


A new genus of Triassic discinid brachiopod and re-evaluating the taxonomy of the group—evolutionary insights into autecological innovation of post-Palaeozoic discinids
YOSHINO ISHIZAKI and YUTA SHIINO
Ishizaki, Y. and Shiino, Y. 2024. A new genus of Triassic discinid brachiopod and re-evaluating the taxonomy of the group—evolutionary insights into autecological innovation of post-Palaeozoic discinids. Acta Palaeontologica Polonica 69 (3): 529–548.
The discinid brachiopod from the Lower Triassic Osawa Formation in the Southern Kitakami Terrane, Japan, exhibited a unique morphological combination of a narrow pedicle track (listrium) and a V-shaped large depressed area, thereby suggesting an intermediate form between the Palaeozoic Orbiculoidea and the extant Discinisca. Based on these characteristics, we propose Bronzoria recta gen. et sp. nov., a genus that appeared in the late Permian and was widely distributed during the Triassic period. Morphological analysis of extant discinids revealed that the pedicle area showed an arrowhead-shaped median plate and a pair of semilunar plates, equivalent to the inner and outer listrial plates of Palaeozoic-type discinids, respectively. Consequently, there are great differences in the development of the pedicle area, i.e., the large pedicle area of extant discinids is suitable for robust pedicle attachment, whereas the narrow pedicle area of Bronzoria gen. nov. suggests a free-lying mode of life. Given the relationship between pedicle-related structures and the mode of life, we hypothesised that the evolution of the large depressed area preceded the development of the pedicle area. Subsequently, the large depressed area accommodated a larger pedicle, facilitating an autecological innovation for pedicle attachment, as observed in extant species.
Key words: Brachiopoda, Linguliformea, Discinidae, Orbiculoidea, Discinisca, exaptation, living fossil, stabilomorph, Olenekian, Triassic.
Yoshino Ishizaki [y-ishizaki@g.ecc.u-tokyo.ac.jp; ORCID: https://orcid.org/0009-0003-9914-2059 ], Department of Earth and Planetary Science, Graduate School of Science, the University of Tokyo; 7-3-1, Hongo, Bunkyo-ku, Tokyo, 113-0033, Japan.
Yuta Shiino [y-shiino@geo.sc.niigata-u.ac.jp; ORCID: https://orcid.org/0000-0001-8534-0941 ], Department of Geology, Faculty of Science, Niigata University; 8050, Ikarashi 2-no-cho, Nishi-ku, Niigata, 950-2181, Japan.
Received 18 April 2024, accepted 27 July 2024, published online 30 September 2024.
Copyright © 2024 Y. Ishizaki and Y. Shiino. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Understanding the morphological stability of fossil organisms provides fundamental insights into the constraints of phenotypic evolution over geological timescales, a subject traditionally referred to as living fossils (e.g., Lidgard and Love 2018; Wood et al. 2020). Organisms with a conserved phenotype are often considered to have successful forms owing to the lack of necessity to change their forms in response to environmental changes. Functionally sophisticated morphology could be maintained with minimal phenotypic variations over the long term, as observed in the case of stabilomorphs (Kin and Błażejowski 2014). However, morphological conservation does not necessarily imply ecological conservation (e.g., Kin and Błażejowski 2014; Werth and Shear 2014). Even small morphological differences, which may seem minor, can produce significant genetic, anatomical, and functional variations. These variations can potentially provide new adaptation capabilities (e.g., Koehl 1996; Shiino and Kuwazuru 2010). Considering their long-term evolutionary history, it is crucial to understand why certain organisms maintain conservative morphologies and whether species with conservative morphology are ecologically conservative.
Lingulate brachiopods are a group with a static morphology that persists from the Cambrian to the present (Holmer and Popov 2000; Liang et al. 2023). Geologically, lingulates, including lingulids and discinids, often occur in dysoxic/anoxic lithofacies and sometimes form monospecific assemblages (e.g., Dustira et al. 2013; Masunaga and Shiino 2021). To understand redox conditions, the fossil occurrences of lingulates have frequently been reported in geological studies described as “Lingula” for lingulid, “Orbiculoidea” for Palaeozoic discinid, and “Discinisca” for Mesozoic discinid. However, detailed information on their morphology and taxonomy has not been discussed (e.g., Souza et al. 2003). Recent anatomical information on lingulids, such as muscle scars and mantle canals, has allowed for a re-evaluation of the taxonomy of “Lingula”, thereby leading to the conclusion that the true Lingula Bruguière, 1791, existed only since the Cenozoic (Emig 2003). It could be expected that discinids were taxonomically variable as well, but their static morphology of simple discoidal shells prevented the understanding of the diversity and evolution of discinids over time.
Among discinid brachiopods, it has been assumed that the extant genus Discinisca Dall, 1871, evolved from the Palaeozoic genus Orbiculoidea d’Orbigny, 1847, around the Permian/Triassic boundary, which suggests a monophyletic relationship based on fossil records (Holmer and Popov 2000; Fig. 1). Both Discinisca and Orbiculoidea exhibit a sedentary mode of life; however, the extant Discinisca differs from Orbiculoidea in having a thick pedicle protruding from a large pedicle foramen for attachment to hard substrates (LaBarbera 1985; Mergl 2010). In the case of late Palaeozoic Orbiculoidea, having a smaller pedicle foramen located posteriorly, is interpreted as a free-lying mode of life (Masunaga and Shiino 2021). Based on the fossil records available thus far, evolution at the generic level has led to autecological innovation, specifically the acquisition of pedicle attachment to hard substrates. Otherwise, Mesozoic “Discinisca” may encompass a broader range of forms and adaptation capabilities than previously documented.
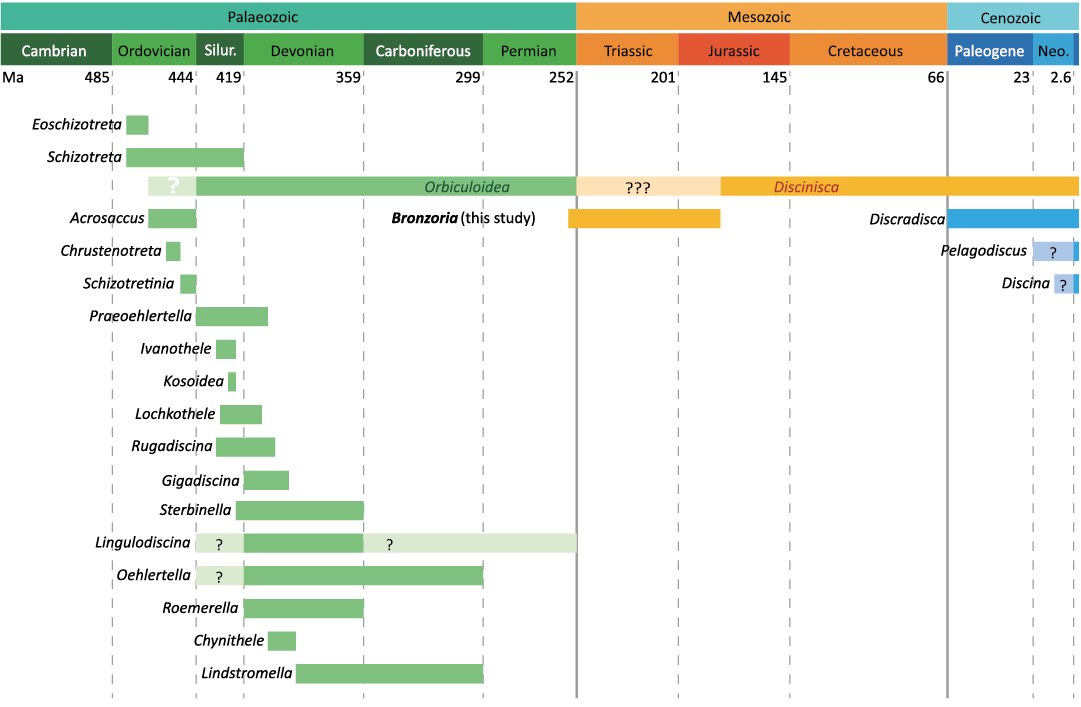
Fig. 1. Range chart of discinid genera. Based on Mergl and Massa (2005), Mergl (2006), and Curry and Brunton (2007). Abbreviations: Neo., Neogene; Silur., Silurian.
Understanding the autecological innovation of the pedicle attachment mode of life is to explore the morphology and taphonomy of Mesozoic discinids, which may bridge the gap between Orbiculoidea and extant Discinisca. Seilacher (1982) reported the Jurassic discinid Discinisca papyracea (Münster in Goldfuss, 1831) (= Orbiculoidea papyracea in Seilacher 1982), which shows cluster occurrences on ammonoid shells (Seilacher 1982). This clustering suggests the attachment of a pedicle protruding from a large arrowhead-shaped pedicle foramen in the ventral valve of Discinisca papyracea (see Seilacher 1982). Although reliable evidence exists regarding Jurassic discinids, whether Triassic discinids realised sessile pedicle attachment remains unclear. In addition, reports on fossil occurrences generally lack detailed taphonomic examinations.
Recently, we discovered discinid specimens in the Lower Triassic Osawa Formation in the Southern Kitakami Terrane, Japan, some of which were autochthonous (Ishizaki and Shiino 2023b). In a previous study (Murata 1973), the discinids from the Osawa Formation were identified as Orbiculoidea sp. cf. O. sibirica. However, some researchers have referred to them as Discinisca without formally describing their taxonomy (Radwański and Summesberger 2001). In this study, the taxonomy of Triassic discinid specimens from the Osawa Formation is re-examined, focusing on their ventral morphology. The ventral morphology of the present specimens was compared with that of the extant Discinisca and Discradisca Stenzel, 1964. Based on the present and previous descriptions, the morphological characteristics of post-Palaeozoic discinid brachiopods were reviewed. Finally, we discuss the relationship between the adaptation capability of discinids and their habitats, specifically addressing the likely process of autecological innovation within a seemingly conservative morphology.
Institutional abbreviations.—IGPS, Institute of Geology and Paleontology, Tohoku University, Sendai, Miyagi, Japan; UMUT, the University Museum, the University of Tokyo, Bunkyo-ku, Tokyo, Japan.
Nomenclatural acts.—This published work and the nomenclatural acts it contains have been registered in ZooBank: urn:lsid:zoobank.org:pub:DF44713F-9F10-442C-B922-99EE20BFACAE
Discinid terminology and diagnostic problems
Discinid brachiopods have been classified based on their convexity, with special reference to the anterior and posterior slopes, shape of the pedicle track, shell ornamentation, and pedicle-related structures (e.g., Mergl 2006, 2010; Zhang et al. 2014). Among these, there seems to be an inconsistency in the terminology of pedicle-related structures. Discinids generally have a slit-like opening just posterior to the ventral apex. This opening is for the pedicle, which is a crucial organ for anchoring or attaching individuals to the substratum (Rudwick 1970; James et al. 1992). In Orbiculoidea, the slit-like structure was sealed during growth, and the small pedicle foramen was positioned at the posterior end of the slit-like structure. This sealed structure forms a furrow-like surface called listrium (Williams and Rowell 1965; Holmer 1987). Since the external surface of the listrium is easily filled with sediment particles, many fossil discinids only exhibit an outline of the depression (e.g., Zhang et al. 2014). Instead, the term “pedicle track” is often used to describe the outline of the depression, regardless of the fossil preservation of the actual listrium (e.g., Zhang et al. 2014; Masunaga and Shiino 2021). Following previous studies, we adopted the term “pedicle track” as the outline of the listrium, and as the “listrium” when we see the plate-like shell inside the pedicle track. In Triassic discinids, a slit-like structure just posterior to the ventral apex extends to the posterior margin and is sometimes referred to as the “pedicle fissure” (Biernat 1995). Given the correspondence, the term “pedicle track” is used herein instead of the “pedicle fissure.”
The extant genera Discinisca, Discradisca, and Pelagodiscus Dall, 1908, are characterised by a “large depressed area” around the pedicle track. Triassic discinids also have a depressed area called the “adherent area” around the pedicle track (Biernat 1995), which may be compared with a large depressed area or pedicle area. We here adopted “large depressed area” and avoided using “adherent area” due to less morphological information in previous photographs (Biernat 1995).
Schematic illustrations of the valve morphology and terminology adopted herein are based on Williams et al. (1997) (Fig. 2). In this study, the term “pedicle-related structure” includes “pedicle foramen”, “pedicle track”, “listrium”, “pedicle area” and “large depressed area”.
