


The largest ghost shrimps ever: evidence from the fossil record and implications for the maximum size estimate of callianassoid burrowing ghost shrimps
MATÚŠ HYŽNÝ, DOMINIK KNEER, and SYLVAIN CHARBONNIER
Hyžný, M., Kneer, D., and Charbonnier, S. 2025. The largest ghost shrimps ever: evidence from the fossil record and implications for the maximum size estimate of callianassoid burrowing ghost shrimps. Acta Palaeontologica Polonica 70 (1): 97–113.
Callianassoid burrowing ghost shrimps are mostly small animals, with a total length (from the tip of the rostrum to the end of the tailfan) typically not exceeding a few centimetres. Representatives of some species in the families Anacalliacidae, Callianassidae, Callichiridae, Ctenochelidae, and possibly also Callianopsidae, however, may grow to relatively large sizes, reaching 10 and more centimetres in length. The maximum size each of these species can attain remains a mere estimate because it is difficult to catch ghost shrimps, particularly the large-sized tropical representatives. Since large individuals have a greater fossilization potential, the ghost shrimp fossil record could contribute to our knowledge about how large these animals can grow. The largest extant ghost shrimp reported to date is an individual of the species Glypturus armatus (Callichiridae), with an estimated total length of 175 mm (based on the extrapolation from an isolated ischium). The existence of even larger animals reaching a total length of approximately 200 mm is documented herein from the Maastrichtian of Madagascar and the middle Eocene of Hungary, with both fossil individuals belonging to the genus Karumballichirus (Callichiridae) and appearing to be closely related to the extant Karumballichirus karumba. An overview of both extant and fossil ghost shrimp species suggests that a total length of 200 mm is rarely, if ever, exceeded by these animals. We suggest that physiological limits imposed by the specialized burrowing lifestyle might prevent ghost shrimp from growing any larger.
Key words: Malacostraca, Decapoda, Axiidea, body length, burrowing shrimps, decapod crustaceans, fossilization potential, physiological limits.
Matúš Hyžný [hyzny.matus@gmail.com; ORCID: https://orcid.org/0000-0002-8960-2846 ], Department of Geology and Paleontology, Faculty of Natural Sciences, Comenius University, Ilkovičova 6, SK-842 15 Bratislava, Slovakia.
Dominik Kneer [dmkneer@gmail.com; ORCID: https://orcid.org/0000-0001-9874-4793 ], Alfred Wegener Institute, Helmholtz Centre for Polar and Marine Research, Wadden Sea Station Sylt, Hafenstraße 43, D-25992 List, Germany.
Sylvain Charbonnier [sylvain.charbonnier@mnhn.fr; ORCID: https://orcid.org/0000-0003-2343-6897 ], Centre de Recherche en Paléontologie – Paris (CR2P, UMR 7207), CNRS-MNHN-Sorbonne Université, Muséum national d’Histoire naturelle, 8 rue Buffon, Paris, France.
Received 23 October 2023, accepted 24 January 2025, published online 19 March 2025.
Copyright © 2025 M. Hyžný et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Burrowing ghost shrimps (Decapoda: Axiidea: Callianassoidea sensu Poore et al. 2019 and Robles et al. 2020) are major components of many fossil shallow marine decapod crustacean assemblages (Hyžný and Klompmaker 2015), which reflects the fact that they, as major inter- and subtidal bioturbators (Rowden and Jones 1993; Curran and Martin 2003; Dworschak et al. 2012; Kneer et al. 2013), often live in high densities (Ziebis et al. 1996; Stamhuis et al. 1997; Bishop and Williams 2005). Many of the known ghost shrimps, both fossil and modern, are relatively small animals with a total length (from the tip of the rostrum to the end of the tailfan) not exceeding a few centimetres. Just a few species grow to relatively large sizes. The largest ghost shrimp alive today are found in the family Callichiridae Manning & Felder, 1991 (Fig. 1, Table 1), with species in several genera exceeding a total length of 100 mm, i.e., Audacallichirus Poore et al., 2019; Callichirus Stimpson, 1866; Corallianassa Manning, 1987; Glypturus Stimpson, 1866; Karumballichirus Poore et al., 2019; Lepidophthalmus Holmes, 1904; and Neocallichirus Sakai, 1988 (e.g., Rodrigues 1971; Saint Laurent and Le Loeuff 1979; Vaugelas and Saint Laurent 1984; Vaugelas 1985; Poore and Suchanek 1988; Bishop and Bishop 1992; Sakai 1999, 2011; Anker and Dworschak 2007; Dworschak 2011a, 2018, 2022; Sakai and Türkay 2014; Komai et al. 2015). Within other axiidean families, such a body length was also documented in the Anacalliacidae (Anacalliax Saint Laurent, 1973), Callianassidae (Neotrypaea Manning and Felder, 1991) and Ctenochelidae (Ctenocheles Kishinouye, 1926) (e.g., Ward 1945; Powell 1949; Biffar 1971a; Holthuis 1991; Wicksten 2011), and possibly also Callianopsidae (Callianopsis Saint Laurent, 1973) (Table 1). Also, the fossil record might provide some clues regarding maximum size estimates of ghost shrimps.
