show neither a medial curvature nor a mesial cutting edge resulting from the compression of the tooth tip, which are present in S. macrodens gen. et sp. nov. Another early actinopterygian fish with a single tooth row, Wendyichthys dicksoni Lund & Poplin, 1997, from the lower Carboniferous of Montana, shows a peculiar anterior inclination of the tooth shafts along the dentary length (Lund and Poplin 1997), thus markedly differ from the upright dentition of S. macrodens gen. et sp. nov. Therefore, based on the comparisons presented above, it can be concluded that none of the known Permo-Carboniferous actinopterygian species match the unique tooth morphology described in S. macrodens gen. et sp. nov.
Stratigraphic and geographic range.—Type horizon and locality only.
Actinopterygii indet.
Fig. 7.
Material.—NMP M546, isolated right maxilla with teeth in lateral view and a possible infraorbital bone; NHMW-Geo-2023/0311/0001, isolated left cleithrum in medial view, both from the Middle Pennsylvanian (Moscovian) of the Nýřany locality, Czech Republic.
Description.—NMP M546 represents an isolated right maxilla exposed in lateral view with an associated fragment of a possible infraorbital bone (Fig. 7A). The preserved portion of the maxilla is fairly large and measures about 61 mm. The elongated suborbital ramus is 40 mm long and it appears to be broken transversally in the middle part, resulting in a slight displacement of its anterior portion ventrally relative to the bone posterior part. The anterior portion of the suborbital ramus is slightly curved and terminates in a rounded tip with lateral rugosity. The postorbital plate of the maxilla is well developed and consists of a high and rounded dorsal part, preserved as an imprint of the bone medial surface, and a ventral projection extending below the toothed ventral border. The latter structure appears to be incomplete, based on the sharply bevelled posteroventral margin of the bone. The postorbital plate is elongated dorsoventrally, short anteroposteriorly, and forms nearly right angle with both the dorsal and ventral borders of the suborbital ramus. The length/height ratio of its preserved part is equal to 0.9. The lateral surface of the maxilla is in the posterior part formed by the granular ornamentation, while its medial surface consists of fine radial grooves. The maxilla preserves about twelve tooth imprints, which are restricted to the ventral margin of the suborbital ramus and do not extend behind the anterior level of the postorbital plate. The largest teeth are located in the central part of the toothed ventral margin and reach about 3 mm in height and 2 mm in length at the base. The posterior-most teeth rapidly decrease in size and reach only about half the height of the largest teeth. There is also some indication on the tooth reduction towards the anterior end of the suborbital ramus, although it is unclear whether teeth were truly absent in this part of the bone. The dentition is homodont and consists of a single row of densely spaced, cone-like to bulbous upright teeth, with some of them showing a longitudinal groove along the centre of the tooth shaft. In large and well-preserved teeth, the ratio of the tooth height/longitudinal diameter varies from about 1.4 to 1.6 and the distance between adjacent tooth positions/tooth longitudinal diameter is about 0.2. An elongated bone fragment of subrectangular shape located dorsal to the maxillary suborbital ramus is tentatively interpreted here as an infraorbital based on its form and position.
NHMW-Geo-2023/0311/0001 represents a fragment of the isolated left cleithrum exposed in medial view, which can be clearly referred to a large actinopterygian fish (Fig. 7B). It forms a massive dermal bone of the pectoral girdle, with a preserved portion of the element reaching 88 mm in height and 41 mm in length. The principal parts of the preserved cleithrum fragment consist of an elongate dorsal process and a broadly expanded central region. The ventral and posterior parts of the bone, including the notch for the connection of the pectoral fin and the ventral lamina, are broken-off in the specimen. The dorsal process is narrow, triangular in shape and distinctly elongate; it measures 43 mm in height and its base is 11 mm long. The longitudinal axis of the process is directed anterodorsally, and it gradually narrows along its vertical length to a pointed tip, which bears an oblique, rugose surface. A prominent ridge present on the medial side of the cleithrum in many actinopterygian forms (e.g., Pearson and Westoll 1979; Long 1988; Štamberg 2007) extends along the midline of the dorsal process and continues ventrally on the medial surface of the expanded central region, dividing the bone into two parts. The posterior part of the dorsal process is narrow and consists of the convex margin with remnants of the medial lamina forming a caudal border of a shallow groove posterior to the medial ridge of the cleithrum. The medial lamina is broken-off along most of its length, so its original extent is unknown in the specimen. The anterior margin of the dorsal process is slightly concave, and the bone surface in front of the medial ridge is moderately convex, particularly on its base. The expanded central region of the bone is strongly extended anteriorly, and its convex margin joins the dorsal process of the cleithrum at an almost right angle. The medial ridge, extending from the dorsal process of the cleithrum ventrally to the expanded central region, curves at the mid-length of the bone anteriorly, where it gradually diminishes and is replaced by the distinct groove directed anteroventrally. The medial surface of the cleithrum is roughened at some places, and comprises of numerous very fine pits and grooves.
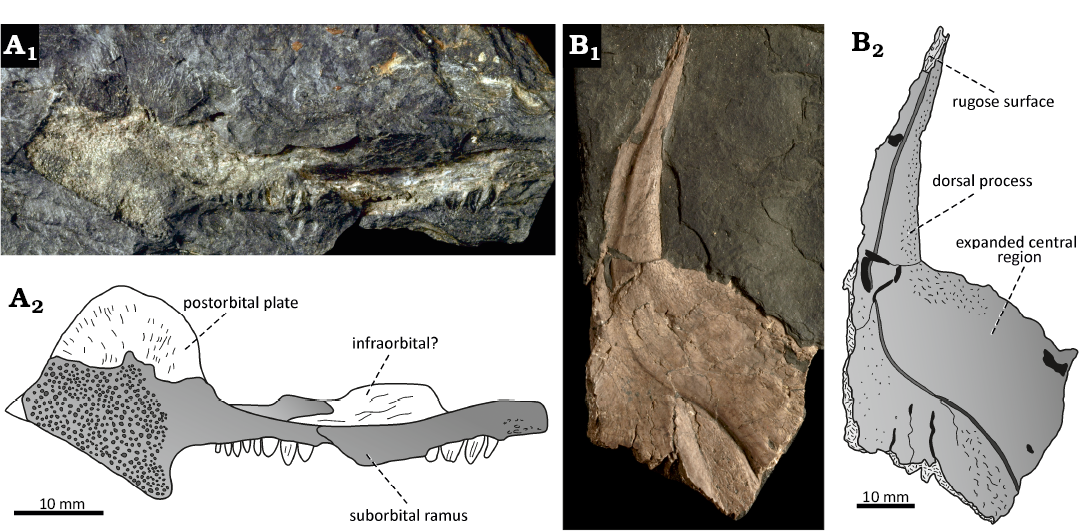
Fig. 7. Isolated skeletal elements referred to indeterminate large actinopterygian fishes from Moscovian, Middle Pennsylvanian, Nýřany Member, Kladno Formation, Nýřany, Czech Republic. A. NMP M546, right maxilla in lateral view, photography (A1) and interpretative drawing (A2). B. NHMW-Geo-2023/0311/0001, left cleithrum in medial view, photography (B1) and interpretative drawing (B2).
Remarks.—NMP M546 was originally briefly described and figured by Fritsch (1885: pl. 61: 5) as a putative palatal bone associated with the early tetrapod “Gaudrya latistoma”, although a possible affinity to actinopterygian fishes was also noted. The morphology of this isolated element is reassessed here and interpreted as belonging to the right maxilla of a large actinopterygian fish. Fritsch (1885) noted the resemblance of NMP M546 to “Amblypterus-like” actinopterygians, i.e., various amblypterids and a sceletophorid (sensu Štamberg 2013). The postorbital plate of the maxilla has a rounded dorsal margin and is anteroposteriorly short compared to its height, the condition which indeed most resembles to amblypterids (Gad 1988; Dietze 1999; Heyler 2000), Sphaerolepis Frič, 1877, and Sceletophorus Fritsch, 1894 (Gardiner 1967; Štamberg 1991, 2013) and gonatodids (Traquair 1907; Gardiner 1967; Elliott 2018). However, the dentition of NMP M546 markedly differs from the distinctive tubular marginal teeth of Amblypteridae, although exhibits some resemblance with their more robust, cone-like coronoid teeth, and it neither can be associated with species of Sphaerolepis, Sceletophorus, and Gonatodidae based on the details of the dentition (Gardiner 1967; Štamberg 2020). In addition, the large dimensions of the maxilla do not support its attribution to any of the taxa mentioned above. The distinct rounded morphology of the postorbital plate differs from the large tetragonal-shaped bone present in most other large-bodied genera and likely represents a unique feature of the specimen, although this morphology might be affected by taphonomic processes to some degree. The slightly bulbous tooth shafts, oriented perpendicular to the suborbital ramus, are densely spaced and arranged in a single row, as also documented in S. macrodens gen. et sp. nov. In addition, both the teeth proportions and dimensions of the maxilla would also support the attribution to the new taxon. However, its unambiguous reference to this species is precluded by the absence of overlapping material, thus until the more complete specimens are discovered, it is retained here as an indeterminate actinopterygian fish.
The general morphology of the cleithrum in NHMW-Geo-2023/0311/0001 conforms that of other Palaeozoic early actinopterygians, and most resembles to Progyrolepis heyleri, Brachydegma caelatum, Angatubichthys mendesi Figueiredo & Carvalho, 2004, and Elonichthys germari Giebel, 1848 (Figueiredo and Carvalho 2004; Schindler 2018a; Štamberg 2018; Argyriou et al. 2022) in that it has elongated and upright dorsal process, and distinctly expanded central region. The dorsal process differs in Cosmoptychius striatus (Agassiz, 1835), in which it has been reconstructed as markedly arched anteriorly (Gardiner 1963). The anterior border of the dorsal process and the expanded central region form an almost right angle, the condition that differs from the smooth, concave transition between the two parts seen in Progyrolepis heyleri and other forms, where the bone region is well-documented (e.g., Pearson and Westoll 1979; Gardiner 1984; Štamberg 2007, 2018; Coates and Tietjen 2019; Argyriou et al. 2022), but somewhat resembles Elonichthys germari (Schindler 2018a). This and the anteroventrally curved medial ridge of the expanded central region, which gradually diminishes to be replaced by a deep groove, may represent the distinctive characters of the specimen. Furthermore, the dimensions of the preserved dorsoventral part of the cleithrum (88 mm in height) indicate a very large individual, of which length would have probably exceeded that of most Palaeozoic actinopterygian fishes. For example, the complete cleithrum of Progyrolepis heyleri reached only about 50 mm in total height (Štamberg 2018). Based on a rather peculiar morphology of the bone not seen in most other early actinopterygians, there is a possibility that the specimen could belong to a larger individual of S. macrodens gen. et sp. nov. However, due to the lack of more complete material, it is currently not possible to compare NHMW-Geo-2023/0311/0001 with the holotype of the latter, thus it is provisionally retained here as an indeterminate large-bodied actinopterygian.