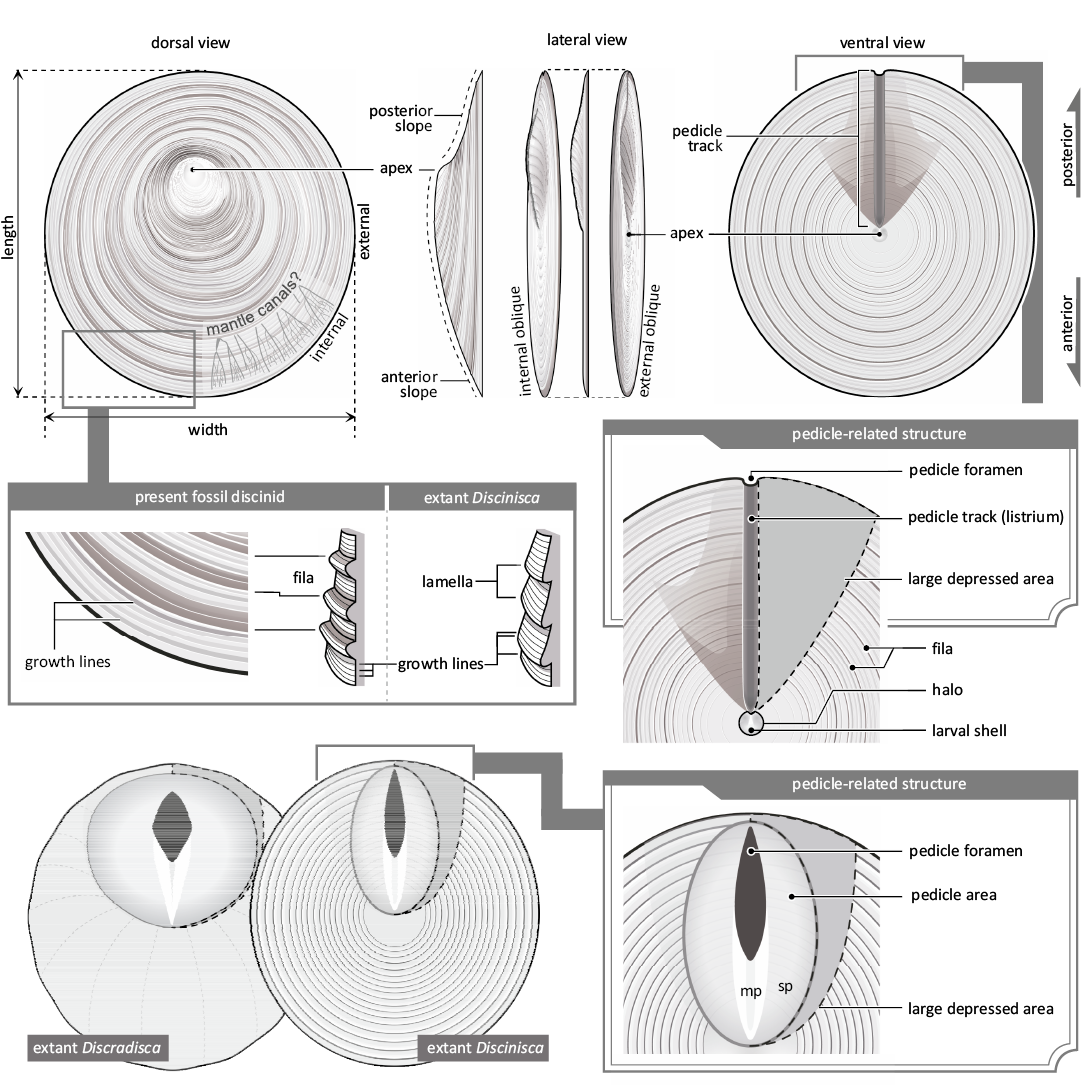
Fig. 2. Morphological terminology of discinid brachiopods. Based on the present fossil specimens and extant discinids of Discinisca and Discradisca. Abbreviations: mp, median plate (inner listrial plate); sp, semilunar plate (outer listrial plate).
Geological setting
The Lower Triassic strata, which yield discinid brachiopods, are well exposed in the Tatezaki area of Minamisanriku Town, Miyagi Prefecture, Japan (Fig. 3). The strata of the study area correspond to the Osawa Formation of the Inai Group in the Southern Kitakami Terrane. The Inai Group in the study area, which is a Lower–Middle Triassic sequence, consists of four formations: the Hiraiso, Osawa, Fukkoshi, and Isatomae, in ascending order (Kamada 1989). The Osawa Formation in the study area is subdivided into three units (Fig. 4, unit-1 to unit-3 in ascending order; Ishizaki and Shiino 2023b). Unit-1 is characterised by alternating beds of poorly sorted silty sandstone and current-rippled sandstone, unit-2 is characterised by sandy faintly laminated mudstone and turbidite successions, and unit-3 is characterised by alternating beds of laminated mudstone and current-rippled sandstone (Kamada 1993; Ehiro et al. 2019; Ishizaki and Shiino 2023b). All units are thought to have been deposited in a prodelta setting (Ishizaki and Shiino 2023b).
The discinid specimens occurred at three localities in unit-2, and there were four stratigraphic horizons of fossil occurrences (Figs. 3, 4). A variety of ammonoid, bivalve, thylacocephalan, ichthyopterygian, coprolite, and plant fossils also occurs from unit-2. Based on biostratigraphic studies using ammonoids, the Osawa Formation has been correlated with the Spathian (upper Olenekian) (Ichikawa 1951; Onuki and Bando 1959; Bando 1964; Bando and Ehiro 1982; Ehiro et al. 2019; Shigeta 2022).
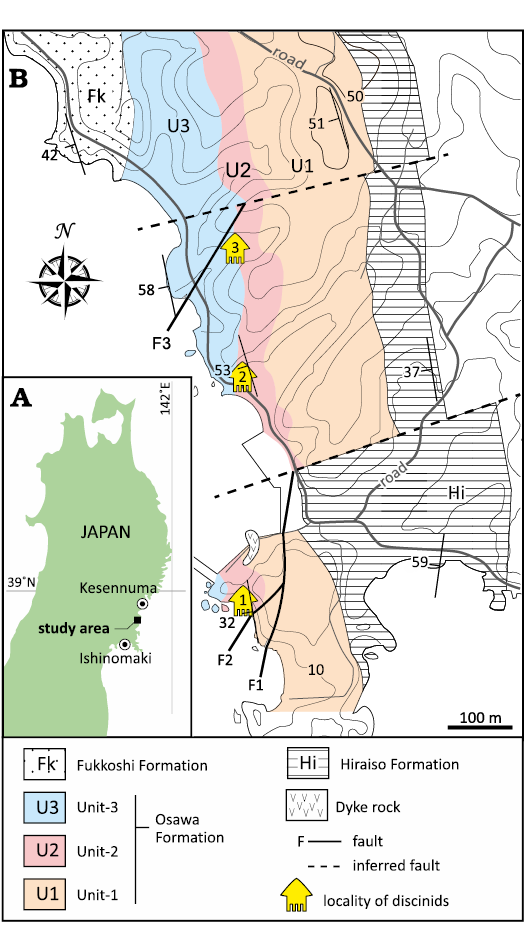
Fig. 3. A. Location of study area in the Tatezaki area, Japan. B. Geological map of the Tatezaki area, showing fossil localities (modified from Ishizaki and Shiino 2023b).
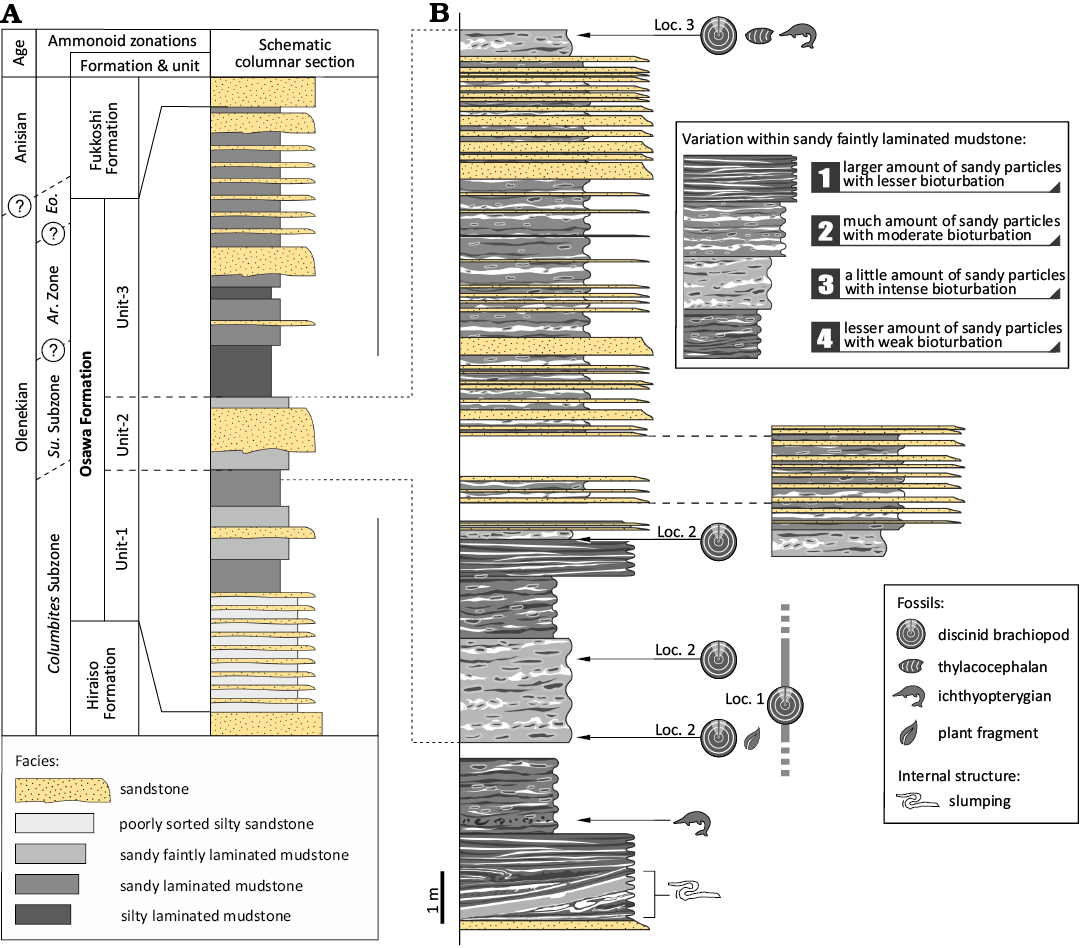
Fig. 4. A. Schematic columnar section and stratigraphic age based on Ishizaki and Shiino (2023b). B. Detailed columnar section of unit-2 based on the continuous outcrop at Locality 2. The stratigraphic horizons of localities 1 and 3 were inferred from Ishizaki and Shiino (2023b). Abbreviations: Ar., Arnautoceltites; Eo., Eodanubites Zone; Su., Subcolumbites.
Material and methods
Fossil specimens.—For morphological analysis, 57 discinid specimens from unit-2 of the Osawa Formation were used. In addition, we used four specimens (IGPS 92672–92675) previously reported by Murata (1973). The external and internal morphologies of specimens were observed in colour and greyscale photographs using a Sony α7R IV digital camera (Sony Corporation, Japan). Prior to greyscale photography, the specimens were whitened with ammonium chloride. A total of 57 discinid specimens from the Tatezaki area are housed at the Tohoku University Museum (IGPS 112854–112910).
Extant specimens.—To compare the pedicle-related structures of fossils and extant discinids, two dead specimens of Discinisca laevis (Sowerby, 1822) and 11 living specimens of Discradisca stella (Gould, 1862) were used. The specimens of Discinisca laevis are housed in the University Museum, the University of Tokyo (UMUT RB17215-1, -2), while those of Discradisca stella were collected from a tombolo (land-tied bar) on Hashira Island, Yamaguchi, Japan (34°00’56.6”N, 132°25’41.9”E; the sampling locality was followed that of Kato 1996). The beach of the tombolo is composed of boulders and gravel, and the interspaces are filled with coarse sand. The specimens attached to the underside of the boulders were slightly buried in the sand in the intertidal zone.
Discradisca stella was observed by removing the pedicle attached to a boulder, using a cutting knife. The ventral and dorsal valves were separated. After observing the relationship between pedicle and shell morphology, the soft parts were removed. Shell ornamentation in the pedicle area was observed. The external morphology of the specimen was examined in photographs using a Sony α7R IV digital camera (Sony Corporation, Japan) and images using a scanning electron microscope VE-8800 (Keyence, Japan).
Review.—Based on previous descriptions and the present morphological analysis, the morphological characteristics of post-Palaeozoic discinid brachiopods (four species of Triassic Orbiculoidea and 51 species of Mesozoic, Cenozoic, and extant discinid brachiopods) were reviewed. Here, we divided the morphology of post-Palaeozoic discinid brachiopods into two categories: dorsal and ventral forms.