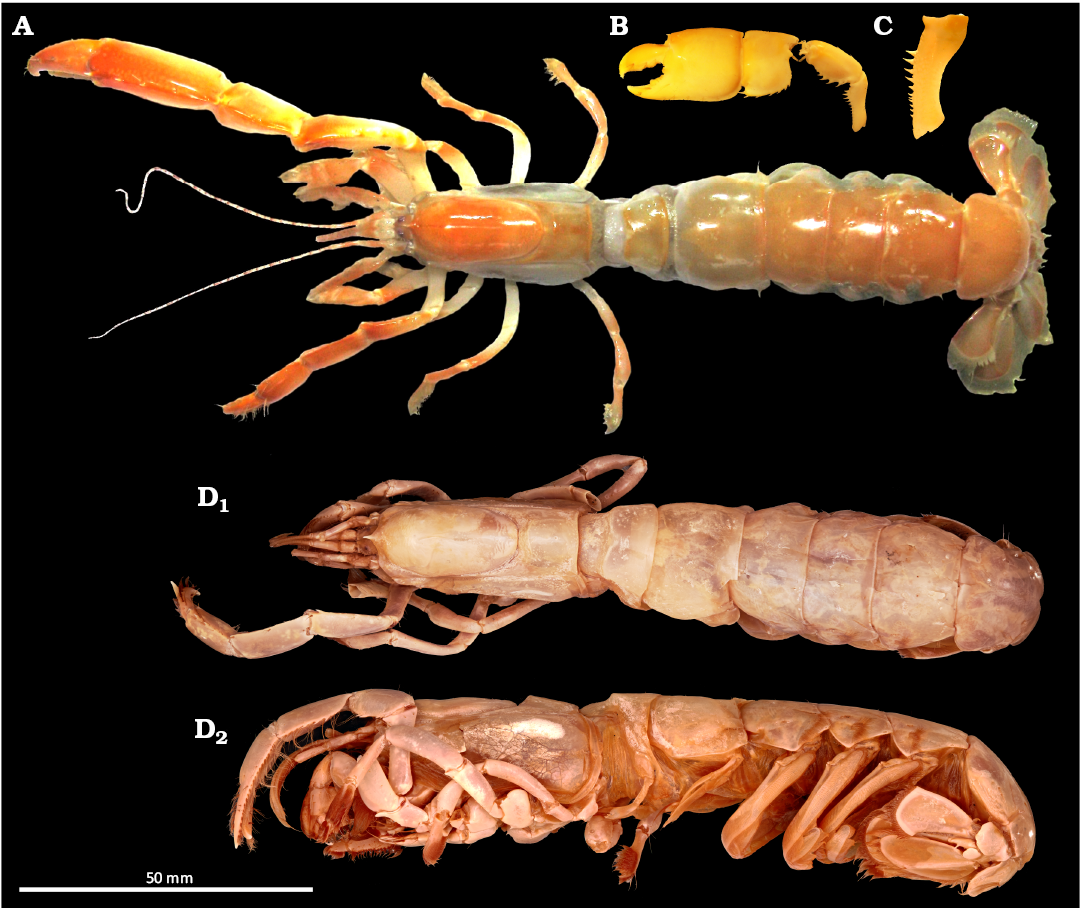
Fig. 1. Ghost shrimp Glypturus armatus (Milne-Edwards, 1870), the largest documented extant ghost shrimp species. Specimens shown here are smaller representatives. A. ZMB 32002 (total length = 107 mm), dorsal view of entire animal; Barrang Lompo, Sulawesi, Indonesia. B. NMCR 39031 (total length = 82 mm), outer lateral view of left major cheliped; Panglao Island, the Philippines. C. NHMW 25460 (total length = 175 mm, based on a regression), outer lateral view of left major cheliped ischium; Panglao Island, the Philippines. D. ZMH K 38197 (total length = 147 mm), dorsal (D1) and lateral (D2) views of entire animal; Somalia, unknown locality. Photo: ©LIB, Mercado-Salas.