Discussion
Distribution, morphology and function of plicidentine in actinopterygians.—The internal structure of the actinopterygian teeth is principally formed by the central pulp cavity surrounded by the layer of dentine, which is externally covered with the collar enamel on most of the tooth shaft, and the cap enameloid (= acrodin) at the tooth tip (Ørvig 1978b; Sasagawa et al. 2009). The dentine of actinopterygians has long been considered to form a simple layer around the large pulp cavity, contrasting with a complex tooth internal structure of early sarcopterygians and some early tetrapods, in which the dentine formed numerous folds projecting into the pulp cavity, known as plicidentine (Bystrow 1938, 1939; Schultze 1970). Until recently, the only extant representative of Actinopterygii with the well-documented presence of plicidentine was the neopterygian fish Lepisosteus along with some extinct relatives (Tomes 1878; Schultze 1969; Preuschoft et al. 1991). Another occurrence of plicidentine in the actinopterygian dentition has been reported by Meunier et al. (2013) in the basibranchial toothplate of Arapaima gigas, followed by its description in the marginal teeth of Hoplias aimara (Meunier et al. 2015a). The latter authors defined a new type of plicidentine, called simplexodont, characterised by its simple form, where branches of the first degree occur on the tooth base, do not extend far into the pulp cavity free of mineralization, and bone of attachment does not extend between folds externally. Since then, the plicidentine has been documented through the histological sections and virtual micro-CT slices in teeth of various extant actinopterygian fishes (for the recent summary, see Viviani et al. 2022). The form of plicidentine in most of the actinopterygian taxa is consistent with the simplexodont type, although in Lepisosteidae the more complex polyplocodont and dendrodont types have been documented (Schultze 1969, 1970; Brito et al. 2017; Meunier and Brito 2017). In extinct actinopterygians, reports on the presence of plicidentine are much less frequent, in part because the internal structure of the well-preserved dentitions are insufficiently examined, especially those in Palaeozoic forms, and the methods used for their studying are destructive. Although the latter issue can be overcome using non-invasive tomographic methods (Germain et al. 2016), these provide less detailed information compared to the histological sections. Thus, the plicidentine has been so far reported only in the Devonian actinopterygian Cheirolepis (Meunier et al. 2018b), the undetermined isolated teeth from the lower Permian of Brazil (Alves et al. 2021), the Early Triassic and Early Jurassic saurichthyids Saurichthys and Saurorhynchus (Maxwell and Stumpf 2017; Argyriou et al. 2018), and some Mesozoic and Cenozoic lepisosteids (Schultze 1969; Brito et al. 2017; Otero 2022). Here, we expand the known record of the plicidentine to another extinct actinopterygian genus from the Middle Pennsylvanian (late Carboniferous), and compare it to other taxa known to exhibit this feature.
In Stambergichthys macrodens gen. et sp. nov., the tooth outer surface is smooth, and there are no external ridges visible on its base to indicate the presence of dentine infolding. This contrasts with the condition seen in some extant predatory fishes like Lepisosteus, Hydrocynus and Hoplias, in which the longitudinal striations occur on the tooth base in correlation with the internal organisation of dentine (Meunier et al. 2015a; Meunier and Brito 2017; Texereau et al. 2018). However, the longitudinal ridges on the tooth external surface may not be necessarily linked to the presence of plicidentine, or indicative of its form and extent (Schultze 1969). Some of the actinopterygian teeth show the presence of the external ridges, which are formed by the thickened enamel layer, resulting in a crenulated tooth surface unrelated to the internal dentine structure (Brito et al. 2017; Alves et al. 2021). On the contrary, the smooth outer surface of the teeth does not preclude the presence of the plicidentine (Texereau et al. 2018), as is also exemplified by Stambergichthys macrodens gen. et sp. nov., even though the plicated external morphology can be hidden in shallow sockets due to tooth implantation. Despite the absence of the prominent ridges on the tooth external surface, the plicidentine of S. macrodens gen. et sp. nov. is well developed and similar to that of Polypterus senegalus (Germain and Meunier 2019), Arapaima gigas (Meunier et al. 2013) and Hoplias aimara (Meunier et al. 2015a), but differs from the rudimentary plicidentine organisation of Cheirolepis canadensis (Fig. 4B; Meunier et al. 2018b).
The previous authors suggested the plicidentine may have served to increase the attachment surface in the teeth implanted in shallow sockets, which lacked a strong fixation to the jaw bone through the tooth roots (Preuschoft et al. 1991). This interpretation has been in actinopterygians often linked to the predatory diet, since the plicidentine is commonly found in the large fangs which are supposed to resist a great strain during the biting (Meunier et al. 2013, 2015a, 2018b). This functional hypothesis would be consistent with the predatory diet inferred from the tooth morphology of S. macrodens gen. et sp. nov., in which homodont dentition was likely subjected to uniformly distributed loading during the predation, thus the plicidentine is developed equally in all teeth of the jaw. However, the presence and relative complexity of the simplexodont plicidentine might be at least in part related also to the tooth dimensions, as documented in the dentition of temnospondyl amphibians (Warren and Davey 1992). The plicidentine is relatively poorly developed in slender medial fangs of the Devonian predatory fish Cheirolepis, while it is completely absent in its diminutive lateral teeth (Meunier et al. 2018b). Similarly, the absence of plicidentine in the fangs of extant predatory stomiids has been interpreted as a result of their slender morphology (Germain et al. 2019), contrasting with the well-developed cone-like fangs of Hoplias and Hydrocynus (Meunier et al. 2015a). The strong conical dentition in the large Carboniferous genus Stambergichthys gen. nov. also shows a well-developed dentine plication. Interestingly, Viviani et al. (2022) noticed that the plicidentine in the parrotfish dentition occurs only in large fangs not involved in food processing, but they concluded that these were probably subjected to unknown source of external stress. We hypothesize that the larger teeth in a jaw were likely to receive more strain during the food processing than smaller ones, resulting in their more complex plicidentine architecture relative to the diminutive teeth, which may lack plicidentine altogether. Thus, there would be a functional correlation between the size of the teeth and the amount of stress it received. Although the source of the increased stress in the actinopterygian dentition would be indeed in many cases associated with the predatory diet, it is not necessarily limited to it, since the plicidentine has been documented in other forms with varied dietary preferences (Meunier et al. 2015b, 2018a; Viviani et al. 2022). The future research on the internal structure of the marginal teeth in the Permo-Carboniferous actinopterygians could provide more data to test the hypothesis on a linkage between size of the teeth and the presence and relative complexity of the simplexodont plicidentine.
Stratigraphy, palaeoenvironment and palaeoecology of the large actinopterygians at Nýřany.—Until now, only small-bodied actinopterygian fishes were known from the Moscovian, Middle Pennsylvanian, of the Nýřany Member, Kladno Formation, including Pyritocephalus sculptus, Sceletophorus biserialis, and Sceletophorus verrucosus, none of which reached the total body length over 150 mm (Štamberg 1991, 2013). Although fragmentary and isolated, here described material, including a new genus and species, S. macrodens gen. et sp. nov., not only complements the knowledge on the diversity of the group in Nýřany, but also represents the oldest stratigraphic record of the large actinopterygian fishes in the continental basins of the Czech Republic. Until now, in the Central and West Bohemian basins, the known occurrences of large-bodied actinopterygians were restricted to the stratigraphically much higher Slaný and Líně formations (Štamberg and Zajíc 2008), corresponding to the Upper Pennsylvanian, Gzhelian, and the Gzhelian/Asselian boundary, respectively (Opluštil et al. 2016). Among these, the earliest records according to Štamberg and Zajíc (2008) come from the Jelenice Member, being represented by Progyrolepis speciosus, although more complete and better documented materials occur in the overlaying Mšec Member, and include moderate to very large species Zaborichthys fragmentalis, Progyrolepis speciosus, and Acrolepis gigas (Štamberg 1991, 2013). The putative finding of A. gigas in the Radnice Member (Štamberg and Zajíc 2008) is erroneous (Jaroslav Zajíc, personal comunication 2024), and only a single isolated scale of indeterminate actinopterygian fish with prominent ganoin ridges has been so far documented from this stratigraphic unit (Zajíc in Pešek et al. 2001: pl. 23: 1; Zajíc 2006). The new actinopterygian material from the Nýřany Member, Kladno Formation, predates the oldest appearance of large-bodied actinopterygians from the Jelenice and Mšec members of the Pilsen Basin by about 5–6 Ma, weakening a possible species-level taxonomic association of this fragmentary material with the better known actinopterygian fauna discussed above.
The Nýřany Member consists of fluviolacustrine sediments of a large alluvial plain (Pešek 1994; Pešek et al. 1998), where a relatively small and shallow freshwater lake with anoxic conditions near the bottom was formed (Milner 1980). At the Nýřany locality, the lacustrine deposits are represented by the Main Nýřany Coal Seam with the sapropelic beds on the bottom, which contain abundant vertebrate remains. According to Milner (1980), the preserved vertebrate fauna consists of a mixture of autochthonous forms inhabiting the swamp environment, and the allochthonous taxa transported to the basin from the nearby areas. The Nýřany assemblage was divided into the three different faunal associations based on their original hypothetical environments: (i) open water/lacustrine association, (ii) shallow water/swamp-like association, and (iii) terrestrial/marginal association (Milner 1980). However, it is important to note here that these palaeoenvironmental conclusions were mostly based on palaeobiological inferences and only limited geological data, largely deduced from the interpretations of the historical sedimentary profile documented by Fritsch (1879) from the Humboldt mine. For instance, there is currently no sedimentological evidence on the facial transition from a swamp to a large stratified lake in Nýřany, nor the existence of such an environment in the Nýřany Member (Pešek 1994; Pešek et al. 1998; Opluštil et al. 2022; Stanislav Opluštil and Jaroslav Zajíc, personal comunications 2024). A detailed palaeoenvironmental analysis of the locality, assembling all available sedimentary and palaeontological data, is beyond the scope of this study, thus the significance of the newly described actinopterygian material is discussed below mainly in relation to other well-known Permo-Carboniferous actinopterygian faunas and their environmental context, with the special focus on the large-bodied taxa.