In the dorsal valve, fossil species of Discinisca are traditionally subdivided into three types A, B, and C (Dall 1920). Type A features a large, lamellose shell ornamentation without costellae (e.g., Discinisca lamellosa [Broderip, 1833] and Discinisca laevis). In contrast, Type B exhibits a large, weakly lamellose shell ornamentation with faint, irregular costellae (e.g., Discradisca strigata [Broderip, 1833] and Discradisca cumingi [Broderip, 1833]). Type C is distinct for its small size and non-lamellose shell ornamentation with regular costellae (e.g., Discradisca stella and Discradisca antillarum [d’Orbigny, 1846]). Among these morphological characteristics, shell size is not a suitable morphological indicator, as each species can vary in size during growth and can be included in either large- or small-sized groups. Stenzel (1964) suggested dividing the genus based on the existence of costellae; therefore, some species in types B and C should be the derivative Discradisca rather than Discinisca. Recently, this suggestion has been followed, and costellae have been preferentially used for identification (Bitner and Cahuzac 2013; Dulai and Hocht 2020; Ishizaki and Shiino 2023a). Following this method, we divided the discinid dorsal form based on striation morphology.
The ventral valves of discinids are not well documented in living individuals or fossils. Species with information on the ventral valve were classified based on the morphology of the pedicle-related structure, with special reference to the characteristics of Orbiculoidea and extant Discinisca. This study particularly focused on the size and shape of the pedicle track and the large depressed area.
Systematic palaeontology
Class Lingulata Gorjansky and Popov, 1985
Order Lingulida Waagen, 1885
Superfamily Discinoidea Gray, 1840
Family Discinidae Gray, 1840
Genus Bronzoria nov.
Zoobank LSID: urn:lsid:zoobank.org:act:D7CB0815-D9EB-44BE-8046-194F01627DA5
Type species: Bronzoria recta gen. et sp. nov., see below.
Species included: Only those discinid species with ventral morphology reported can be compared to Bronzoria gen. nov. and either included into the genus, or excluded from it. Members of Bronzoria gen. nov. are at the least Orbiculoidea taskrestensis Dagys in Dagys and Kurushin, 1985, Bronzoria recta gen. et sp. nov., Discinisca sibirica (Moisseiev, 1947), Discinisca bosniaca (Kittl, 1904), Discinisca discoides (Schlotheim, 1820), Discinisca townshendi (Davidson, 1851) (= Discinisca babeana? [d’Orbigny 1849]), Discinisca rhaetica (Andreae, 1893), Discinisca zapfei Radwański and Summesberger, 2001, and Discinisca spitsbergensis Biernat, 1995.
Etymology: From the Bronzor in the Japanese videogame Pokémon, modelled after the ancient circular bronze mirror. The pattern of concentric circles and the posterior slit on the ventral side of the Bronzor resembles the morphology of the present genus.
Diagnosis.—The shell convexity is convexoplane-convexoconcave. The dorsal apex is located subposteriorly. The ventral apex is located subcentral. In the posterior part of the ventral valve, there is a V-shaped large depressed area and a narrow pedicle track with a small pedicle foramen. The outer surface ornamentation shows smooth to concentric ridges of the fila and is not lamellose.
Remarks.—Bronzoria gen. nov. is similar to Discinisca in having a large depressed area (e.g., Holmer and Popov 2000). However, the present genus, which is characterised by a narrow pedicle track, differs from Discinisca, which has a U-shaped or elliptical pedicle track (Table 1). Bronzoria gen. nov. also differs from Discinisca in that it lacks a lamellose shell. The present genus is similar to Orbiculoidea in having an elongate pedicle track with a small pedicle foramen (e.g., Mergl 2006; Zhang et al. 2014). However, the shape of the pedicle track in the genus is narrower than that of Orbiculoidea. A large depressed area is also unique to the present genus, which is not developed in Orbiculoidea (Table 1).
Table 1. Diagnostic characters of three discinid genera.
|
Pedicle-related structure |
Orbiculoidea |
Bronzoria |
Discinisca |
|
Large depressed area |
– |
V-shaped |
wide, U-shaped |
|
Pedicle track |
elongate oval |
narrow |
U- or elliptical-shaped |
|
Pedicle foramen |
small |
small |
large, oval |
|
Shell convexity |
strongly dorsibiconvex-convexoplane |
convexoplane-convexoconcave |
dorsibiconvex-convexoconcave |
|
Surface ornamentation |
well-developed concentric fila |
fine growth lines and fila |
fine growth lines and lamellae |
The internal posterior surface of the ventral valve is the potential attachment site of muscles (Holmer and Popov 2000). In this case, the presence of a large depressed area alters the valve opening and closure system, influencing the length and direction of muscle contractions. Additionally, the morphology of the pedicle track reflects the sedentary mode of life. Therefore, the morphological differences in pedicle-related structures among Bronzoria gen. nov., Discinisca and Orbiculoidea are considered to be generic-scale differences (Table 1).
Stratigraphic and geographic range.—The oldest species of this genus is Bronzoria bosniaca (Kittl, 1904) from the upper Permian, and the other species are from the Triassic and Middle Jurassic. The youngest species of this genus is Bronzoria townshendi (Davidson, 1851). Discinids assigned to Bronzoria have been reported from the United Kingdom, Spitsbergen, France, Germany, Austria, Slovenia, Bosnia, Siberia (Russia), and Japan (see SOM: table S1, Supplementary Online Material available at http://app.pan.pl/SOM/app69-Ishizaki_Shiino_SOM.pdf).
Bronzoria recta sp. nov.
Figs. 5–8.
1973 Orbiculoidea sp. cf. O. sibirica Moisseiev; Murata 1973: pl. 29: 16, 17.
2023 Orbiculoidea sp. cf. O. sibirica Moisseiev; Ishizaki and Shiino 2023b: fig. 12G.
ZooBank LSID: urn:lsid:zoobank.org:act:73725EC9-9A3E-41DF-85 B5-6D3A6948E166
Etymology: From Latin recta, straight; a narrow and straight pedicle track is the most notable characteristic of this species.
Type material: Holotype: dorsal and ventral valves, originally conjoined shell, IGPS 112854 (Fig. 5A). Paratypes: four supposed conjoined shells, IGPS 92672, 112855–112857 (Figs. 5B, C, 6B, E); three dorsal valves, IGPS 112858–112860 (Fig. 6); two ventral valves, IGPS 112861–112862 (Figs. 7, 8); all from type locality and horizon.
Type horizon: Middle part of the Osawa Formation (unit-2: Spathian, Olenekian), Lower Triassic.
Type locality: Tatezaki area in Minamisanriku Town, Miyagi Prefecture, Japan (Fig. 3).
Material.—Eight articulated shells, 33 dorsal valves, 15 ventral valves and one unknown valve (dorsal or ventral) were collected (IGPS 112854–112910). We also used four specimens (IGPS 92672–92675) reported as Orbiculoidea sp. cf. O. sibirica by Murata (1973). Although the original height of the shell and the 3D morphology of the shell slope were not preserved due to deformation, the overall morphology was well preserved. The shells were thin and blackish in colour.
Diagnosis.—Convexoplane to convexoconcave shell with a low conical dorsal valve. Both dorsal and ventral valves have comparatively dense, regularly arranged fila on the outer surface. The dorsal valve has a convex anterior slope and a concave posterior slope. The dorsal apex is subposterior to the centre. The ventral apex is almost central, with a posterior slope curving dorsally. The pedicle track is narrow and straight, extending to the posterior margin along the midline of a V-shaped large depressed area.
Description.—The shell convexity is convexoplane–convexoconcave with a subcircular outline (Figs. 5–8). The length and width of the shell are 7–13 mm and 6–14 mm, respectively. The outline of the smaller specimens tends to be a true circle, while that of the larger specimens changes to a weak trapezoid with a short, straight posterior margin (Figs. 5, 6).
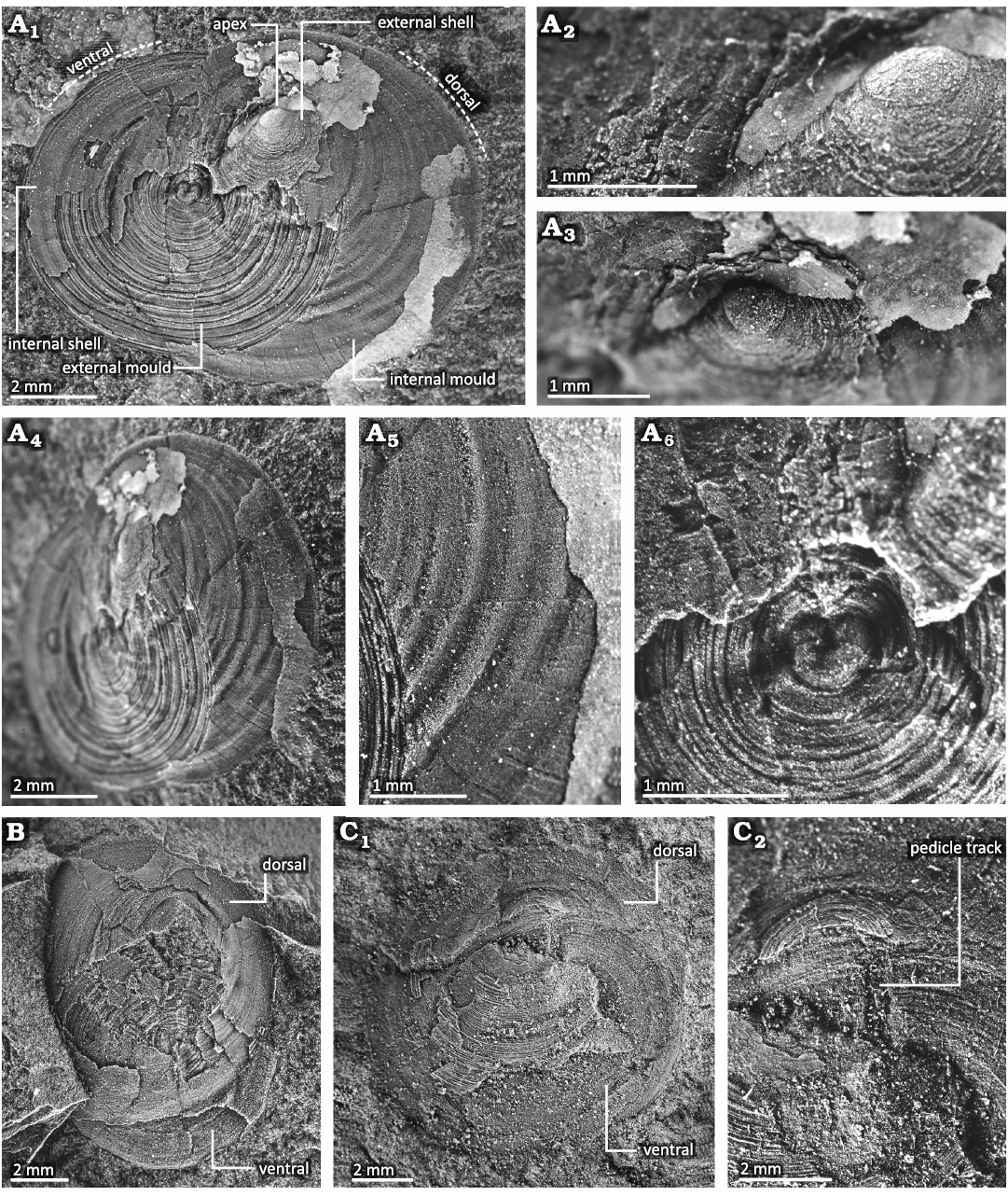
Fig. 5. Conjoined shell of discinid brachiopod Bronzoria recta gen. et sp. nov. from the Tatezaki area, Miyagi, Japan, Osawa Formation, Olenekian, Lower Triassic. A. IGPS 112854, holotype, internal surface of the dorsal valve and external surface of the ventral valve. The dorsal valve shows a smooth larval shell at its apex (A2, A3) and network-like thinner furrows on the internal mould (A4, A5). The centre of the ventral valve shows an external mould of the larval shell (A6). B. IGPS 112855, internal mould of the dorsal valve and external mould of the ventral valve. C. IGPS 112856, external mould of the dorsal valve and internal mould of the ventral valve (C1), and detail showing a pedicle track on the ventral valve (C2).