The incomplete mandible of S. macrodens gen. et sp. nov., as well as the other skeletal remains described here, represent at least one large-bodied actinopterygian species currently known to occur in Nýřany. The rarity and fragmentary nature of these elements indicate they were likely subjected to post-mortem transportation. This contrasts with the small-bodied haplolepid Pyritocephalus sculptus and sceletophorids Sceletophorus biserialis and S. verrucosus, known at the locality from tens of often completely preserved specimens (Štamberg 1978, 1991). These taxa were the only actinopterygians recorded at Nýřany until now, and their relative abundance, completeness of their material and small size all support the interpretation they were autochthonous inhabitants of the shallow lake with low diversity of species. The Linton locality in Ohio, USA, is known for its abundant and mostly autochthonous vertebrate fauna of the Middle Pennsylvanian, Moscovian age, which represents freshwater deposits of abandoned river channel rich in organic matter, and with anoxic conditions near the bottom (Hook and Ferm 1985, 1988; Hook and Baird 1988), providing thus a good example for the comparison. This locality documents abundant and diversified association of haplolepid actinopterygians (Lowney 1980; Hook and Baird 1988), which are also frequent in Nýřany, and, most likely, represent dwellers of swamp lakes (Westoll 1944), although they might not be confined to them. The shared component of both actinopterygian faunas is Pyritocephalus, which is less common in Linton, but in contrast to Nýřany, the large-bodied actinopterygians are entirely unknown, even though the sampling bias can play a role. On the contrary, the Bashkirian, Lower Pennsylvanian, coal deposits at Newsham, Northumberland, UK have previously been interpreted as large stratified body of water, either freshwater and extending in abandoned river channel (Boyd 1984; Hook and Ferm 1985), or brackish and representing a delta channel (Milner 1987), with few sedimentary data available to favour one of these hypotheses. Regardless, a diversified actinopterygian assemblage has been recorded from the locality. Unlike the situation in Nýřany and Linton, the actinopterygian fauna at Newsham is almost devoid of haplolepids, which are represented only by the four fragmentary specimens attributed to Protohaplolepis attheyi, Parahaplolepis aff. anglica, and Pyritocephalus rudis (Westoll 1944; Lowney 1983; Huber 1992; Elliott 2014), the scarce remains of which were interpreted as probably transported to the deepwater basin from a hypothetical nearby swamp lake (Boyd 1984). Besides the haplolepids, actinopterygian fauna at Newsham is dominated by the deep-bodied platysomids, rhadinichthyids, “elonichthyids”, and acrolepids (Traquair 1905; Land 1974). The two latter groups are represented at the locality by the large-bodied taxa “Elonichthys” semistriatus (Traquair 1877, 1905; Woodward 1891) and Acrolepis hopkinsi (Traquair 1905, 1907, 1909), the latter of which is known from the large articulated specimen (Traquair 1907: pl. 23). The fine preservation of its material, with articulated dermal skull bones, mandible and pectoral girdle, could support the interpretation this species was autochthonous in deposits of the deepwater basin.
Another example of a well-documented actinopterygian fauna of a large freshwater lake with no marine influence is represented by the Mšec Member, Slaný Formation, Gzhelian, Upper Pennsylvanian in the Czech Republic, of which sediments can be found through the continental basins of the west, central and northeast Bohemia. It consists of organic-rich claystones and siltstones representing the deposits of a large stratified lake which extended on the area of 5000–10 000 km2 (Skoček 1990; Lojka et al. 2009). The actinopterygian assemblage is relatively diverse, and at least six species belonging to five families are present, including small-bodied aeduellid Spinarichthys dispersus, elonichthyid Elonichthys krejcii and trissolepid Sphaerolepis kounoviensis, as well as moderate-sized pygopterid Zaborichthys fragmentalis and large-bodied acrolepids, i.e., Progyrolepis speciosus and Acrolepis gigas. Similarly to Newsham, and contrary to the relatively shallow lake deposits of Linton and Nýřany, the haplolepids are completely absent in the Mšec Member. Although most of the findings are represented by isolated elements only, this may be the result of some peculiar taphonomic processes of autolysis discussed by Milner (1980: 446–448), and the ichthyofauna of the Mšec Member is indeed considered to be autochthonous (Lojka et al. 2009). This can be additionally confirmed by a complete specimen of Acrolepis gigas from the large siderite concretion at Žilov, which can only be formed if a rapid burial under anoxic conditions is present (Perrier and Charbonnier 2014). Furthermore, Harris and Lucas (2017) reported a gigantic actinopterygian fish from the Kasimovian, Upper Pennsylvanian black shale deposits of the Tinajas Member, Atrasado Formation in New Mexico, USA, which has been interpreted as deposited in a large freshwater lake with an anoxic bottom (Lerner et al. 2009). The comparison to the other upper Carboniferous localities historically considered to represent limnic freshwater environments, and containing well-documented actinopterygian faunas, is difficult, mainly because many of them have recently been argued to be marine-connected (e.g., Mazon Creek; Clements et al. 2018), or their marine influence is being debated (e.g., Montceau-les-Mines: Poplin et al. 2001; Charbonnier et al. 2008; Schultze 2009; Mickle 2011; Perrier and Charbonnier 2014; Puertollano Basin: Jiménez et al. 1999; Soler-Gijón and Moratalla 2001; Schultze 2009; Fisher et al. 2013; Soler-Gijón and Díez Ruiz 2023). However, the Mazon Creek and Montceau-les-Mines appear to lack large-bodied actinopterygians in their assemblages, whereas in Puertollano Basin, Spain, the remains of Progyrolepis speciosus have recently been uncovered in sediments assumed to represent the estuarine-deltaic environment, indicating potentially euryhaline adaptations of this species (Soler-Gijón and Díez Ruiz 2023). The shallow coastal environments of Mazon Creek and Montceau-les-Mines, either freshwater or brackish (Charbonnier et al. 2008; Schultze 2009; Clements et al. 2018), were possibly not preferred by the large actinopterygian forms, which may have inhabited the deeper areas of the basin.
In addition to the Carboniferous localities, some medium to large-sized freshwater actinopterygians have also been reported from the Permian deposits of the Central and Western Europe. The remains of Progyrolepis heyleri were described from the Asselian, lower Permian, locality of Buxieres-les-Mines, France (Poplin 1999; Štamberg 2018), of which setting has recently been interpreted as a large, open and deep freshwater lake with no significant marine influence (Steyer et al. 2000; Luccisano et al. 2023). A peculiar isolated mandible of a large actinopterygian fish, Usclasichthys macrodens, assigned to Pygopteridae by Štamberg and Steyer (2021), as well as remains of Pygopterus sp., were described from the lower Permian of Lodѐve Basin, Spain (Heyler 1977; Lopez et al. 2008). The stratigraphic position of the findings has not been specified, except for the note it comes from the Autunian Group of the Usclas region (Heyler 1977). However, according to Lopez et al. (2008), the fish remains are derived from the lowermost part of the Tuilliѐres-Loiras Formation, represented by the black shale deposits, indicating a deepwater lacustrine environment with anoxic conditions near the bottom (Lopez et al. 2008; Pochat and Van Den Driessche 2011). Finally, the medium-sized (<400 mm total length) elonichthyid Rhabdolepis macropterus is known from the lower Permian limnic deposits of the Saar-Nahe Basin, Germany (Gardiner 1963; Schindler 2018b). Its frequent occurrence has been documented from the clay ironstone facies of the Humberg Black Shale, which are generally considered to contain the autochthonous fauna in the deepest part of the extensive Humberg-Lebach Lake (Boy 1987; Schoch 2009, 2014). Thus, based on the overview presented above, it could be concluded that the large freshwater actinopterygians are commonly found in deepwater environments across the Permo-Carboniferous continental basins in the central Pangea (Fig. 8). Although the occurrence of such fishes at Nýřany indeed could indicate a presence of deep and extensive water of body, as previously suggested by Milner (1980), we note that palaeobiological evidence alone is ambiguous and insufficient to interpret depositional environment, and other lines of evidence should be taken in account.
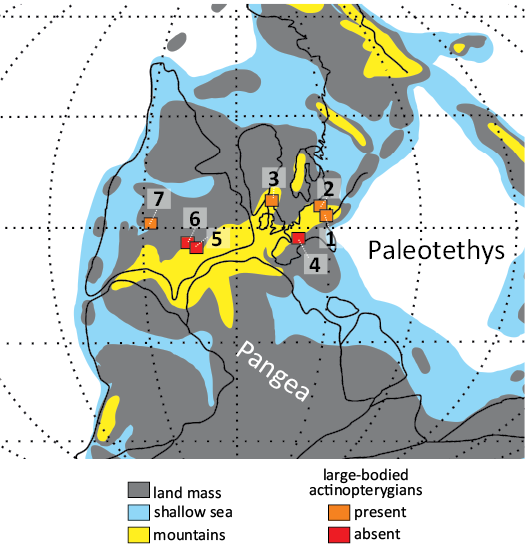
Fig. 8. The late Carboniferous (Pennsylvanian) palaeogeographic map showing the occurrence of large-bodied actinopterygians in selected freshwater or marine-influenced localities within continental basins of central Pangea. 1, Nýřany, Czech Republic; 2, Mšec, Czech Republic; 3, Newsham, Northumberland, UK; 4, Montceau-les-Mines, France; 5, Linton, Ohio, USA; 6, Mazon Creek, Illinois, USA; 7, Tinajas, New Mexico, USA.