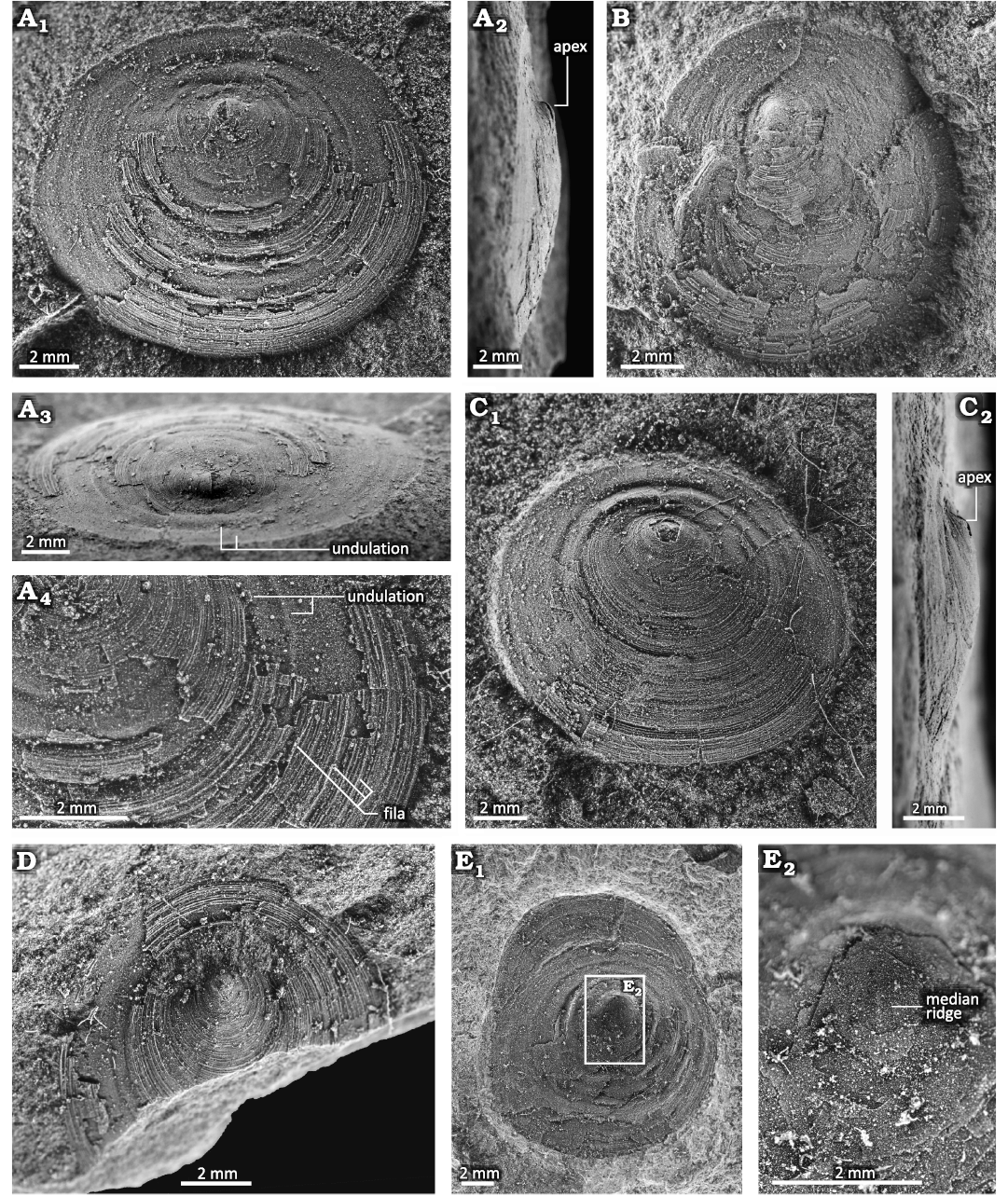
Fig. 6. Dorsal valve of discinid brachiopod Bronzoria recta gen. et sp. nov. from the Tatezaki area, Miyagi, Japan, Osawa Formation, Olenekian, Lower Triassic. A. IGPS 112858, internal mould with partially preserved external shell in the anterior half; dorsal (A1), lateral (A2), postero-oblique (A3) views and detail (A4). B. IGPS 112857, internal mould of the posterior half with external shell in part of the anterior half. The ventral shell is preserved anteriorly. C. IGPS 112859, external shell of anterior half and internal mould of posterior half; dorsal (C1) and lateral (C2) views. D. IGPS 112860, external shell in dorsal view. E. IGPS92672, internal surface of the dorsal valve (E1) as described by Murata (1973), detail (E2) shows a faint median ridge.
The dorsal valve is low conical in shape with 1.08–2.09 mm height, which is supposed to be lower than the original height. The apex is located posteriorly at one-third to one-fourth of its length from the posterior margin (Figs. 5, 6). The anterior slope is moderately convex (Fig. 6A2, C2). The posterior slope is overall concave (Fig. 6A–D), though its convexity changes during growth. The slope just posterior to the apex is moderately convex, and subsequently bent posteriorly to become a plane to a gently concave slope (Fig. 6A2, A3, C). The shell margin, including the posterior slope, is slightly bent ventrally (Fig. 6A2, C). In this configuration, the position of the apex moves backwards, with the larval shell facing the posterior direction (Figs. 5A, 6A, C). The lateral slopes are gently convex and smoothly transition into the convex anterior slope and concave posterior slope. The external surface of the dorsal valve exhibits numerous, distinct, and slightly elevated concentric growth lines. These growth lines are somewhat discontinuous and are arranged at intervals of approximately 0.06–0.07 mm (Fig. 6A4, D). Several growth lines are inserted into the anterior part. The growth lines periodically develop into higher-elevated concentric fila. The concentric fila interval is the broadest in the antero-median part, with 2–3 fila of 1 mm in length (Fig. 6A4). Both the growth lines and concentric fila are faint around the apex, while they are distinct near the shell margin. The concentric fila are more continuous than the growth lines. The internal surface of the dorsal valve is comparatively smooth with broad concentric undulations (Fig. 6A3). The undulations show low-height wavy outlines in cross sectional view, of which the width increases from 0.1 mm to 0.35 mm toward the shell margin (Figs. 5, 6). The larger specimen has a median septum just anterior to the apex (Fig. 6E). In the peripheral part of the internal surface, narrow and shallow furrows 0.02–0.04 mm in width extend radially (Fig. 5A5). From these furrows, network-like thinner furrows branch and extend radially, all of which are similar to mantle canals (Mergl and Massa 2005; Williams et al. 1997).
The ventral valve is flattened overall, and its apex is situated at the centre of the ventral valve (Figs. 5, 7). The margin of the ventral valve is slightly geniculated toward the ventral direction (Figs. 5A1, B, 7A1). The anterior and lateral slopes are flattened, while the posterior slope is concave, with its concavity change during growth (Fig. 7A, B). Along the median line, the posterior slope just posterior to the larval shell is strongly depressed and flattened (Figs. 7B, 8). In the posterior part, the posterior slope bends ventrally, forming a concave outline (Figs. 7B, 8). The peripheral part of the posterior slope is flattened with a geniculated margin. In the horizontal view, the concave area of the posterior slope exhibits a symmetric, V-shaped depression known as the large depressed area (Fig. 7A3, B3). The angle of the V shape is approximately 70°. In the anterior part of the large depressed area, the boundary with the lateral slope is geniculated, while in the posterior part is smooth (Fig. 7A, B). Similar to the dorsal valve, the external surface of the ventral valve exhibits numerous and distinct, slightly elevated concentric growth lines (Fig. 7A, B). These growth lines are irregular and arranged in approximately 0.02–0.04 mm intervals. The growth lines periodically develop into higher elevated and concentric fila, with intervals of 3–4 ridges of 1 mm in length (Fig. 7B4). The concentric growth lines and the fila of the ventral valve are elevated more vertically than those of the dorsal valve. Growth lines and fila are faint around the apex, while they become distinct near the shell margin.
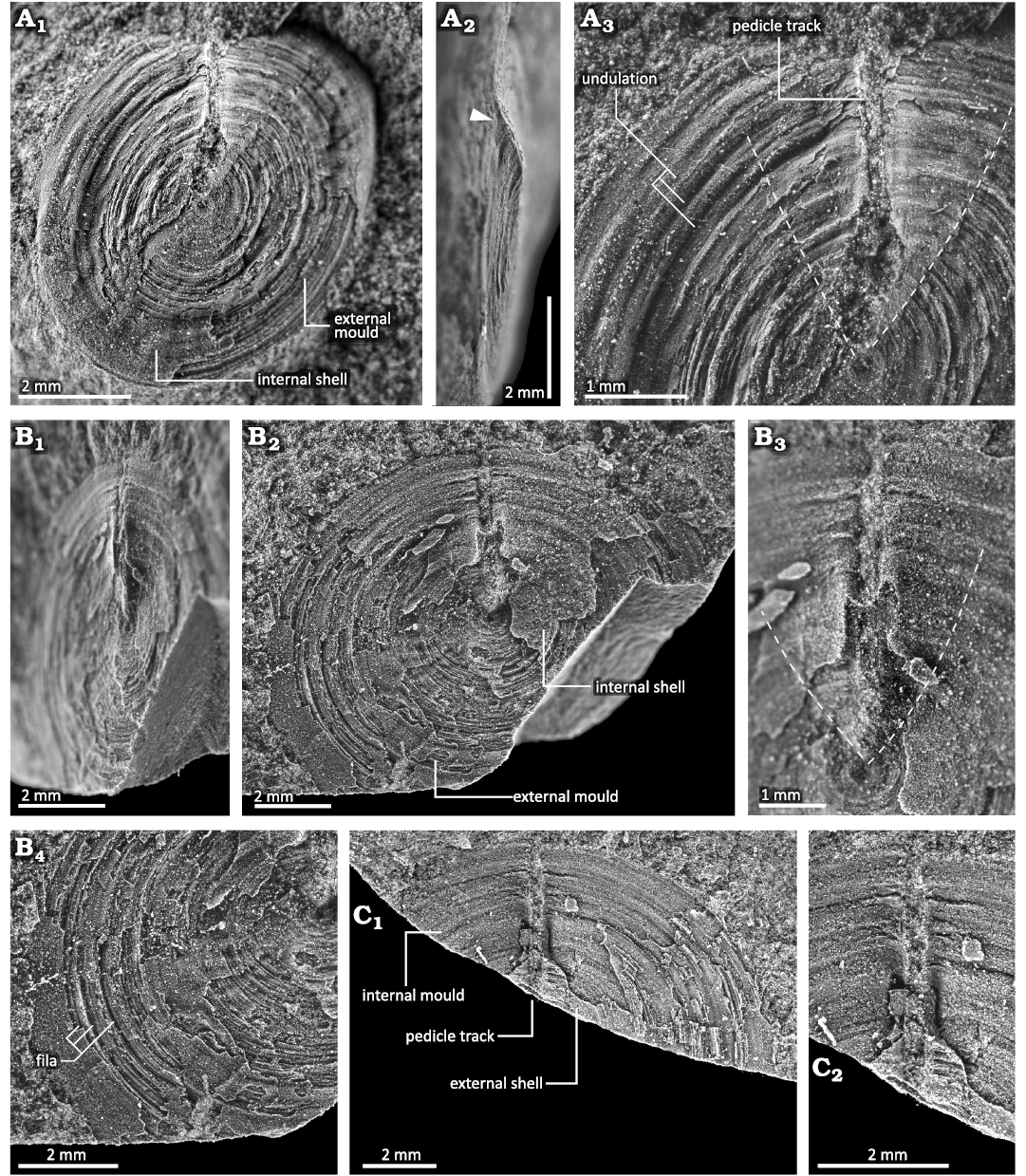
Fig. 7. Ventral valve of discinid brachiopod Bronzoria recta gen. et sp. nov. from the Tatezaki area, Miyagi, Japan, Osawa Formation, Olenekian, Lower Triassic. A. IGPS 112861, internal shell with a partially exposed external mould on the anterior right part (A1).The white arrowhead in the lateral view (A2) shows the posterior end of the ventral valve, which remains a furrow of the pedicle track. The area around the pedicle track posterior to the dashed line (A3) is slightly elevated, forming V-shaped large depressed area in the external view. B. IGPS 112862b, internal shell around the pedicle track and external mould in the anterior half, in oblique (B1) and dorsal (B2) views. The area around the pedicle track posterior to the dashed line (B3) is slightly elevated, similar to that in A3. Anterior half of B2 showing fila (B4). C. IGPS 112862a, internal mould with partially preserved external shell at the anterior part around the listrium (C1, counterpart of B). Detail showing a pedicle track (C2).

Fig. 8. Colour photographs of ventral morphology around the pedicle track of discinid brachiopod Bronzoria recta gen. et sp. nov. from the Tatezaki area, Miyagi, Japan, Osawa Formation, Olenekian, Lower Triassic. A. IGPS 112862a, internal mould with partially preserved external shell at the anterior part around the listrium (the same specimen as in Fig. 7C), in ventral (A1), oblique (A2), and anterior (A3) views. B. IGPS 112862b, internal shell around pedicle track (the same specimen in Fig. 7B), in dorsal (B1), oblique (B2), and antero-oblique (B3) views. Note that the listrium has a convex shape just posterior to the apex, a ridge shape in the middle, and a flat shape in the posterior area (B2, B3: three white arrowheads).
The narrow, longitudinal pedicle track is positioned just posterior to the ventral apex, which is approximately 0.3 mm wide, extending to the posterior margin (Figs. 5C, 7, 8). On the external surface, the listrium is in the depressed area toward the midline of the posterior slope, although it is filled with muddy sediments (Figs. 7C, 8A). Thus, detailed information regarding the morphology and surface ornamentation of the listrium is unknown. The pedicle foramen is also invisible due to filled sediments. However, given that the pedicle track extends to the shell margin, the pedicle foramen is undoubtedly located at the posterior margin and opens outwards (Figs. 7A2, B, 8). On the internal surface, the listrium has a shape different from that seen in the external view. An anterior part of the listrium is convex, corresponding to the depression observed on the external surface (Fig. 8B: arrowhead near the apex). In the middle part of the listrium, around the most depressed part on the posterior slope, the shells beside the pedicle track are elevated to the ridges; therefore, the middle part of the listrium appears depressed (Fig. 8B: arrowhead of the middle). In the posterior part, around the shell margin, the elevated ridges parallel to the listrium become weak and obscure (Fig. 8B: arrowhead near the posterior margin).