As noted above, sedimentological data support neither the presence of deepwater environment laterally to the shallow swamp lake, nor subsidence of the basin, allowing the accumulation of a large water column. Despite this, an allochthonous aquatic vertebrate association apparently occurs at the locality (Table 1). These taxa can be recognized based on the criteria established by Milner (1980), including the relative abundance of specimens, degree of their completeness and articulation, functional morphology, as well as comparison to other faunal associations from elsewhere. The large xenacanthiform shark Orthacanthus bohemicus has been described at the locality from some skull and postcranial skeleton materials, isolated dorsal spines and numerous isolated teeth (Fritsch 1889; Heidtke 1998; Soler-Gijón 2004). Previous authors noted the wide geographic and stratigraphic distribution of this and other xenacanthiform genera across the Permo-Carboniferous limnic basins of the Central and Western Europe, and proposed a palaeogeographical scenario of their continental dispersion through the river channels interconnecting these basins (Schneider and Zajíc 1994; Schneider 1996; Luccisano et al. 2022). This is supported by the recent isotopic analyses of xenacanthiform teeth and spines (Fisher et al. 2013; Luccisano et al. 2023), indicating that large-bodied forms like Orthacanthus were restricted to large and deep freshwater lakes and river systems. Similarly, the dipnoan fish Sagenodus sp., known in Nýřany from several isolated scales (Fritsch 1888; Watson and Gill 1923), has been documented not only from various European continental basins (Fritsch 1888; Schultze 1993; Olive et al. 2012; Beeby et al. 2020), but also from numerous localities in the USA (Schultze and Chorn 1997). The latter authors proposed that the genus Sagenodus was euryhaline and spread from the North America to Europe via a marine connection, followed by its dispersal across the European continental basins through the river channels. Its remains are frequently found at Newsham (Beeby et al. 2020) and occur, among others, in the Mšec and Kounov members (Fritsch 1888; Lojka et al. 2009), indicating the genus might have preferred a deepwater environment, although not being necessarily restricted to it. The aquatic specializations of the baphetoids, in Nýřany represented by Baphetes orientalis, are well supported by the various morphological adaptations on the skull and mandible (Beaumont 1977; Beaumont and Smithson 1998). Westoll (1944) noted that members of the group often co-occur with haplolepids in deposits interpreted as stagnant shallow waters, while Milner (1987) assumed the baphetoids inhabited coastal brackish deepwater environments, although he pointed to their possible wide range of ecological adaptations. Although the Baphetoidea represents a rare faunal component in both the Linton (Hook and Baird 1986) and Nýřany (Milner 1980) locality, the complete articulated skulls have been reported from both sites (Romer 1930; Steen 1938; Beaumont 1977; Milner et al. 2009), indicating that their materials did not undergo a long transport to the place of deposition. We hypothesize that the scarce occurrences of the baphetoids in Nýřany reflect their ecological preferences, and these forms might have indeed primarily inhabited the river systems, with only occasional excursions to the relatively shallow lake to forage or breed. Finally, Diplovertebron punctatum is currently known from a single incomplete specimen (Fritsch 1885: pl. 52) which represents scattered skeletal remains of a possible embolomere (Carroll 1970; Klembara et al. 2014). The nature of its preservation, as well as sparse occurrence, indicate it is allochthonous at the locality (Milner 1980), although too incomplete to infer its possible habitat. The allochthonous aquatic vertebrate association documented in deposits of the relatively shallow lake of Nýřany thus does not require the presence of a nearby large and deep freshwater lake, as previously hypothesized (Milner 1980), but it can equally be interpreted as originating from the braided river system known to occur across the Nýřany Member (Opluštil et al. 2005). In context of this, the mandible of Stambergichthys macrodens gen. et sp. nov. and remains of the same or other large actinopterygian fishes in the locality are interpreted here also as derived from a river channel that could have been connected to the shallow lake for some time (Pešek 1994; Pešek et al. 1998). It is also interesting to point out here that, although largely incomplete, the allochthonous aquatic vertebrate association of Nýřany seems to resemble in general composition to some other Carboniferous localities interpreted as deepwater environments (Fig. 9). This faunal concordance can be perceived as partly artificial and reflecting a wide distribution of its constituents in various depositional settings. In other words, certain aquatic vertebrates (e.g., xenacanthiform sharks, actinopterygian and dipnoan fishes) might have used the river channels primarily to reach the more suitable destinations such as deep freshwater basins, where their occurrence is indeed well-documented. This might also be the case of large-bodied actinopterygians like Progyrolepis, i.e., Progyrolepis speciosus and Progyrolepis heyleri, which have been recorded in the Permo-Carboniferous basins of the Czech Republic (Štamberg 1991, 2013; Lojka et al. 2009), France (Poplin 1999; Štamberg 2018; Štamberg and Steyer 2021) and Spain (Soler-Gijón and Díez Ruiz 2023). The absence of S. macrodens gen. et sp. nov. from other continental basins of Europe may thus be related either to its endemism, sampling bias, or the simple lack of European basins of the corresponding age.
Table 1. The list of aquatic taxa considered in this study to be allochthonous at the locality of Nýřany (Czech Republic).
|
Taxon |
Material |
References |
|
Xenacanthiformes |
||
|
Orthacanthus bohemicus Frič, 1875 |
two fairly complete juvenile individuals; articulated skull with mandibles and pectoral girdle; mandible with skull elements; pelvic girdle; numerous isolated spines and teeth |
|
|
Actinopterygii |
||
|
Actinopterygii indet. |
isolated maxilla with infraorbital, cleithrum |
this study |
|
Stambergichthys macrodens gen. et sp. nov. |
isolated mandible with teeth |
this study |
|
Dipnoi |
||
|
Sagenodus sp. |
isolated scales |
|
|
Tetrapoda |
||
|
Baphetes orientalis Milner et al., 2009 |
two articulated skulls, mandibles and pectoral girdle |
|
|
Diplovertebron punctatum Frič, 1879 |
disarticulated cranial and postcranial elements |
|
The dentition of S. macrodens gen. et sp. nov. consists of large, massive, uniform teeth with pointed tips and smooth surface, which are arranged in a single row, and clearly display the carnivorous adaptations. The well-developed simplexodont form of plicidentine, found in all teeth of the jaw, indicates the dentition was adapted to withstand the high pressure loads associated with prey capturing. These morphological and structural features, combined with the relatively large dimensions of the species (estimated total length up to 700 mm), support the interpretation it occupied high trophic levels in the ecosystems it inhabited. However, the remains of much larger actinopterygians are documented in Nýřany (NHMW-Geo-2023/0311/0001; the estimated total body length probably no less than 1200 mm). Due to the lack of overlapping material, it currently cannot be resolved whether this material can be attributed to S. macrodens gen. et sp. nov., or it represents another so far unknown large-bodied species. In any case, this form would have represented a gigantic actinopterygian fish likely occupying top-most positions of the food web (Fig. 9), and probably able to compete with large-bodied xenacanthiform sharks and early tetrapods co-occurring at the locality.
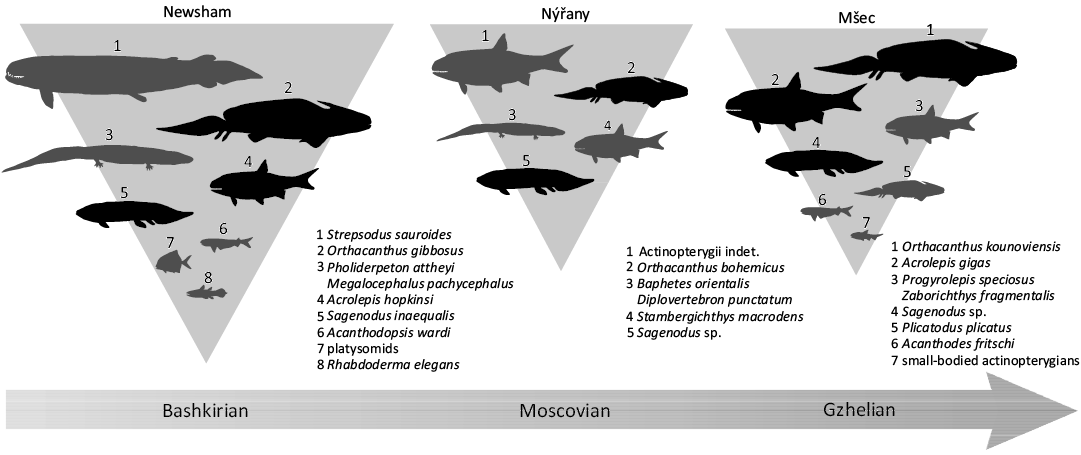
Fig. 9. The comparison of Pennsylvanian aquatic vertebrate associations of deepwater environment (Newsham and Mšec) with allochthonous aquatic vertebrate association of Nýřany, interpreted here as originating from braided river system. Black silhouettes represent shared faunal components at the genus level. Faunal lists based on Lojka et al. (2009) for Mšec, on various sources for Newsham (note that listed fauna of Newsham is not exhaustive).
Conclusions
The isolated skeletal remains of large actinopterygian fishes are reported here for the first time from the Moscovian, Middle Pennsylvanian of Nýřany locality, Czech Republic. These include a new genus and species, Stambergichthys macrodens gen. et sp. nov., based on a single lower jaw with well-preserved teeth, which has been scanned through the micro-computed tomography and shows a presence of a complex internal neurovascular system innerving the lower jaw and teeth, and supplying both with blood vessels. The marginal dentition of the new species exhibits a single row of massive, homodont and conical teeth with the presence of simplexodont plicidentine on their base, the features that support its predatory ecology. The occurrence of isolated and fragmentary remains of large-bodied actinopterygians in the coal deposits of a relatively shallow lake indicates that their remains were probably derived from the braided river system flowing through the alluvial plain of the Nýřany Member, and represent the oldest stratigraphic record of large actinopterygian fishes in the Permo-Carboniferous sediments of Czech Republic. These results underline a potential taxonomic and palaeoecological importance of some isolated and fragmentary specimens found in coal-bearing deposits of the Central and Western Europe, being a part of the classic and well-known late Carboniferous vertebrate assemblages.
Acknowledgements
We are indebted to Nela Doláková (Masaryk University, Brno, Czech Republic), Ursula Göhlich (NHMW), Boris Ekrt (NMP), and Petr Budil (CGS) for the permission to study the materials in their care and assistance in the collections. Stanislav Štamberg (Museum of Eastern Bohemia, Hradec Králové, Czech Republic), Frederik Spindler (Dinosaurier Museum Altmühltal, Denkendorf, Germany) and Arjan Mann (National Museum of Natural History, Smithsonian Institution, Washington, USA) are thanked for the fruitful discussion on the taxonomic assignment of the Nýřany material. We are also thankful to Jakub Sakala and Stanislav Opluštil (both Charles University, Prague, Czech Republic), as well as Jaroslav Zajíc (Czech Academy of Sciences, Prague, Czech Republic) for the discussion on the palaeoenvironment of the Nýřany locality. Valuable comments from two reviewers, Adriana López-Arbarello (Ludwig-Maximilians-Universität München, Germany) and Sam Giles (University of Michigan, USA), helped to greatly improve the initial draft of the manuscript. This study was supported by the Specific Research Project MUNI/A/1261/2022 of the Faculty of Science at Masaryk University in Brno and the Czech Science Foundation (grant no. GA22-25799S).
Author contributions’
PB, conceived the study, conducted analyses, wrote the text and prepared figures; MI, conceived and supervised the study, wrote the text; ET, MO, conducted analyses; VN, conducted analyses and wrote the text.
References
Agassiz, L. 1833–1844. Recherches sur les poissons fossiles. 1420 pp. Petitpierre, Neuchatel. Crossref
Ahlberg, P.E. and Clack, J.A. 1998. Lower jaws, lower tetrapods—a review based on the Devonian genus Acanthostega. Transactions of the Royal Society of Edinburgh: Earth Sciences 89: 11–46. Crossref
Aldinger, H. 1937. Permische Ganoidfische aus Ostgrönland. Meddelelser om Grønland 102: 1–392.
Allis, E.P. 1922. The cranial anatomy of Polypterus, with special reference to Polypterus bichir. Journal of Anatomy 56: 189–294.
Alves, Y.M., Gama Junior, J.M., and Cupello, C. 2021. Palaeoniscoid remains from the Lower Permian Pedra de Fogo Formation (Parnaíba Basin): insights from general morphology and histology. Historical Biology 33: 1933–1943. Crossref
Argyriou, T., Giles, S., Friedman, M., Romano, C., Kogan, I., and Sánchez-Villagra, M.R. 2018. Internal cranial anatomy of Early Triassic species of †Saurichthys (Actinopterygii: †Saurichthyiformes): implications for the phylogenetic placement of †saurichthyiforms. BMC Evolutionary Biology 18: 161. Crossref
Argyriou, T., Giles, S., and Friedman, M. 2022. A Permian fish reveals widespread distribution of neopterygian-like jaw suspension. eLife 11: e58433. Crossref
Arratia, G. and Cloutier, R. 1996. Reassessment of the morphology of Cheirolepis canadensis (Actinopterygii). In: H.-P. Schultze and R. Cloutier (eds.), Devonian Fishes and Plants of Miguasha, Quebec, Canada, 165–197. Verlag Dr. Friedrich Pfeil, München.
Bačovský, V., Čegan, R., Tihlaříková, E., Neděla, V., Hudzieczek, V., Smrža, L., Janíček, T., Beneš, V., and Hobza, R. 2022. Chemical genetics in Silene latifolia elucidate regulatory pathways involved in gynoecium development. Journal of Experimental Botany 73: 2354–2368. Crossref
Barták, P. 2020. Přehled výzkumů zástupců skupin Temnospondyli Zittel, 1888 a Lepospondyli Zittel, 1888 ze svrchního karbonu České republiky. Acta Musei Moraviae, Scientiae Geologicae 105: 97–151.
Beaumont, E.H. 1977. Cranial morphology of the Loxommatidae (Amphibia: Labyrinthodontia). Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 280: 29–101. Crossref
Beaumont, E.H. and Smithson, T.R. 1998. The cranial morphology and relationships of the aberrant Carboniferous amphibian Spathicephalus mirus Watson. Zoological Journal of the Linnean Society 122: 187–209. Crossref
Beeby, E.L., Smithson, T.R., and Clack, J.A. 2020. Systematics and description of the lungfish genus Sagenodus from the Carboniferous of the UK. Earth and Environmental Science Transactions of the Royal Society of Edinburgh 111: 47–74. Crossref
Botella, H., Blom, H., Dorka, M., Ahlberg, P. E., and Janvier, P. 2007. Jaws and teeth of the earliest bony fishes. Nature 448: 583–586. Crossref
Boy, J.A. 1987. Die Tetrapoden-Lokalitäten des saarpfälzischen Rotliegenden (?Ober-Karbon–Unter-Perm; SW-Deutschland) und die Biostratigraphie der Rotliegend-Tetrapoden. Mainzer Geowissenschaftlichen Mittelungen 16: 31–65.
Boyd, M.J. 1984. The Upper Carboniferous tetrapod assemblage from Newsham, Northumberland. Palaeontology 27: 367–392.
Brito, P.M., Alvarado-Ortega, J., and Meunier, F.J. 2017. Earliest known lepisosteoid extends the range of anatomically modern gars to the Late Jurassic. Scientific Reports 7: 17830. Crossref
Bystrow, A.P. 1938. Zahnstruktur der Labyrinthodonten. Acta Zoologica 19: 387–425. Crossref
Bystrow, A.P. 1939. Zahnstruktur der Crossopterygier. Acta Zoologica 20: 283–338. Crossref
Cabrera, A. 1944. Dos nuevos peces ganoideos del Triásico argentino. Notas del Museo de La Plata, Paleontología 9: 569–576.
Carroll, R.L. 1970. The ancestry of reptiles. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 257: 267–308. Crossref
Carroll, R.L. and Baird, D. 1972. Carboniferous stem-reptiles of the family Romeriidae. Bulletin of the Museum of Comparative Zoology 143: 321–364.
Charbonnier, S., Vannier, J., Galtier, J., Perrier, V., Chabard, D., and Sotty, D. 2008. Diversity and paleoenvironment of the flora from the nodules of the Montceau-les-Mines biota (Late Carboniferous, France). Palaios 23: 210–222. Crossref
Clements, T., Purnell, M., and Gabbott, S. 2018. The Mazon Creek Lagerstätte: a diverse late Paleozoic ecosystem entombed within siderite concretions. Journal of the Geological Society 176: 1–11. Crossref
Coates, M.I. and Tietjen, K. 2019. “This strange little palaeoniscid”: a new early actinopterygian genus, and commentary on pectoral fin conditions and function. Earth and Environmental Science Transactions of the Royal Society of Edinburgh 109: 15–31. Crossref
Cope, E.D. 1887. General notes: geology and palaeontology. American Naturalist 21: 1014–1019. Crossref
Cox, C.B. and Hutchinson, P. 1991. Fishes and amphibians from the Late Permian Pedra de Fogo Formation of northern Brazil. Palaeontology 34: 561–573.
Dietze, K. 1999. Paramblypterus duvernoyi (Actinopterygii): skull morphology and intra-specific variation, and its implications for the systematics of paramblypterid fishes. Journal of Vertebrate Paleontology 19: 247–262. Crossref
Dunkle, D.H. 1939. A new paleoniscid fish from the Texas Permian. American Journal of Science 237: 262–274. Crossref
Elliott, F.M. 2014. A new haplolepid fauna (Osteichthyes: Actinopterygii) from the Lower Coal Measures of Scotland: Westphalian A; Langsettian, Carbonicola communis chronozone (Bashkirian). Earth and Environmental Science Transactions of the Royal Society of Edinburgh 105: 207–225. Crossref
Elliott, F.M. 2018. An early actinopterygian ichthyofauna from the Scottish Lower Coal Measures Formation: Westphalian A (Bashkirian). Earth and Environmental Science Transactions of the Royal Society of Edinburgh 107: 351–394. Crossref
Figueiredo, F.J. and Carvalho, B.C.M.C. 2004. A new actinopterygian fish from the Late Permian of the Paraná Basin, Southern Brazil. Arquivos do Museu Nacional 62: 531–547.
Figueroa, R.T. and Andrews, J.V. 2023. Fitting fangs in a finite face: a novel fang accommodation strategy in a 280‐million‐year‐old ray‐finned fish. Journal of Anatomy 242: 525–534. Crossref
Figueroa, R.T., Friedman, M., and Gallo, V. 2019. Cranial anatomy of the predatory actinopterygian Brazilichthys macrognathus from the Permian (Cisuralian) Pedra de Fogo Formation, Parnaíba Basin, Brazil. Journal of Vertebrate Paleontology 39: e1639722. Crossref
Fischer, J., Schneider, J.W., Voigt, S., Joachimski, M.M., Tichomirowa, M., Tütken, T., Götze, J., and Berner, U. 2013. Oxygen and strontium isotopes from fossil shark teeth: environmental and ecological implications for Late Palaeozoic European basins. Chemical Geology 342: 44–62. Crossref
Frič, A. 1875. Über die Fauna der Gaskohle des Pilsner und Rakonitzer Beckens. Sitzungsberichte der Königlichen Böhmischen Gesellschaft der Wissenschaften in Prag, Mathematisch-naturwissenschaftliche Classe 1875: 70–79.
Frič, A. 1877. Zur Fauna der Gaskohle von Zaboř bei Schlan, Kroučová bei Řenč und Třemošná bei Pilsen, sowie über die Sphaerosideritkugeln von Žilov. Sitzungsberichte der Königlichen Böhmischen Gesellschaft der Wissenschaften in Prag, Mathematisch-naturwissenschaftliche Classe 1877: 45–52.
Frič, A. 1879. Neue Uebersicht der in der Gaskohle und der Kalksteinen der Permformation in Böhmen vorgefundenen Thierreste. Sitzungsberichte der Königlichen Böhmischen Gesellschaft der Wissenschaften in Prag, Mathematisch-naturwissenschaftliche Classe 1879: 184–195. Crossref
Friedman, M., Pierce, S.E., Coates, M., and Giles, S. 2019. Feeding structures in the ray-finned fish Eurynotus crenatus (Actinopterygii: Eurynotiformes): implications for trophic diversification among Carboniferous actinopterygians. Earth and Environmental Science Transactions of the Royal Society of Edinburgh 109: 33–47. Crossref
Fritsch, A. 1870. Ueber das Auffinden von neuen Thierresten aus der so genannten Brettelkohle von Nyřan bei Pilsen. Sitzungsberichte der Königlichen Böhmischen Gesellschaft der Wissenschaften in Prag, Mathematisch-naturwissenschaftliche Classe 1870: 33–35.
Fritsch, A. 1879. Fauna der Gaskohle und der Kalksteine der Permformation Böhmens. Band 1, Heft 1. 1–92. Selbstverlag, Prag. Crossref
Fritsch, A. 1885. Fauna der Gaskohle und der Kalksteine der Permformation Böhmens. Band 2, Heft 1. 1–32. Selbstverlag, Prag.
Fritsch, A. 1888. Fauna der Gaskohle und der Kalksteine der Permformation Böhmens. Band 2, Heft 3. 65–92. Selbstverlag, Prag.