The larval shells are well-preserved in the dorsal and ventral valves, both of which have unique ornamentation with distinct boundaries (Fig. 5A). In the dorsal valve, the larval shell has a subcircular outline and was approximately 600 µm in diameter (Fig. 5A2, A3). All slopes of the larval shell are convex with a smooth surface. In the ventral valve, the larval shell has a subcircular outline, approximately 600 µm in diameter (Fig. 5A6). In the best-preserved specimen, the outline of the larval shell is heart-shaped. Similar to the dorsal larval shell, the ventral larval shell has a smooth surface but shows a variety of convexities. The peripheral part of the anterior to postero-lateral slope is moderately concave. The postero-median part has a slit-like ridge 40 µm wide, extending from the centre to the posterior margin of the halo. The slit-like ridge changes wider and lower toward the posterior margin of the halo, connecting to the pedicle track. The central part of the larval shell is gently convex.
Remarks.—Bronzoria recta gen. et sp. nov. is similar to Bronzoria sibirica (Moisseiev, 1947), in having a subcircular outline approximately 10 mm in size with convexoplane convexity. In particular, the narrow, longitudinal pedicle track extending to the posterior margin with the V-shaped large depressed area in Bronzoria sibirica is extremely similar to that of the present species. Murata (1973) described this species as Orbiculoidea sp. cf. O. sibirica based on its size, flattened ventral valve, and narrow pedicle track. However, the position of the dorsal apex in Bronzoria recta gen. et sp. nov. is more anterior than that in Bronzoria sibirica, with its apex located posteriorly at one-fifth to one-ninth of its length (Dagys 1965). Moreover, the dorsal valve of the present species has a posterior slope with a concave outline, which differs from the gently convex posterior slope of Bronzoria sibirica. The dorsal convexity around the apex can be strongly related to the muscular system and the volume of soft parts (Masunaga and Shiino 2021); therefore, this is not Bronzoria sibirica but a previously undescribed species.
In the Early Triassic, some discinid brachiopods exhibited a ventral morphology similar to that of Bronzoria recta gen. et sp. nov. (Kittl 1904; Wagner 1913; Dagys 1965). According to Radwański and Summesberger (2001), three discinid species are categorised as “slightly (?) older Triassic forms”: Bronzoria sibirica, Bronzoria bosniaca (Kittl, 1904) and Bronzoria major (Wagner, 1913). Bronzoria bosniaca and Bronzoria major are characterised by a narrow, longitudinal pedicle track extending to the posterior margin with the V-shaped large depressed area, as observed in Bronzoria recta gen. et sp. nov. (Kittl 1904; Wagner 1913). These two species are also similar to this species in their low conical, convexoplane shell and the position of the dorsal apex, but they differ in their larger shell size (Bronzoria bosniaca: 30 mm in diameter, Bronzoria major: 43 mm in diameter) and wider intervals of the fila compared to the present species. A similar morphology of convexity has also been reported in the Late Triassic Bronzoria rhaetica (Andreae, 1893). However, the latter species differs from Bronzoria recta gen. et sp. nov. in its larger size (32 mm in diameter), a pedicle track widening toward the posterior margin, and a trapezoid outline with a long, straight posterior margin (Andreae 1893).
Stratigraphic and geographic range.—Osawa Formation, Spathian (upper Olenekian, Lower Triassic); probably within the Southern Kitakami Terrane, Japan.
Pedicle-related structure in extant and fossil discinids
Morphology of pedicle-related structures in extant discinids.—Discinisca laevis and Discradisca stella have a U-shaped large depressed area in the posterior half of the ventral valve (Fig. 9). A large pedicle area develops within this depression, but it is smaller than the large depressed area (Fig. 9A1, A4 B2, C3). The pedicle area is oval in Discinisca laevis (Fig. 9A, B), whereas it is heart-shaped in Discradisca stella (Fig. 9C).
In Discinisca laevis, the pedicle area is strongly concave with its external surface displaying growth lines (Fig. 9A4, B3). In contrast, the external surface, except for the pedicle area is characterised by concentric lamellae (Fig. 9A4, B3), showing a great disparity in surface ornamentation. The pedicle area is subdivided into two regions: a narrow, arrowhead-shaped median plate just anterior to the elongate oval pedicle foramen (Fig. 9A4, B3) and a pair of semilunar plates on the right and left sides (Fig. 9A4, B3). The former median plate is concave as it is a furrow-like depression (Fig. 9A4, B3). The growth lines on the median plate are correlated with the anterior curvature of the pedicle foramen (Fig. 9B3).
In living Discradisca stella, the pedicle covers the entire external surface of the pedicle area. The pedicle area is slightly concave and milky-white colour (Fig. 9C2). The external surface of the pedicle area appears smooth but exhibits faint growth lines (Fig. 9C2), while the outside of the external surface has radial ridges, the so-called costellae (Fig. 9C2, C3). The pedicle foramen is elongated oval located posteriorly in the pedicle area (Fig. 9C3). Similar to Discinisca laevis, the pedicle area of Discradisca stella shows an arrowhead-shaped median plate (Fig. 9C4) and a pair of semilunar plates (Fig. 9C4). The median plate is comparatively flat, with a thin ridge along the midline (Fig. 9C4).
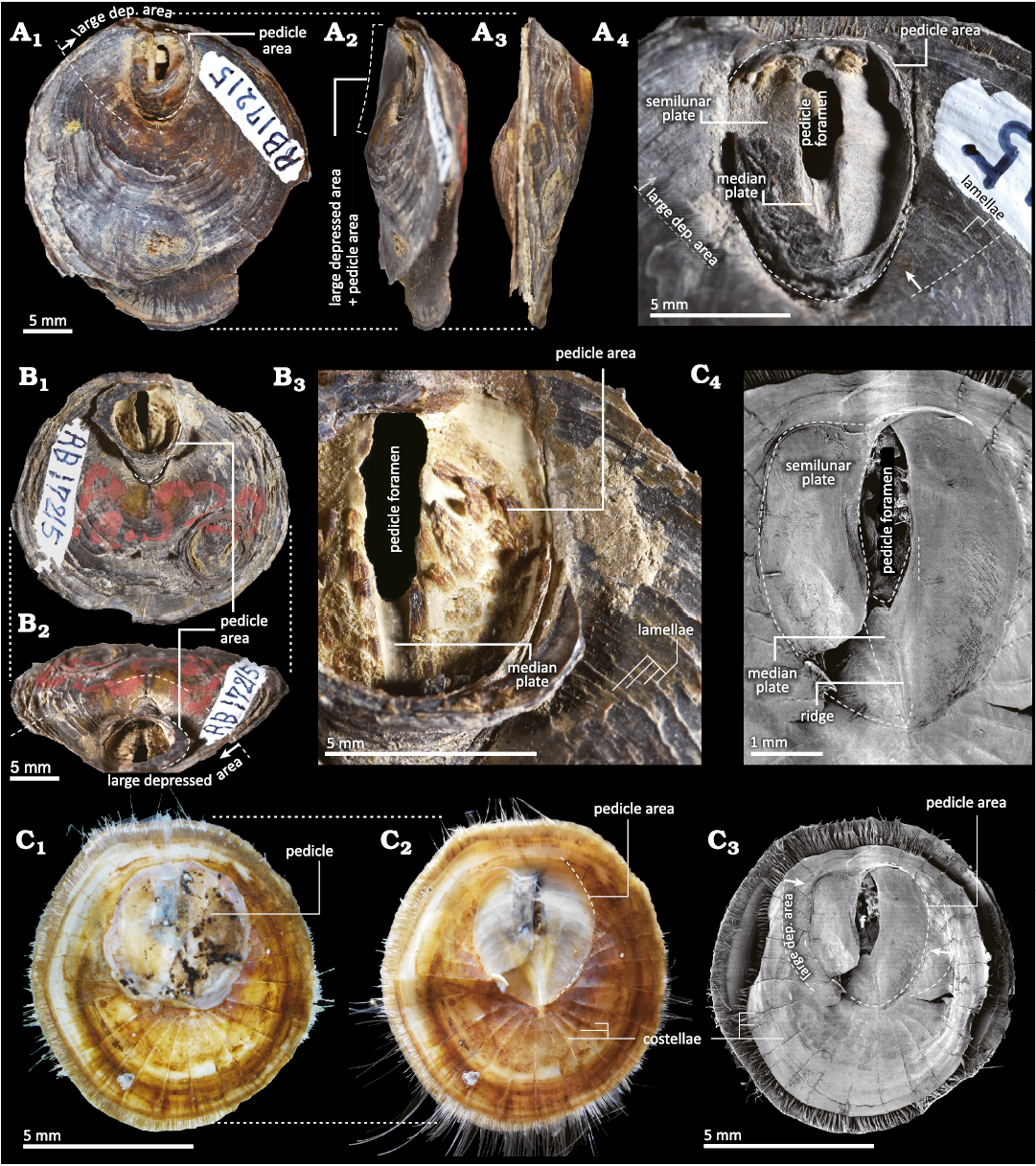
Fig. 9. Morphology of extant discinids. A, B. Discinisca laevis (Sowerby, 1822) from Calles, Peru, Recent. A. UMUT RB17215-1 in ventral (A1), oblique lateral (A2), and lateral (A3) views, detail of the large depressed area (A4). B. UMUT RB17215-2 in ventral (B1) and oblique posterior (B2) views, detail of the large depressed area (B3). C. Discradisca stella (Gould, 1862) from Hashira Island, Yamaguchi, Japan, Recent. UMUT RB34200 in ventral view. Colour photographs with (C1) and without (C2) pedicle and SEM images (C3, C4) showing the relationship among pedicle-related structures. The shell outline in C3 is slightly deformed owing to drying. Abbreviation: dep., depressed.
Review of post-Palaeozoic discinid morphology.—The morphological characteristics of four species of the Triassic Orbiculoidea and 55 species of the Mesozoic, Cenozoic, and extant discinid genera are shown in SOM: table S1.
In previous studies, Triassic Orbiculoidea included Orbiculoidea yangkangensis Xu and Liu, 1983, Orbiculoidea qieermaensis Xu and Liu, 1983, Orbiculoidea taskrestensis Dagys in Dagys and Kurushin, 1985, and Orbiculoidea winsnesi Gobbett, 1963. Of these, Orbiculoidea winsnesi was identified by Foster et al. (2017) using only the morphology of the dorsal valve, which seems doubtful for identifying it as O. winsnesi was originally described by Gobbett (1963) based on specimens from the middle Carboniferous. The four species of Triassic Orbiculoidea are characterised by subcircular outlines, low conical shapes, and fine concentric growth lines (Xu and Liu 1983; Dagys and Kurushin 1985; Foster et al. 2017). Orbiculoidea yangkangensis and O. qieermaensis have a biconvex shell with short, discontinuous costellae in their dorsal valve, while O. taskrestensis and O. winsnesi of Foster et al. (2017) have a convexoconcave shell. Their ventral valves, except for those of O. winsnesi described by Foster et al. (2017), exhibit different morphological characteristics of pedicle-related structure. Orbiculoidea yangkangensis and O. qieermaensis are characterised by short, narrow pedicle tracks just posterior to the ventral apex without a V-shaped large depressed area (Xu and Liu 1983), as seen in Bronzoria recta gen. et sp. nov. These pedicle-related structural characteristics are typical of Orbiculoidea. In contrast, O. taskrestensis has a short, inverted triangle-shaped pedicle track just posterior to the ventral apex, with a V-shaped large depressed area (Dagys and Kurushin 1985), similar to Bronzoria recta gen. et sp. nov.
In this review, all species of discinid brachiopods, except Orbiculoidea are characterised by having convexoplane–convexoconcave shells with fine, concentric growth lines (see SOM: table S1). Among these, the ventral valves of three genera (Discinisca, Discradisca, and Pelagodiscus) definitely have a pedicle track within a large depressed area, although information regarding the ventral valve is lacking for many species. The dorsal form was subdivided into three types based on the development of the costellae (types A, B, and C), while the ventral form of the pedicle track and large depressed area was subdivided into three types (types 1, 2, and 3) (Fig. 10; see also SOM: table S1).
Among the 55 species from the late Permian to the present, there are 28 species of type A, 9 species of type B, and 18 species of type C (Fig. 10; see also SOM: table S1). The dorsal valve of type A, which is characterised by a lack of costellae, is 1.5–43 mm in size, with a subcentral to posterior (1/3–1/20 of the length from the posterior margin) apex position. The dorsal valve of type B, characterised by weak or partly visible costellae, is 6.5–24 mm in size, with a subcentral to posterior (1/3–1/10 of the length from the posterior margin) apex position. The dorsal valve of type C, characterised by distinct fine costellae, is 2.3–35 mm in size with a subcentral–posterior (central–1/7 of the length from the posterior margin) apex position. The dorsal valve is slightly larger than the ventral valve.