Fritsch, A. 1889. Fauna der Gaskohle und der Kalksteine der Permformation Böhmens. Band 2, Heft 4. 93–114. Selbstverlag, Prag.
Fritsch, A. 1894. Fauna der Gaskohle und der Kalksteine der Permformation Böhmens. Band 3, Heft 3. 81–104. Selbstverlag, Prag.
Fritsch, A. 1895. Fauna der Gaskohle und der Kalksteine der Permformation Böhmens. Band 3, Heft 4. 105–132. Selbstverlag, Prag.
Fritsch, A. 1907. Miscellanea Palaeontologica. I. Palaeozoica. 23 pp. Selbstverlag, Prag. Crossref
Gad, J. 1988. Der Schädel und Schultergürtel von Paramblypterus gelberti (Actinopterygii) und seine Verwandtschaft mit den Paramblypteriformes und Redfieldiiformes. Paläontologische Zeitschrift 62: 147–164. Crossref
Gardiner, B.G. 1963. Certain palaeoniscoid fishes and the evolution of the snout in actinopterygians. Bulletin of the British Museum (Natural History), Geology 8: 255–325.
Gardiner, B.G. 1967. Further notes on palaeoniscoid fishes with a classification of the Chondrostei. Bulletin of the British Museum (Natural History), Geology 14: 143–206.
Gardiner, B.G. 1984. The relationships of the palaeoniscid fishes, a review based on new specimens of Mimia and Moythomasia from the Upper Devonian of Western Australia. Bulletin of the British Museum (Natural History), Geology 37: 173–428.
Gegenbaur, C. 1874. Grundriss der Vergleichenden Anatomie. 660 pp. Wilhelm Engelmann, Leipzig. Crossref
Germain, D. and Meunier, F.J. 2019. Teeth of extant Polypteridae and Amiidae have plicidentine organization. Acta Zoologica 100: 119–125. Crossref
Germain, D., Mondéjar-Fernández, J., and Meunier, F.J. 2016. The detection of weakly developed plicidentine in teleost teeth using 3D tomography. Cybium 40: 75–82.
Germain, D., Schnell, N.K., and Meunier, F.J. 2019. Histological data on bone and teeth in two dragonfishes (Stomiidae; Stomiiformes): Borostomias panamensis Regan & Trewavas, 1929 and Stomias boa Reinhardt, 1842. Cybium 43: 103–107.
Giebel, C.G.A. 1848. Fauna der Vorwelt mit steter Berücksichtigung der lebenden Thiere. Erste Band: Wirbelthiere. Dritte Abtheilung: Fische. 467 pp. Brockhaus, Leipzig. Crossref
Giles, S., Darras, L., Clément, G., Blieck, A., and Friedman, M. 2015. An exceptionally preserved Late Devonian actinopterygian provides a new model for primitive cranial anatomy in ray-finned fishes. Proceedings of the Royal Society B: Biological Sciences 282: 20151485. Crossref
Goodrich, E.S. 1930. Studies on the Structure and Development of Vertebrates. 837 pp. Macmillan and Co., Ltd., London. Crossref
Hamel, M.-H. 2005. A new lower actinopterygian from the Early Permian of the Paraná Basin, Brazil. Journal of Vertebrate Paleontology 25: 19–26. Crossref
Harris, S.K. and Lucas, S.G. 2017. Preliminary description of a large, three-dimensionally preserved lower actinopterygian skull from the Upper Pennsylvanian Atrasado Formation of New Mexico. New Mexico Museum of Natural History and Science Bulletin 77: 109–112.
Hay, O.P. 1900. Descriptions of some vertebrates of the Carboniferous age. Proceedings of the American Philosophical Society 39: 96–123.
Heidtke, U.H.J. 1998. Revision der Gattung Orthacanthus Agassiz 1843 (Chondrichthyes: Xenacanthida). Paläontologische Zeitschrift 72: 135–147. Crossref
Heyler, D. 1977. Découvertes ichthyologiques dans le Permien de Lodève; une nouvelle structure dentaire. Géologie Méditerranéenne 4: 189–204. Crossref
Heyler, D. 2000. Les actinoptérygiens stéphaniens et autuniens du Massif central (France) dans les collections du M.N.H.N. (Paris) et du Muséum d’Autun: compléments, mises au point, bilan. Bulletin de la Société d’Histoire naturelle d’Autun 169: 7–44.
Hook, R.W. and Baird, D. 1986. The Diamond coal mine of Linton, Ohio, and its Pennsylvanian-age vertebrates. Journal of Vertebrate Paleontology 6: 174–190. Crossref
Hook, R.W. and Baird, D. 1988. An overview of the Upper Carboniferous fossil deposit at Linton, Ohio. Ohio Journal of Science 88: 55–60.
Hook, R.W. and Ferm, J.C. 1985. A depositional model for the Linton tetrapod assemblage (Westphalian D, Upper Carboniferous) and its palaeoenvironmental significance. Philosophical Transactions of the Royal Society of London 311: 101–109. Crossref
Hook, R.W. and Ferm, J.C. 1988. Paleoenvironmental controls on vertebrate-bearing abandoned channels in the Upper Carboniferous. Palaeogeography, Palaeoclimatology, Palaeoecology 63: 159–181. Crossref
Huber, P. 1992. Pyritocephalus lowneyae n. sp., the youngest haplolepiform (Pisces: Actinopterygii) from the Pennsylvanian of central New Mexico. New Mexico Bureau of Mines & Mineral Resources Bulletin 138: 183–187.
Huxley, T.H. 1880. On the application of the laws of evolution to the arrangement of the Vertebrata, and more particularly of the Mammalia. Proceedings of the Zoological Society of London 43: 649–662.
Jambura, P.L., Kindlimann, R., López-Romero, F., Marramà, G., Pfaff, C., Stumpf, S., Türtscher, J., Underwood, C.J., Ward, D.J., and Kriwet, J. 2019. Micro-computed tomography imaging reveals the development of a unique tooth mineralization pattern in mackerel sharks (Chondrichthyes; Lamniformes) in deep time. Scientific Reports 9: 9652. Crossref
Jeffery, J.E. 2002. Mandibles of rhizodontids: anatomy, function and evolution within the tetrapod stem-group. Transactions of the Royal Society of Edinburgh: Earth Sciences 93: 255–276. Crossref
Jiménez, A., Martinez-Tarazona, R., and Suárez-Ruiz, I. 1999. Paleoenvironmental conditions of Puertollano coals (Spain): petrological and geochemical study. International Journal of Coal Geology 41: 189–211. Crossref
Klembara, J., Clack, J.A., Milner, A.R., and Ruta, M. 2014. Cranial anatomy, ontogeny, and relationships of the Late Carboniferous tetrapod Gephyrostegus bohemicus Jaekel, 1902. Journal of Vertebrate Paleontology 34: 774–792. Crossref
Land, D.H. 1974. Geology of the Tynemouth District. 176 pp. Memoirs of the Geological Survey of Great Britain, London.
Lerner, A.J., Lucas, S.G., Spielmann, J.A., Krainer, K., Dimichele, W.A., Chaney, D.S., Schneider, J.W., Nelson, W.J., and Ivanov, A. 2009. The biota and paleoecology of the Upper Pennsylvanian (Missourian) Tinajas locality, Socorro County, New Mexico. In: V.W. Lueth, S.G. Lucas, and R.M. Chamberlin (eds.), 60th Field Conference, Geology of the Chupadera Mesa Region, Guidebook, 267–280. New Mexico Geological Society, Socorro. Crossref
Leuzinger, L., Cavin, L., López-Arbarello, A., and Billon‐Bruyat, J.-P. 2020. Peculiar tooth renewal in a Jurassic ray‐finned fish (Lepisosteiformes, †Scheenstia sp.). Palaeontology 63: 117–129. Crossref
Lojka, R., Drábková, J., Zajíc, J., Sýkorová, I., Franců, J., Bláhová, A., and Grygar, T. 2009. Climate variability in the Stephanian B based on environmental record of the Mšec Lake deposits (Kladno-Rakovník Basin, Czech Republic). Palaeogeography, Palaeoclimatology, Palaeoecology 280: 78–93. Crossref
Long, J.A. 1988. New palaeoniscoid fishes from the Late Devonian and Early Carboniferous of Victoria. Memoirs of the Association of Australasian Palaeontologists 7: 1–64.
Lopez, M., Gand, G., Garric, J., Körner, F., and Schneider, J. 2008. The playa environments of the Lodève Permian basin (Languedoc-France). Journal of Iberian Geology 34: 29–56.
López-Arbarello, A., Rogers, R., and Puerta, P. 2006. Freshwater actinopterygians of the Los Rastros Formation (Triassic), Bermejo Basin, Argentina. Fossil Record 9: 238–258. Crossref
López-Arbarello, A., Rauhut, O.W.M., and Cerdeño, E. 2010. The Triassic fish faunas of the Cuyana Basin, western Argentina. Palaeontology 53: 249–276. Crossref
Lowney, K.A. 1980. A revision of the family Haplolepidae (Actinopterygii, Paleonisciformes) from Linton, Ohio (Westphalian D, Pennsylvanian). Journal of Paleontology 54: 942–953.
Lowney, K.A. 1983. The earliest known (Namurian A, E1) haplolepids (Osteichthyes: Actinopterygii). Transactions of the Royal Society of Edinburgh: Earth Sciences 74: 69–78. Crossref
Luccisano, V., Pradel, A., Amiot, R., Pouillon, J.-M., Kindlimann, R., Steyer, J.-S., and Cuny, G. 2022. Systematics, ontogeny and palaeobiogeography of the genus Orthacanthus (Diplodoselachidae, Xenacanthiformes) from the lower Permian of France. Papers in Palaeontology 8: e1470. Crossref
Luccisano, V., Cuny, G., Pradel, A., Fourel, F., Lécuyer, C., Pouillon, J.-M., Lachat, K., and Amiot, R. 2023. Palaeoenvironmental and palaeoecological reconstructions based on oxygen, carbon and sulfur isotopes of Early Permian shark spines from the French Massif central. Palaeogeography, Palaeoclimatology, Palaeoecology 628: 111760. Crossref
Lund, R. 2000. The new actinopterygian order Guildayichthyiformes from the Lower Carboniferous of Montana (USA). Geodiversitas 22: 171–206.
Lund, R. and Melton, W.G. 1982. A new actinopterygian fish from the Mississippian Bear Gulch Limestone of Montana. Palaeontology 25: 485–498.