Ventral valve morphology was described in 24 species: type 1 for 9 species, type 2 for 9 species, and type 3 for 7 species.
The type 1 ventral valve has a narrow, longitudinal pedicle track on the midline of the V-shaped large depressed area, as seen in the present Bronzoria recta gen. et sp. nov. Some type 1 species exhibit pedicle track widening toward the posterior margin (see SOM: table S1). The external surface of the large depressed area exhibits fine concentric growth lines that are continuous with those in the peripheral part of the large depressed area. The pedicle foramen is invisible, possibly due to filled sediment.
The type 2 ventral valve has a pedicle area slightly inside the large depressed area. The pedicle area in smaller individuals is U-shaped, with its pedicle foramen opened posteriorly, while that in larger individuals shows an elliptical shape by closing the pedicle foramen (Mergl 2010). In larger individuals, the pedicle foramen is large and oval, with an arrowhead-shaped plate anterior to the pedicle foramen. The external surface of the pedicle area exhibits faint concentric growth lines, which differ greatly from those of other areas with distinct growth lines. The ventral valve of Discinisca papyracea (Münster in Goldfuss, 1831) is illustrated as having a V-shaped pedicle area and costellae on the peripheral part of the large depressed area (Seilacher 1982). These features are different from those of type 2; however, the shape of the pedicle area is most similar to that of type 2. In Discinisca elslooensis Radwańska and Radwański, 2003, the pedicle area seems to be U-shaped, though the posterior parts of the specimens are broken (Radwańska and Radwański 2003). This leads to the possibility that the species belongs to the genus Discina Lamarck, 1819, which is characterised by a thick shell, short slit-like structure (= pedicle track of Discina in Holmer and Popov 2000; = pedicle slit of D. elslooensis in Radwańska and Radwański 2003), and a well-developed median septum in the ventral valve. However, we adopted the original descriptions of the authors and subdivided this species into type 2 based on the ventral valve.
The type 3 ventral valve has an oval to heart-shaped pedicle area, which is much wider than type 2. The pedicle foramen is also larger and wider than that in type 2. Similar to type 2, an arrowhead-shaped plate anterior to the pedicle foramen is present. The external surface of the pedicle area appears smooth but exhibits faint concentric growth lines, which differ greatly from those of the others with distinct growth lines and costellae.
Discussion
Occurrence and ecology of Bronzoria recta gen. et sp. nov.—All specimens occur in sandy faintly laminated mudstone layers in unit-2 of the Osawa Formation (Fig. 4: facies 1 and 3), with a large amount of small organic matter and plant fragments (Ishizaki and Shiino 2023b). There are four stratigraphic horizons of fossil occurrences, and the layers show slight variations in the amount of sandy particles and the intensity of bioturbation (Fig. 4). Among the layers, the lower two layers and the upper layer of fossil occurrences are characterised by a small amount of sandy particles with intense bioturbation (Fig. 4: facies 3), while the middle layer is characterised by a larger amount of sandy particles with less bioturbation (Fig. 4: facies 1).
The present specimens are autochthonous or parautochthonous elements with a preserved life posture based on the presence of conjoined valves and their occurrence of convex-up orientation (Ishizaki and Shiino 2023b). According to previous studies, extant species of Discinisca are present on hard substrates by way of pedicle attachment (e.g., Dall 1920). Due to this mode of life, extant species occasionally exhibit cluster assemblages on surfaces such as small pebbles and bioclasts when other attachment site is dispersed and restricted (Mergl and Massa 2005; Mergl 2010). However, in the present specimens, we did not find any other pebbles or bioclasts near the discinid specimens that could have been candidates for hard substrates for pedicle attachment in adult stage. Given that the specimens show a dispersed arrangement but are not densely colonised, the present species had a free-lying mode of life to muddy sediment, as observed in the Palaeozoic discinid Orbiculoidea (Mergl 2006; Masunaga and Shiino 2021).
Sedimentary analyses revealed that the habitat of the present species was near the fan lobe of the prodelta (distal outer shelf) (Ishizaki and Shiino 2023b). Muddy substrates were comparatively oxic because the sandy supply modified benthic redox conditions episodically. As a result, inhabitation by the opportunistic Bronzoria recta gen. et sp. nov. could have occurred during oxic episodes in the vicinity of the fan lobe, supplying a larger amount of food matter. However, the environment without the sandy supply was originally dysoxic (Ishizaki and Shiino 2023b).
Comparison of pedicle-related structures of fossil and extant discinids.—Although the terminology differs, the morphological combination of pedicle-related structures in extant discinids is very similar to that of the Palaeozoic discinid Orbiculoidea. Based on the present observation of extant discinids, the arrowhead-shaped median plate anterior to the pedicle foramen and the pair of semilunar plates are comparable to the inner and outer listrial plates, respectively (Masunaga and Shiino 2021; Fig. 11). As observed in extant discinids, the width of the arrowhead-shaped median plate is similar to that of the pedicle foramen. Given the pattern of the growth lines, the formation of the median plate is a result of stepwise secretions to seal the anterior part of the pedicle foramen as growth.
Following the morphological characteristics observed in extant discinids, we realised that Bronzoria recta gen. et sp. nov. has an extremely narrow pedicle area, as seen in the pedicle track. It is clear from the surface ornamentation that the pedicle area exists only within the pedicle track and does not reach a large depressed area. A similar morphology with a small pedicle foramen is abundant in Palaeozoic discinids, such as Acrosaccus and Orbiculoidea (e.g., Holmer 1987; Mergl 2001; Masunaga and Shiino 2021), which may have a similar pedicle.
Morphology and adaptation capability of the pedicle-related structure.—Based on fossil occurrences, Bronzoria recta gen. et sp. nov. adopted a free-lying mode of life on muddy bottoms. This autecological property is similar to that of Orbiculoidea species such as Orbiculoidea verum Masunaga and Shiino, 2021. However, it has been suggested that most Mesozoic discinids were attached to hard substrates such as ammonite shells (Tate 1867; Boehm 1911; Seilacher 1982). Pedicle attachment to hard substrates such as shells and boulders is also common in extant discinids (e.g., LaBarbera 1985; Mergl 2010), which appears to contradict Bronzoria recta gen. et sp. nov. Understanding discinid adaptation requires examining the relationship between pedicle-related structures and the use of pedicles.
Extant Discinisca and Discradisca adopt a sessile mode of life by attaching with a thick, short pedicle onto a hard substrate (Emig 1997; Mergl 2010). Morphological observations of Discradisca stella revealed that the pedicle broadly covers a large depressed area, with the coated surface of the ventral valve exhibiting different ornamentation. This large depressed area accommodates a substantial amount of pedicle tissues as seen in pedicle area, ensuring a rigid attachment with no gap between the ventral valve and the substrate. In the present species, the ventral valve has no wide pedicle area but a narrow pedicle track, suggesting no feasibility for a sessile mode of life similar to extant discinids (Fig. 11).
In late Palaeozoic Orbiculoidea, the ventral valve typically has a narrow, long pedicle track with a pedicle foramen at the posterior end. The pedicle foramen, which is small (possibly less than a millimetre), suggests the presence of a thin, elongate pedicle. This pedicle indicates penetrating into firm but supple substrates and soft substrates, such as loose fine skeletal debris and mud, respectively (Mergl 2001; Masunaga and Shiino 2021). The direction of extension of the supposed pedicle may play a role in maintaining posture, even if the supposed pedicle is not as thick (Masunaga and Shiino 2021). Such a well-known relationship between the mode of life and the development of the pedicle track in Orbiculoidea fits the present conclusion that the present species adapted to soft substrates as a free-lying mode of life.
The narrow, long pedicle track is described in the other “Discinisca” from the Triassic and Jurassic (Fig. 10; see also SOM: table S1). Such type 1 morphology may reflect adaptation to soft substrates as a free-lying mode of life. In turn, discinids with type 2 ventral valves began to attach to ammonoid and bivalve shells from the Jurassic at the latest (Seilacher 1982). As a result, the morphology of a large depressed area would have been converted to host a larger volume of pedicle, making it possible for the animal to achieve a sessile mode of life, as observed in extant discinids (Fig. 11).
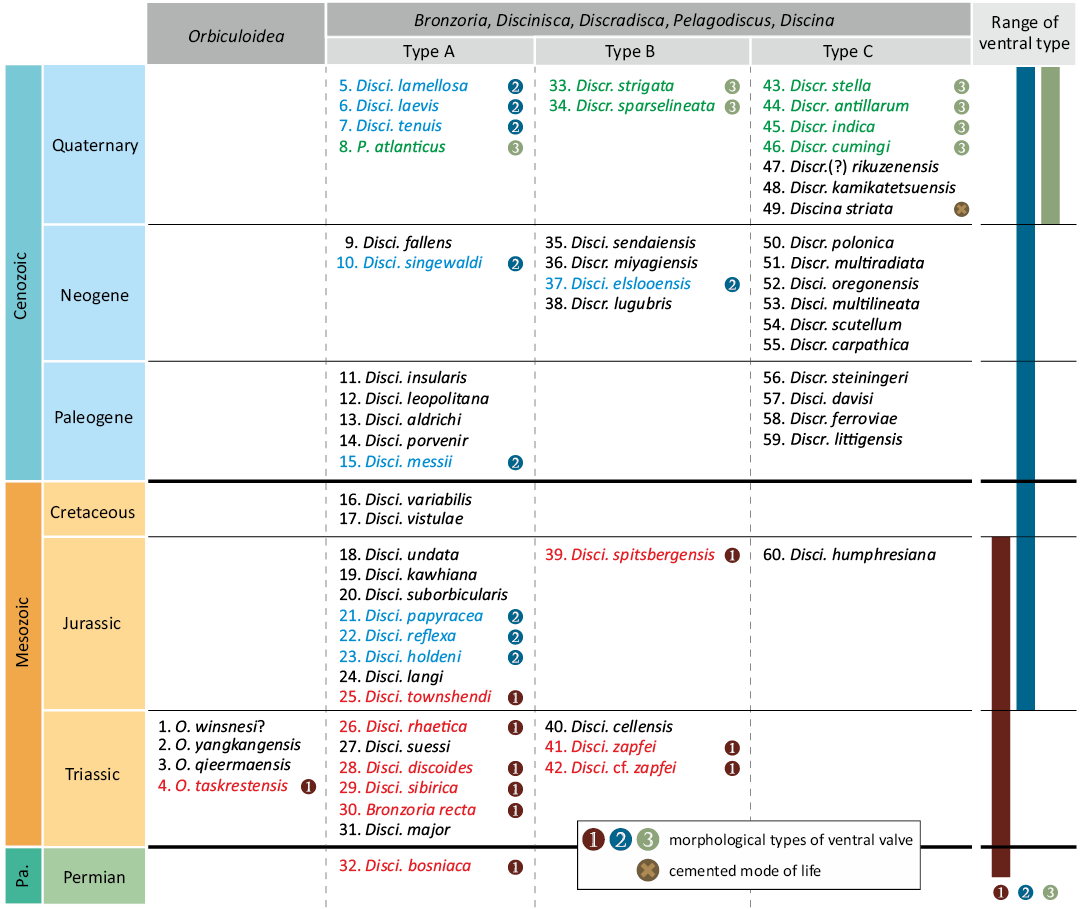
Fig. 10. Morphological types of post-Palaeozoic discinid brachiopods and selected Palaeozoic species. All discinids with type 1 ventral valves classified as Bronzoria gen. nov. The width of each stratigraphic unit not to scale. Each square and the position of the species name does not reflect its order of appearance; however, the order of the list is provided in the SOM. Abbreviations: Disci., Discinisca; Discr., Discradisca; Pa., Palaeozoic; O., Orbiculoidea; P., Pelagodiscus.
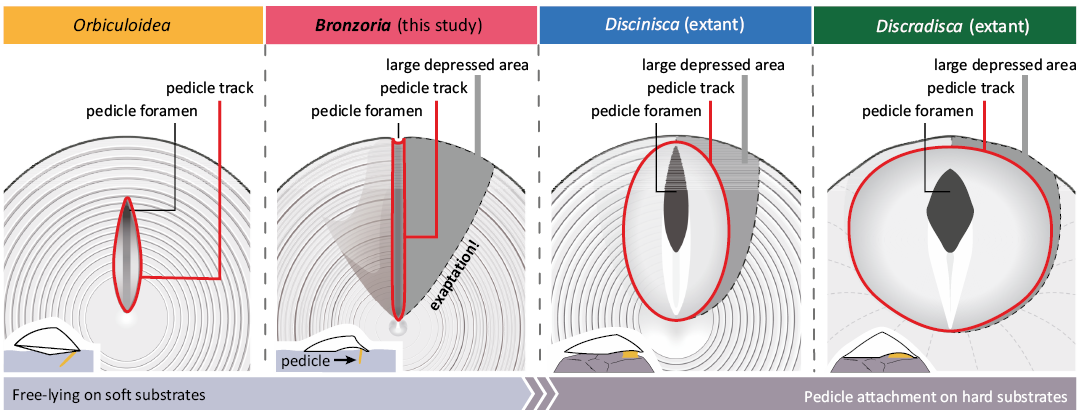
Fig. 11. Ventral morphology and related life postures of four discinid genera.
Acquisition and ecological significance of large depressed area.—Based on the morphological combination of pedicle-related structures, the type 1 ventral valve could be functional for the free-lying mode of life on soft substrates. A prime constraint of the free-lying mode of life is a narrow pedicle track (pedicle area). The supposed thickness of the pedicle shows a considerable degree of uniformity in its construction, regardless of the presence or absence of a large depressed area. However, the large depressed area is likely for functional pedicle attachment of the extant species of Discinisca and Discradisca, which provide a larger pedicle area and accommodate a thick pedicle between the ventral valve and the attachment substratum. This difference between the fossil and extant discinids suggests that the large depressed area may have evolved independently of the sessile mode of life.
Morphologically, the presence of a large depressed area alters the shell outline and convexity. The ventral valve of Bronzoria gen. nov. generally shows flat regions anteriorly and laterally, with the V-shaped large depressed area posteriorly, resulting in the formation of a concave valve. As the posterior space inside the shell is used as a storage of internal structures such as muscles and digestive tracts, the acquisition of the V-shaped large depressed area enables the reduction of the volume of the soft parts. This leads to the possibility that the oxygen consumption of Bronzoria gen. nov. was lower than that of the other discinids with no large depressed area, resulting in a lower metabolic rate.
The present review revealed that discinids bearing large depressed area persisted from the late Permian and thrived mainly in the Triassic (Fig. 10). Since the Guadalupian-Lopingian mass extinction event (Isozaki 1997), marine dysoxic-anoxic conditions have developed around the sea bottom, which lasted until the end of the Early Triassic (e.g., Hallam 1991; Twitchett and Wignall 1996; Chen et al. 2005; Dai et al. 2021). During this period, tolerance to poorly oxygenated conditions is required, as suggested previously (e.g., Twitchett et al. 2004). According to He et al. (2017), the end Permian to Early Triassic brachiopods in southern China decreased in size owing to anoxic, oligotrophic, and high-temperature environments. In addition to the decrease in size, a smaller amount of soft parts (by means of a large depressed area) could result from tolerance to an oxygen-poor environment. The present species Bronzoria recta gen. et sp. nov. was adapted to the dysoxic sea bottom as an opportunistic occurrence (Ishizaki and Shiino 2023b), which is consistent with the present interpretation.
In summary, the sedentary mode of life of discinids changed over time in response to changes in the benthic environment and related morphological evolution. First, Triassic-type discinids such as Bronzoria gen. nov., which enabled the reduction of soft parts, survived in the oxygen-poor environment of the end Permian to early Triassic owing to their lower metabolism. Subsequently, a large depressed area of the ventral valve could provide an extensive pedicle area, facilitating a robust attachment using pedicle, as seen in the extant discinids. In this scenario, the V-shaped large depressed area in Bronzoria gen. nov. is a trait of exaptation, leading to the hypothesis that the oxygen-poor environment of the Triassic resulted in the ecological innovation of discinids.
Remarks on the discinid diversity and evolution.—It is apparent that the acquisition of large depressed area results in the decrease of soft parts, possibly helping the adaptation to the oxygen-poor environment as discussed so far. From a morpho-functional viewpoint, the other discinid species with similar morphology to Bronzoria gen. nov. may also have the same adaptation capability, such as observed in Devonian discinids (e.g., a small, triangular depressed area of Orbiculoidea tarda [Barrande, 1879], in Mergl 2001: pl. 17: 4). However, the scenario is not so simply explained, as brachiopod morphology is designed to be a multi-functional body, which ensures consistency with any inferred autecological properties. There is a possibility to evolve similar morphology through entirely different pathways. In the case of a large depressed area, the concavity around the posterior part of the ventral valve enables not only the decrease of the amount of soft parts inside the shell but also the alteration of the direction of muscle contraction. Such a modification of the musculatory system has the potential for ecological differentiation (Shiino and Suzuki 2007; Masunaga and Shiino 2021), the hypothesis that has not yet been discussed.
Evolutionarily, a trait of exaptation exhibits a shift in function over time. If the habitat for discinids and its related environmental changes are closely similar, one would expect a corresponding pattern of morphological evolution. However, there is no evidence of ecological innovation as observed in Bronzoria gen. nov., even in species with similar morphology. The exaptation of a large depressed area would have caused unique environmental changes for Bronzoria gen. nov. that the Palaeozoic discinids have never experienced. Although we have no reliable methods to ascertain evolutionary dynamic forces, careful examinations of morphology, ecology, environment, and their changes over time may provide plausible hypotheses to understand the evolution of discinids.
Conclusions
The current discinid brachiopod from the Lower Triassic Osawa Formation appears to exhibit a narrow pedicle track (listrium) and a V-shaped large depressed area, thus representing an intermediate form between Orbiculoidea and Discinisca. Based on these characteristics, we propose a new genus, Bronzoria gen. nov., which mainly includes discinids well-known from the Triassic. Morphological analysis of extant discinids revealed that the pedicle area consists of an arrowhead-shaped median plate and a pair of semilunar plates, which can be compared to the inner and outer listrial plates of Palaeozoic discinids, respectively. Based on the morphological relationship of pedicle-related structures, we hypothesised that the large depressed area has evolved regarding the tolerance of an oxygen-poor environment and has secondarily been diverted as a pedicle area, which provides a larger volume of pedicle with sufficient distance from the attachment site. The V-shaped large depressed area, as observed in Bronzoria gen. nov., is a case of exaptation for autecological innovation of the pedicle attachment mode of life.
Acknowledgements
We gratefully acknowledge Masayuki Ehiro (Tohoku University Museum, Sendai, Japan), Atsushi Matsuoka, Toshiyuki Kurihara, Hayato Ueda (all Niigata University, Japan), Yutaro Suzuki and Akira Tsukagoshi (both Shizuoka University, Japan) for thorough discussion. Takenori Sasaki (University of Tokyo, Japan) helped us to search discinid specimens. We are also grateful to the Board of Education, Minamisanriku town, and Naoya Takahashi (Konpiramaru, Umishokunin, Minamisanriku, Japan) for their helpful support of our investigations. The Ministry of the Environment, Japan, and the Agency for Cultural Affairs, Japan permitted the present field surveys of a nature reserve. We sincerely thank Maria Aleksandra Bitner (Institute of Paleobiology PAS, Warsaw, Poland) and Michal Mergl (University of West Bohemia, Plzeň, Czech Republic) for their reviews. This study was financially supported in part by JST SPRING Grant Number JPMJSP2108 and the JSPS KAKENHI Grant Number 22K03795.
References
Andreae, A. 1893. Die Brachiopoden des Rhät von Malsch. Mitteilungen der Grossherzoglich Badischen Geologischen Landesanstalt 3: 11–17.
Bando, Y. 1964. The Triassic stratigraphy and ammonite fauna of Japan. The Science Reports of the Tohoku University, Second Series, Geology 36: 1–137.
Bando, Y. and Ehiro, M. 1982. On some Lower Triassic Ammonites from the Osawa Formation at Asadanuki, Towa-cho, Tome-gun, Miyagi prefecture, Northeast Japan. Transactions and Proceedings of the Palaeontological Society of Japan, New Series 1982: 375–385.
Barrande, J. 1879. Systême Silurien du Centre de la Bohême. V. 226 pp. Barrande, J., Prague.
Biernat, G. 1995. A new Jurassic discinid brachiopod from Spitsbergen. Polish Polar Research 16: 37–46.
Bitner, M.A. and Cahuzac, B. 2013. New record of Discradisca (Brachiopoda: Discinidae) from the Early Miocene of the Aquitaine Basin, south-western France. Comptes Rendus Palevol 12: 23–29. Crossref
Boehm, G. 1911. Grenzschichten zwischen Jura und Kreide von Kawhia (Nordinsel Neuseelands). Neues Jahrbuch für Mineralogie, Geologie und Paläeontologie Band 1: 1–24.
Broderip, W.J. 1833. Descriptions of some new species of Cuvier’s family of Brachiopoda. Proceedings of the Zoological Society of London 1: 141–144. Crossref
Bruguière, J.G. 1791. Tableau encyclopédique et méthodique des trois règnes de la nature. Vers, coquilles, mollusques et polypiers. 180 pp. Panckoucke, Paris.
Chen, Z.Q., Kaiho, K., and George, A.D. 2005. Early Triassic recovery of the brachiopod faunas from the end-Permian mass extinction: A global review. Palaeogeography, Palaeoclimatology, Palaeoecology 224: 270–290. Crossref
Curry, G.B. and Brunton, C.H.C. 2007. Stratigraphic distribution of brachiopods. In: P.A. Selden (ed.), Treatise on Invertebrate Paleontology, Part H: Brachiopoda Revised Volume 6, 2901–2965. Geological Society of America and University of Kansas, Boulder and Lawrence. Crossref
Dagys, A.S. 1965. The Triassic Brachiopoda of Siberia [in Russian]. 187 pp. Nauka, Moskva.
Dagys, A.S. and Kurushin, N.I. 1985. Triassic Brachiopods and Bivalves of the North Middle Siberia [in Russian]. 160 pp. Nauka, Moskva.
Dai, X., Yuan, Z., Brayard, A., Li, M., Liu, X., Jia, E., Du, Y., Song, H., and Song, H. 2021. Calibrating the late Smithian (Early Triassic) crisis: New insights from the Nanpanjiang Basin, South China. Global and Planetary Change 201: 103492. Crossref
Dall, W.H. 1871. Report on the Brachiopoda obtained by the United States Coast Survey Expedition, in charge of L.F. de Pourtales, with a revision of the Craniidae and Discinidae. Harvard University, Museum of Comparative Zoology, Bulletin 3:1–45.
Dall, W.H. 1908. Reports on the dredging operations off the west coast of Central America to the Galapagos, to the west coast of Mexico, and in the Gulf of California, in charge of Alexander Agassiz, carried on by the U.S. Fish Commission steamer “Albatross,” during 1891, Lieut.-Commander Z.L. Tanner, U.S.N., commanding. XXXVII. Reports on the scientific results of the expedition to the eastern tropical Pacific, in charge of Alexander Agassiz, by the U.S. Fish Commission steamer “Albatross”, from October, 1904 to March, 1905, Lieut.-Commander L.M. Garrett, U.S.N., commanding. XIV. The Mollusca and Brachiopoda. Bulletin of the Museum of Comparative Zoology 43: 205–487.
Dall, W.H. 1920. Annotated list of the recent Brachiopoda in the collection of the United States National Museum, with descriptions of thirty-three new forms. Proceedings U.S. National Museum 57: 261–377. Crossref
Davidson, T. 1851. A monograph of the British fossil Brachiopoda. Part III. The Oolitic and Liasic Brachiopoda. Monographs of the Palaeontographical Society 4: 1–64. Crossref
Dulai, A. and Hocht, F.V.D. 2020. Upper Oligocene brachiopods from NW Germany, with description of a new Platidiinae genus, Germanoplatidia n. gen. Rivista Italiana di Paleontologia e Stratigrafia 126: 223–248.
Dustira, A.M., Wignall, P.B., Joachimski, M., Blomeier, D., Hartkopf-Fröder, C., and Bond, D.P.G. 2013. Gradual onset of anoxia across the Permian–Triassic Boundary in Svalbard, Norway. Palaeogeography, Palaeoclimatology, Palaeoecology 374: 303–313. Crossref
Ehiro, M., Sasaki, O., Kano, H., and Nagase, T. 2019. Additional thylacocephalans (Arthropoda) from the Lower Triassic (upper Olenekian) Osawa Formation of the South Kitakami Belt, Northeast Japan. Palaeoworld 28: 320–333. Crossref
Emig, C.C. 1997. Ecology of inarticulated brachiopods. In: R.L. Kaesler (ed.), Treatise on Invertebrate Paleontology, Part H: Brachiopoda Revised Volume 1, 473–482. Geological Society of America and University of Kansas, Boulder and Lawrence.
Emig, C.C. 2003. Proof that Lingula (Brachiopoda) is not a living-fossil, and emended diagnoses of the family Lingulidae. Carnets de Geologie 2003, CG2003 (L01), 1–8. Crossref
Foster, W.J., Danise, S., and Twitchett, R.J. 2017. A silicified Early Triassic marine assemblage from Svalbard. Journal of Systematic Palaeontology 15: 851–877. Crossref
Gobbett, D.J. 1963. Carboniferous and Permian brachiopods of Svalbard. Norsk Polarinstitutt Skrifter 127: 1–201.
Goldfuss, G.A. 1831. Petrefacta Germaniae Tam Ea: Quae in Museo Universitatis Regiae Borussicae Fridericiae Wilhelmiae Rhenanae Servantur Quam Alia Quaecunque in Museis Hoeninghusiano Muensteriano Aliisque Extant, Iconibus et Descriptionibus Illustrata, Abbildungen und Beschreibungen der Petrefacten Deutschlands und der Angrenzenden Länder. 164 pp. Arnz & Comp., Düsseldorf.