Lund, R. and Poplin, C. 1997. The rhadinichthyids (paleoniscoid actinopterygians) from the Bear Gulch Limestone of Montana (USA, Lower Carboniferous). Journal of Vertebrate Paleontology 17: 466–486. Crossref
Martínek, K., Pešek, J., and Opluštil, S. 2017. Significant hiatuses in the terrestrial Late Variscan Central and Western Bohemian basins (Late Pennsylvanian–Early Cisuralian) and their possible tectonic and climatic links. Geologica Carpathica 68: 269–281. Crossref
Maxwell, E.E. and Stumpf, S. 2017. Revision of Saurorhynchus (Actinopterygii: Saurichthyidae) from the Early Jurassic of England and Germany. European Journal of Taxonomy 321: 1–29. Crossref
Meunier, F.J. and Brito, P.M. 2017. Histological characteristics of lower jaw bones and oral teeth of the short nose gar, Lepisosteus platostomus Rafinesque, 1820 (Lepisosteidae). Cybium 41: 279–286.
Meunier, F.J., Brito, P.M., and Leal, M.-E.C. 2013. Morphological and histological data on the structure of the lingual toothplate of Arapaima gigas (Osteoglossidae; Teleostei). Cybium 37: 263–271.
Meunier, F.J., Germain, D., and Otero, O. 2018a. A histological study of the lingual molariform teeth in Hyperopisus bebe (Mormyridae; Osteoglossomorpha). Cybium 42: 87–90.
Meunier, F.J., Mayrinck, D.D., and Brito, P.M. 2015a. Presence of plicidentine in the labial teeth of Hoplias aimara (Erythrinidae; Ostariophysi; Teleostei). Acta Zoologica 96: 174–180. Crossref
Meunier, F.J., Mondéjar-Fernández, J., Goussard, F., Clément, G., and Herbin, M. 2015b. Presence of plicidentine in the oral teeth of the coelacanth Latimeria chalumnae Smith 1939 (Sarcopterygii; Actinistia). Journal of Structural Biology 190: 31–37. Crossref
Meunier, F.J., Otero, O., and Laurin, M. 2018b. Histological study of the jaw teeth in the Devonian actinopterygian †Cheirolepis canadensis (Whiteaves). Cybium 42: 67–74.
Mickle, K.E. 2011. The early actinopterygian fauna of the Manning Canyon Shale Formation (upper Mississippian, lower Pennsylvanian) of Utah, U.S.A. Journal of Vertebrate Paleontology 31: 962–980. Crossref
Mickle, K.E., Lund, R., and Grogan, E.D. 2009. Three new palaeoniscoid fishes from the Bear Gulch Limestone (Serpukhovian, Mississippian) of Montana (USA) and the relationships of lower actinopterygians. Geodiversitas 31: 623–668. Crossref
Milner, A.C., Milner, A.R., and Walsh, S.A. 2009. A new specimen of Baphetes from Nýřany, Czech Republic and the intrinsic relationships of the Baphetidae. Acta Zoologica 90: 318–334. Crossref
Milner, A.R. 1980. The tetrapod assemblage from Nýřany, Czechoslovakia. In: A.L. Panchen (ed.), The Terrestrial Environment and the Origin of Land Vertebrates, 439–496. Academic Press, London.
Milner, A.R. 1987. The Westphalian tetrapod fauna; some aspects of its geography and ecology. Journal of the Geological Society 144: 495–506. Crossref
Neděla, V., Tihlaříková, E., Maxa, J., Imrichová, K., Bučko, M., and Gemeiner, P. 2020. Simulation-based optimisation of thermodynamic conditions in the ESEM for dynamical in-situ study of spherical polyelectrolyte complex particles in their native state. Ultramicroscopy 211: 112954. Crossref
Olive, S., Clément, G., and Pouillon, J.-M. 2012. First occurrence of the lungfish Sagenodus (Dipnoi, Sarcopterygii) from the Carboniferous Lagerstätte of Montceau-les-Mines, France. Journal of Vertebrate Paleontology 32: 285–295. Crossref
Opluštil, S., Martínek, K., and Tasáryová, Z. 2005. Facies and architectural analysis of fluvial deposits of the Nýřany Member and the Týnec Formation (Westphalian D–Barruelian) in the Kladno-Rakovník and Pilsen basins. Bulletin of Geosciences 80: 45–66.
Opluštil, S., Schmitz, M., Cleal, C.J., and Martínek, K. 2016. A review of the Middle–Late Pennsylvanian west European regional substages and floral biozones, and their correlation to the Geological Time Scale based on new U-Pb ages. Earth-Science Reviews 154: 301–335. Crossref
Opluštil, S., Zajíc, J., and Svoboda, J. 2022. Pralesy a jezera mladších prvohor: Když uhlí bylo ještě zelené. 423 pp. Academia, Praha.
Ørvig, T. 1978a. Microstructure and growth of the dermal skeleton in fossil actinopterygian fishes: Boreosomus, Plegmolepis and Gyrolepis. Zoologica Scripta 7: 125–144. Crossref
Ørvig, T. 1978b. Microstructure and growth of the dermal skeleton in fossil actinopterygian fishes: Nephrotus and Colobodus, with remarks on the dentition in other forms. Zoologica Scripta 7: 297–326. Crossref
Otero, R.A. 2022. Ray-finned fishes (Actinopterygii) from the Upper Jurassic (Oxfordian) of the Atacama Desert, Northern Chile. PeerJ 10: e13739. Crossref
Patterson, C. 1982. Morphology and interrelationships of primitive actinopterygian fishes. American Zoologist 22: 241–259. Crossref
Pearson, D.M. and Westoll, T.S. 1979. The Devonian actinopterygian Cheirolepis Agassiz. Transactions of the Royal Society of Edinburgh 70: 337–399. Crossref
Perrier, V. and Charbonnier, S. 2014. The Montceau-les-Mines Lagerstätte (Late Carboniferous, France). Comptes Rendus Palevol 13: 353–367. Crossref
Pešek, J. 1994. Carboniferous of Central and Western Bohemia (Czech Republic). 60 pp. Czech Geological Survey, Prague.
Pešek, J., Opluštil, S., Kumpera, O., Holub, V., and Skoček, V. 1998. Paleogeographic Atlas, Late Paleozoic and Triassic Formations, Czech Republic. 53 pp. Czech Geological Survey, Prague.
Pešek, J., Holub, V., Jaroš, J., Malý, L., Martínek, K., Prouza, V., Spudil, J., and Tásler, R. 2001. Geologie a ložiska svrchnopaleozoických limnických pánví České republiky. 243 pp. Český geologický ústav, Praha.
Piotrowski, T. and Northcutt, R.G. 1996. The cranial nerves of the Senegal bichir, Polypterus senegalus [Osteichthyes: Actinopterygii: Cladistia]. Brain, Behavior and Evolution 47: 55–102. Crossref
Pochat, S. and Van Den Driessche, J. 2011. Filling sequence in Late Paleozoic continental basins: a chimera of climate change? A new light shed given by the Graissessac-Lodève basin (SE France). Palaeogeography, Palaeoclimatology, Palaeoecology 302: 170–186. Crossref
Poplin, C. 1999. Un paléoniscoïde (Pisces, Actinopterygii) de Buxières-les-Mines, témoin des affinités fauniques entre Massif central et Bohême au passage Carbonifère–Permien. Geodiversitas 21: 147–155.
Poplin, C. and Heyler, D. 1993. The marginal teeth of the primitive fossil actinopterygians: systematics and evolution. In: U.H.J. Heidtke (ed.), New Research on Permo-Carboniferous Faunas, 113–124. Pollichia-Buch 29, Bad Dürkheim.
Poplin, C. and Lund, R. 2000. Two new deep-bodied palaeoniscoid actinopterygians from Bear Gulch (Montana, USA, Lower Carboniferous). Journal of Vertebrate Paleontology 20: 428–449. Crossref
Poplin, C., Sotty, D., and Janvier, P. 2001. Un Myxinoïde (Craniata, Hyperotreti) dans le Konservat-Lagerstätte Carbonifère supérieur de Montceau-les-Mines (Allier, France). Comptes Rendus de l’Académie des Sciences, Series IIA—Earth and Planetary Science 332: 345–350. Crossref
Porro, L.B., Rayfield, E.J., and Clack, J.A. 2015a. Computed tomography, anatomical description and three‐dimensional reconstruction of the lower jaw of Eusthenopteron foordi Whiteaves, 1881 from the Upper Devonian of Canada. Palaeontology 58: 1031–1047. Crossref
Porro, L.B., Rayfield, E.J., and Clack, J.A. 2015b. Descriptive anatomy and three-dimensional reconstruction of the skull of the early tetrapod Acanthostega gunnari Jarvik, 1952. PLOS ONE 10: e0118882. Crossref
Preuschoft, H., Reif, W.-E., Loitsch, C., and Tepe, E. 1991. The function of labyrinthodont teeth: big teeth in small jaws. In: N. Schmidt-Kittlerand and K. Vogel (eds.), Constructional Morphology and Evolution, 151–171. Springer-Verlag, Heidelberg. Crossref
Reisz, R. 1975. Pennsylvanian pelycosaurs from Linton, Ohio and Nýřany, Czechoslovakia. Journal of Paleontology 49: 522–527.
Richter, M. 1983. Ultra-estrutura de dentes de Paleoniscídeos (Pisces) do Grupo Passa Dois, RS, Brasil. Iheringia, Série Geología 8: 131–145.
Romer, A.S. 1930. The Pennsylvanian tetrapods of Linton, Ohio. Bulletin of the American Museum of Natural History 59: 77–147.
Sasagawa, I., Ishiyama, M., Yokosuka, H., Mikami, M., and Uchida, T. 2009. Tooth enamel and enameloid in actinopterygian fish. Frontiers of Materials Science 3: 174–182. Crossref
Schaumberg, G. 1996. Über wenig bekannte Acrolepiden aus dem oberpermischen Kupferschiefer und Marl-Slate von Deutschland und NE-England. Philippia 7: 325–354.
Schindler, T. 2018a. Neubeschreibung und erste Rekonstruktion von Elonichthys germari Giebel, 1848 (Pisces, Actinopterygii; Oberkarbon, Mitteldeutschland). Hallesches Jahrbuch für Geowissenschaften 41: 1–33.