Gorjansky, V.Y. and Popov, L.E. 1985. The morphology, systematic position, and origin of inarticulate brachiopods with carbonate shells. Paleontological Journal 1985 (3): 3–13.
Gould, A.A. 1862. Otia Conchologica: Descriptions of Shells and Mollusks, from 1839–1862. 256 pp. Gould and Lincoln, Boston. Crossref
Gray, J.E. 1840. Synopsis of the Contents of the British Museum. 42th Edition. 370 pp. British Museum, London.
Hallam, A. 1991. Why was there a delayed radiation after the end-Palaeozoic extinctions? Historical Biology 5: 257–262. Crossref
He, W., Shi, G. R., Xiao, Y., Zhang, K., Yang, T., Wu, H., Zhang, Y., Chen, B., Yue, M., Shen, J., Wang, Y., Yang, H., and Wu, S. 2017. Body-size changes of latest Permian brachiopods in varied palaeogeographic settings in South China and implications for controls on animal miniaturization in a highly stressed marine ecosystem. Palaeogeography, Palaeoclimatology, Palaeoecology 486: 33–45. Crossref
Holmer, L.E. 1987. Discinacean brachiopods from the Ordovician Kullsberg and Boda limestones of Dalarna, Sweden. Geologiska Föreningen i Stockholm Förhandlingar 109: 317–326. Crossref
Holmer, L.E. and Popov, L.E. 2000. Lingulata. In: R.L. Kaesler (ed.), Treatise on Invertebrate Paleontology, Part H: Brachiopoda Revised Volume 1, 30–146. Geological Society of America and University of Kansas, Boulder and Lawrence.
Ichikawa, K. 1951. The Triassic system in the Southern Kitakami Mountains [in Japanese]. In: T. Mitsuchi (ed.), Triassic Stratigraphy of Japan, Report Special Number, 7–23. Geological Survey of Japan, Tokyo.
Ishizaki, Y. and Shiino, Y. 2023a. A discinid shell on Ikarashi beach, Niigata, Japan. Science Reports of Niigata University (Geology) 38: 1–16.
Ishizaki, Y. and Shiino, Y. 2023b. Sedimentary environment and redox conditions of the Lower Triassic Osawa Formation in the Southern Kitakami Terrane, Japan: insights into ocean redox stratification and faunal recovery. Palaios 38: 210–232. Crossref
Isozaki, Y. 1997. Permo-Triassic boundary Superanoxia and stratified superocean: Records from lost deep-sea. Science 276: 235–238. Crossref
James, M.A., Ansell, A.D., Curry, G.B., Collins, M.J., Peck, L.S., and Rhodes, M.C. 1992. The biology of living brachiopods. Advances in Marine Biology 28: 175–387. Crossref
Kamada, K. 1989. Triassic [in Japanese]. In: Editorial Committee of TOHOKU, Part 2 of Regional Geology of Japan (ed.), Regional Geology of Japan Part 2 TOHOKU, 31–35. Kyoritsu Shuppan, Tokyo.
Kamada, K. 1993. Geology of the Tsuya District. With Geological Sheet Map at 1:50000 [in Japanese]. 70 pp. Geological Survey of Japan, Akita.
Kato, M. 1996. The unique intertidal subterranean habitat and filtering system of a limpet-like brachiopod, Discinisca sparselineata. Canadian Journal of Zoology 74: 1983–1988. Crossref
Kin, A. and Błażejowski, B. 2014. The horseshoe crab of the genus Limulus: living fossil or stabilomorph? PLoS One 9: e108036. Crossref
Kittl, E. 1904. Geologie der Umgebung von Sarajevo. Jahrbuch der Kaiserlich-Königlichen Geologischen Reichsanstalt 53: 511–746.
Koehl, M.A.R. 1996. When does morphology matter? Annual Review of Ecology and Systematics 27: 501–542. Crossref
LaBarbera, M. 1985. Mechanisms of spatial competition of Discinisca strigata (Inarticulata: Brachiopoda) in the intertidal of Panama. Biological Bulletin 168: 91–105. Crossref
Lamarck, J.B.P. de. 1819. Histoire naturelle des animaux sans vertèbres, présentant les caractères généraux et particuliers de ces animaux, leur distribution, leurs classes, leurs familles, leurs genres, et la citation des principales espèces qui s’y rapportent; précédée d’une introduction offrant la détermination des caractères essentiels de l’animal, sa distinction du végétal et des autres corps naturels; enfin, l’exposition des principes fondamentaux de la zoologie. Histoire naturelle des animaux sans vertèbres In: J.B.P. de. Lamarck (ed.), Histoire naturelle des animaux sans vertèbres. 5, 1–711. Lamarck, J.B.P. de., Paris.
Liang, Y., Strotz, L.C., Topper, T.P., Holmer, L.E., Budd, G.E., Chen, Y., Fang, R., Hu, Y., and Zhang, Z. 2023. Evolutionary contingency in lingulid brachiopods across mass extinctions. Current Biology 33: 1565–1572. Crossref
Lidgard, S. and Love, A.C. 2018. Rethinking living fossils. Bioscience 68: 760–770. Crossref
Masunaga, M. and Shiino, Y. 2021. Death or living assemblage? The middle Permian discinid brachiopods in the Kamiyasse area, Southern Kitakami Mountains, northeastern Japan. Paleontological Research 25: 258–278. Crossref
Mergl, M. 2001. Lingulate brachipods of the Silurian and Devonian of the Barrandian (Bohemia, Czech Republic). Acta Musei Nationalis Pragae, Series B – Historia Naturalis 57: 1–49.
Mergl, M. 2006. A review of Silurian discinoid brachiopods from historical British localities. Bulletin of Geosciences 81: 215–236. Crossref
Mergl, M. 2010. Discinid brachiopod life assemblages: Fossil and extant. Bulletin of Geosciences 85: 27–38. Crossref
Mergl, M. and Massa, D. 2005. A new giant discinoid brachiopod from the Lower Devonian of Algeria. Acta Palaeontologica Polonica 50: 397–402.
Moisseiev, A.S. 1947. Brachiopoda [in Russian]. In: L.D. Kiparisova (ed.), Atlas of the Guide Forms of the Fossil Faunas of the USSR, Triassic, Volume VII, 61–81. Gosgeolizdat, State Editorial Office for Geological Literature, USSR Ministry of Geology, Moscow.
Murata, M. 1973. Triassic fossils from the Kitakami Massif, Northeast Japan: Part 1, pelecypods and brachiopods of the Osawa and the Fukkoshi Formations. Science Reports of the Tohoku University, Second Series (Geology). Special Volume 6: 267–275.
Onuki, Y. and Bando, Y. 1959. On the Inai Group of the lower and middle Triassic system (stratigraphical and paleontological studies of the Triassic system in the Kitakami massif, northeastern Japan: –3) [in Japanese with English abstract]. Contributions from the Institute of Geology and Paleontology, Tohoku University 50: 1–69.
d’Orbigny, A. 1846. Molusques. In: M. Ramón de la Sagra (ed.), Histoire, Physique, Politique et Naturelle de l’ile de Cuba, 2: 5–28. A. Bertrand, Paris.
d’Orbigny, A. 1847. Considérations zoologiques et géologiques sur les Brachiopodes ou Palliobranches. Comptes Rendus Hebdomadaires des Séances de l’Académie des Sciences 25: 193–195, 266–269.
d’Orbigny, A. 1849. Prodrome de Paléontologie stratigraphique universelle des animaux Mollusques et Rayonnés, Volume 1. 394 pp. Victor Masson, Paris.
Radwańska, U. and Radwański, A. 2003. Bosquet’s (1862) inarticulate brachiopods: Discinisca elslooensis sp. n. from the Elsloo Conglomerate. Bulletin de l’Institut Royal des Sciences Naturelles de Belqique, Sciences de la Terre 73: 185–194.
Radwański, A. and Summesberger, H. 2001. A new species of inarticulate brachiopods, Discinisca zapfei sp. n., from the Upper Triassic Zlambach Formation (Northern Calcareous Alps, Austria), and a discussion of other Triassic disciniscans. Annalen des Naturhistorischen Museum in Wien 102: 109–129.
Rudwick, M.J.S. 1970. Living and Fossil Brachiopods. 199 pp. Hutchinson University Library, London.
Schlotheim, E.F.B. 1820. Die Petrefactenkunde auf ilem fetzigen standpunkte durch die beschreibung: Seiner sammlung versteinerter und fossiler überreste des thier- und Pflanzenreichs. 614 pp. Becker’s chen Buchhandlung, Gotha.
Seilacher, A. 1982. Ammonite shells as habitats in the Posidonia Shales of Holzmaden-floats or benthic islands? Neues Jahrbuch für Geologie und Paläontologie, Monatshefte 1982 (2): 98–114. Crossref
Shigeta, Y. 2022. Revision of early Spathian (late Olenekian, Early Triassic) ammonoids from the Osawa Formation at Akaushi in the Motoyoshi area, South Kitakami Belt, Northeast Japan. Paleontological Research 26: 405–419. Crossref
Shiino, Y. and Kuwazuru, O. 2010. Functional adaptation of spiriferide brachiopod morphology. Journal of Evolutionary Biology 23: 1547–1557. Crossref
Shiino, Y. and Suzuki, Y. 2007. Articulatory and musculatory systems in a Permian concavo-convex brachiopod Waagenoconcha imperfecta (Productida, Brachiopoda). Paleontological Research 11: 265–275. Crossref
Souza, P.A., Petri, S., and Dino, R. 2003. Late Carboniferous palynology from the Itararé Subgroup (Paraná Basin) at Araçoiaba da Serra, São Paulo State, Brazil. Palynology 27: 39–74. Crossref
Sowerby, G.B. 1822. Remarks on the genera Orbicula and Crania of Lamarck, with description of two species of each genus; and some observations proving the Patella distorta of Montagu to be a species of Crania. Transactions of the Linnean Society of London 13: 465–472. Crossref
Stenzel, H.B. 1964. Stratigraphic and paleoecologic significance of a new Danian brachiopod species from Texas. Geologische Rundschau 54: 619–631. Crossref
Tate, R. 1867. On the fossiliferous development of the zone of Ammonites angulatus, Schloth., in Great Britain. Quarterly Journal of the Geological Society 23: 305–314. Crossref
Twitchett. R.J., Krystyn, L., Baud, A., Wheeley, J.R., and Richoz, S. 2004. Rapid marine recovery after the end-Permian mass-extinction event in the absence of marine anoxia. Geology 32: 805–808. Crossref
Twitchett, R.J. and Wignall, P.B. 1996. Trace fossils and the aftermath of the Permo-Triassic mass extinction: evidence from northern Italy. Palaeogeography, Palaeoclimatology, Palaeoecology 124: 137–151. Crossref
Waagen, W.H. 1885. Salt Range fossils, volume I, part 4. Productus Limestone fossils, Brachiopoda. Memoirs of the Geological Survey of India, Palaeontologia Indica (Series 13) 5: 729–770.
Wagner, G. 1913. Beiträge zur Stratigraphie und Bildungsgeschichte des oberen Hauptmuschelkalks und der unteren Lettenkohle in Franken. Geologische und Paläontologische Abhandlungen 12: 275–452.
Werth, A.J. and Shear, W.A. 2014. The evolutionary truth about living fossils. American Scientist 102: 434–443. Crossref
Williams, A., Brunton, C.H.C., and MacKinnon, D.I. 1997. Morphology. In: R.L. Kaesler (ed.), Treatise on Invertebrate Paleontology, Part H: Brachiopoda Revised Volume 1, 321–422. Geological Society of America and University of Kansas, Boulder and Lawrence.
Williams, A. and Rowell, A.J. 1965. Morphology. In: R.C. Moore (ed.), Treatise on Invertebrate Paleontology, Part H: Brachiopoda Volume 1, 57–138. Geological Society of America and University of Kansas, Boulder and Lawrence.
Wood, D., Besnard, G., Beerling, D.J., Osborne, C.P., and Christin, P.A. 2020. Phylogenomics indicates the “living fossil” Isoetes diversified in the Cenozoic. PLoS One 15: e0227525. Crossref
Xu, G.R. and Liu, G.C. 1983. Brachiopods [in Chinese]. In: Z.Y. Yang, H.F. Yin, G.R. Xu, S.B. Wu, Y.L. He, G.C. Liu, and J.R. Yin (eds.), Triassic of the South Qilian Mountains, 84–128. Geological Publishing House, Beijing.
Zhang, Y., Shi, G.R., He, W.H., Zhang, K.X., and Wu, H.T. 2014. A new Changhsingian (Late Permian) brachiopod fauna from the Zhongzhai section (South China), Part 2: Lingulida, Orthida, Orthotetida and Spiriferida. Alcheringa 38: 480–503. Crossref
Acta Palaeontol. Pol. 69 (3): 529–548, 2024
https://doi.org/10.4202/app.01164.2024