Schindler, T. 2018b. Revision of Rhabdolepis macropterus (Bronn, 1829) (Osteichthyes, lower Actinopterygii; Lower Permian, SW Germany). PalZ 92: 651–660. Crossref
Schneider, J.W. 1996. Xenacanth teeth—a key for taxonomy and biostratigraphy. Modern Geology 20: 321–340.
Schneider, J.W. and Zajíc, J. 1994. Xenacanthiden (Pisces, Chondrichthyes) des mitteleuropäischen Oberkarbon und Perm – Revision der Originale zu Goldfuss 1847, Beyrich 1848, Kner 1867 und Fritsch 1879–1890. Freiberger Forschungsheft, C 452: 101–151.
Schoch, R.R. 2009. Life-cycle evolution as response to diverse lake habitats in Paleozoic amphibians. Evolution 63: 2738–2749. Crossref
Schoch, R.R. 2014. Amphibian Evolution: The Life of Early Land Vertebrates. 264 pp. Wiley-Blackwell, New York. Crossref
Schultze, H.-P. 1969. Die Faltenzähne der Rhipidistiiden Crossopterygier, der Tetrapoden und der Actinopterygier-Gattung Lepisosteus; nebst einer Beschreibung der Zahnstruktur von Onychodus (Struniiformer Crossopterygier). Palaeontographica Italica 65: 63–137.
Schultze, H.-P. 1970. Folded teeth and the monophyletic origin of tetrapods. American Museum Novitates 2408: 1–10.
Schultze, H.-P. 1993. The lungfish Sagenodus (Dipnoi, Osteichthyes) from the Upper Rotliegend (Lower Permian) of the Saar-Nahe Basin, SW-Germany. In: U.H.J. Heidtke (ed.), New Research on Permo-Carboniferous Faunas, 125–132. Pollichia-Buch 29, Bad Dürkheim.
Schultze, H.-P. 2009. Interpretation of marine and freshwater paleoenvironments in Permo-Carboniferous deposits. Palaeogeography, Palaeoclimatology, Palaeoecology 281: 126–136. Crossref
Schultze, H.-P. and Bardack, D. 1987. Diversity and size changes in palaeonisciform fishes (Actinopterygii, Pisces) from the Pennsylvanian Mazon Creek fauna, Illinois, U.S.A. Journal of Vertebrate Paleontology 7: 1–23. Crossref
Schultze, H.-P. and Chorn, J. 1997. The Permo-Carboniferous genus Sagenodus and the beginning of modern lungfish. Contributions to Zoology 67: 9–70. Crossref
Skoček, V. 1990. Stefanská jezernědeltová sekvence ve středních a severovýchodních Čechách. Sborník geologických věd 45: 91–122.
Soler-Gijón, R. 2004. Development and growth in xenacanth sharks: new data from Upper Carboniferous of Bohemia. In: G. Arratia, M.V.H. Wilson and R. Cloutier (eds.), Recent Advances in the Origin and Early Radiation of Vertebrates, 533–562. Verlag Dr. Friedrich Pfeil, München.
Soler-Gijón, R. and Díez Ruiz, A. 2023. “Carbonífero de Puertollano” Natural Monument (Puertollano basin, Spain): a window for the knowledge of early vertebrates. Spanish Journal of Palaeontology 38: 81–94. Crossref
Soler-Gijón, R. and Moratalla, J.J. 2001. Fish and tetrapod trace fossils from the Upper Carboniferous of Puertollano, Spain. Palaeogeography, Palaeoclimatology, Palaeoecology 171: 1–28. Crossref
Stack, J., Hodnett, J.-P., Lucas, S.G., and Sallan, L. 2021. Tanyrhinichthys mcallisteri, a long-rostrumed Pennsylvanian ray-finned fish (Actinopterygii) and the simultaneous appearance of novel ecomorphologies in Late Palaeozoic fishes. Zoological Journal of the Linnean Society 191: 347–374. Crossref
Štamberg, S. 1978. New data on Pyritocephalus sculptus (Pisces) from the Carboniferous of the Plzeň Basin. Paleontologická konference ’77 – Univerzita Karlova Praha 1978: 275–288.
Štamberg, S. 1989. Scales and their utilization for the determination of actinopterygian fishes (Actinopterygii) from Carboniferous basins of central Bohemia. Časopis pro mineralogii a geologii 34: 255–269.
Štamberg, S. 1991. Actinopterygians of the Central Bohemian Carboniferous basins. Acta Musei Nationalis Pragae, B 47: 25–102.
Štamberg, S. 2006. Carboniferous–Permian actinopterygian fishes of the continental basins of the Bohemian Massif, Czech Republic: an overview. In: S.G. Lucas, G. Cassinis, and J.W. Schneider (eds.), Non-marine Permian Biostratigraphy and Biochronology. Geological Society of London Special Publications 265: 217–230. Crossref
Štamberg, S. 2007. Permo-Carboniferous Actinopterygians of the Boskovice Graben, Part 1. Neslovicella, Bourbonnella, Letovichthys, Elonichthys. 155 pp. Muzeum východních Čech v Hradci Králové, Hradec Králové.
Štamberg, S. 2013. Knowledge of the Carboniferous and Permian actinopterygian fishes of the Bohemian Massif—100 years after Antonín Frič. Acta Musei Nationalis Pragae, B 69: 159–182. Crossref
Štamberg, S. 2016. A new actinopterygian species of Igornichthys Heyler, 1972 from the Permian of the Krkonoše Piedmont Basin (Bohemian Massif, Czech Republic), and its relationship to the actinopterygians of other European Permo-Carboniferous basins. Geodiversitas 38: 475–488. Crossref
Štamberg, S. 2018. Actinopterygians of the Permian locality Buxières-les-Mines (Bourbon-l’Archambault Basin, France) and their relationship to other early actinopterygians. Fossil Imprint 74: 245–291. Crossref
Štamberg, S. 2020. Teeth of actinopterygians from the Permo-Carboniferous of the Bohemian Massif with special reference to the teeth of Aeduellidae and Amblypteridae. Bulletin of Geosciences 95: 369–389. Crossref
Štamberg, S. and Steyer, J.-S. 2021. New actinopterygians from the Permian of the Brive Basin, and the ichthyofaunas of the French Massif Central. Fossil Imprint 77: 145–165. Crossref
Štamberg, S. and Zajíc, J. 2008. Carboniferous and Permian Faunas and Their Occurrence in the Limnic Basins of the Czech Republic. 224 pp. Muzeum východních Čech v Hradci Králové, Hradec Králové.
Steen, M.C. 1938. On the fossil Amphibia from the gas coal of Nýřany and other deposits in Czechoslovakia. Proceedings of the Zoological Society of London 108: 205–283. Crossref
Stelate, A., Tihlaříková, E., Schwarzerová, K., Neděla, V., and Petrášek, J. 2021. Correlative light-environmental scanning electron microscopy of plasma membrane efflux carriers of plant hormone auxin. Biomolecules 11: 1407. Crossref
Steyer, J.S., Escuillié, F., Pouillon, J.-M., Broutin, J., Debriette, P., Freytet, P., Gand, G., Poplin, C., Rage, J.-C., Rival, J., Schneider, J.W., Štamberg, S., Werneburg, R., and Cuny, G. 2000. New data on the flora and fauna from the ?uppermost Carboniferous–Lower Permian of Buxieres-les-Mines, Bourbon l’Archambault Basin (Allier, France); a preliminary report. Bulletin de la Société Géologique de France 171: 239–249. Crossref
Texereau, M., Germain, D., and Meunier, F.J. 2018. Comparative histology of caniniform teeth in some predatory ichthyophagous teleosts. Cybium 42: 75–81.
Tomes, C.S. 1878. On the structure and development of vascular dentine. Philosophical Transactions of the Royal Society of London 169: 25–47. Crossref
Traquair, R.H. 1877. The Ganoid fishes of the British Carboniferous Formations. Part I. Palaeoniscidae. Monographs of the Palaeontographical Society 31: 1–60. Crossref
Traquair, R.H. 1905. On the distribution of fossil fish-remains in the Carboniferous rocks of the Edinburgh District. Transactions of the Royal Society of Edinburgh 40: 687–707. Crossref
Traquair, R.H. 1907. The Ganoid fishes of the British Carboniferous Formations. Part I, No. 3. Palaeoniscidae. Monographs of the Palaeontographical Society 61: 87–106. Crossref
Traquair, R.H. 1909. The Ganoid fishes of the British Carboniferous Formations. Part I, No. 4. Palaeoniscidae. Monographs of the Palaeontographical Society 63: 107–122. Crossref
Viviani, J., LeBlanc, A., Rurua, V., Mou, T., Liao, V., Lecchini, D., Galzin, R., and Viriot, L. 2022. Plicidentine in the oral fangs of parrotfish (Scarinae, Labriformes). Journal of Anatomy 241: 601–615. Crossref
Warren, A.A. and Davey, L. 1992. Folded teeth in temnospondyls—a preliminary study. Alcheringa 16: 107–132. Crossref
Watson, D.M.S. and Gill, E.L. 1923. The structure of certain Palaeozoic Dipnoi. Zoological Journal of the Linnean Society 35: 163–216. Crossref
Westoll, T.S. 1944. The Haplolepidae, a new family of Late Carboniferous bony fishes: a study in taxonomy and evolution. Bulletin of the American Museum of Natural History 83: 1–122.
Woodward, A.S. 1891. Catalogue of the Fossil Fishes in the British Museum (Natural History), Part II. 567 pp. British Museum (Natural History), London.
Yushkevich, P.A., Piven, J., Hazlett, H.C., Smith, R.G., Ho, S., Gee, J.C., and Gerig, G. 2006. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. NeuroImage 31: 1116–1128. Crossref
Zajíc, J. 1988. Stratigraphic position of finds of the acanthodians (Acanthodii) in Czechoslovakia. Acta Universitatis Carolinae – Geologica 1986: 145–153.
Zajíc, J. 2006. The main fish communities of the limnic Permian and Carboniferous basins of Czech Republic. Scripta Facultatis Scientiarum Naturalium Universitatis Masarykianae Brunensis 33–34: 99–101.
Zhu, M., Zhao, W., Jia, L., Lu, J. Qiao, T., and Qu, Q. 2009. The oldest articulated osteichthyan reveals mosaic gnathostome characters. Nature 458: 469–474. Crossref
Acta Palaeontol. Pol. 69 (3): 501–522, 2024
https://doi.org/10.4202/app.01162.2024