The fossilization potential of ghost shrimps is relatively high due to well-calcified chelae (Bishop and Williams 2005; Hyžný and Klompmaker 2015) and their burrowing lifestyle enhancing preservation, as at least some moults may remain in the burrow (Hyžný and Klompmaker 2015 and references therein). Although the taxonomic evaluation of isolated cheliped fingers (the most common remains of fossil ghost shrimps) may prove to be difficult (Hyžný and Klompmaker 2015), the information they can provide on the ecology and lifestyle of the fossil animals should not be neglected. The present contribution aims to discuss one specific aspect of ghost shrimps: their potential maximum size. Living ghost shrimps are not easy to catch (Vaugelas 1985; Dworschak 2015), as especially the large ones can burrow more than 1.5 m deep into the substrate (Dworschak et al. 2012, and references therein), so the estimate of their maximum size based on specimens in zoological collections may not be completely informative. However, large individuals with well-sclerotized cuticle and heavily calcified chelipeds, rather than smaller ones, are more easily preserved as fossils (Bishop and Williams 2005; Hyžný and Klompmaker 2015). This is why ghost shrimp fossils could help us find out more about the maximum size these animals can attain.
Although body size is an important biological characteristic affecting the physiology and ecology of organisms (Peters 1983; Bonner 2006; Maszczyk and Brzeziński 2018), only a limited attention has been paid to body size of decapod crustaceans in the fossil record (Klompmaker et al. 2015, 2016b). As for ghost shrimps, their body size is tightly connected with their burrowing activities and consequently the impact on their environment as major bioturbators (Nickel and Atkinson 1995; Felder 2001; Kornienko 2013). The estimate of the maximum body size in selected ghost shrimp taxa throughout their stratigraphic span is thus of great importance in evaluating the role of these animals as ecosystem engineers not only in modern settings (Berkenbusch and Rowden 2003; Kneer et al. 2013) but also in the geological past.
This contribution, based on the examination of rich fossil ghost shrimp material largely consisting of isolated cheliped elements and a comparison with material of extant taxa, aims to: (i) provide an overview of the largest modern ghost shrimp specimens collected to date; (ii) present the fossil material belonging to arguably the largest documented ghost shrimp; and (iii) discuss potential size limitations of these animals.
Table 1. Overview of extant ghost shrimp species with a documented estimated total length of 100 mm or more. Data are largely based on a literature survey. Total length (in mm), i.e., the length from the tip of the rostrum to the end of the tailfan, is followed by carapace length (in mm). For an exhaustive overview of all documented specimens reaching or exceeding a total length of 100 mm see SOM. Abbreviation: N/A, not available.
|
Family |
Species |
Total length |
Carpace length |
Sex |
Repository number |
Reference |
|
Anacalliacidae |
Anacalliax agassizi |
112 |
N/A |
female |
MCZH 12872 (paratype) |
|
|
Anacalliacidae |
Anacalliax argentinensis |
143 |
N/A |
male |
USNM 135056 |
|
|
Callianassidae |
Neotrypaea californiensis |
120 |
N/A |
female |
N/A |
|
|
Callianassidae |
Neotrypaea gigas |
150 |
N/A |
N/A |
N/A |
|
|
Callianopsidae |
Callianopsis goniophthalma |
N/A |
27.5 |
male |
USNM 28354 |
|
|
Callichiridae |
Audacallichirus mirim |
115 |
28 |
female |
RMNH D 37701 |
|
|
Callichiridae |
Audacallichirus monodi |
130 |
N/A |
female |
MNHN Th-390 |
|
|
Callichiridae |
Callichirus adamas |
117 |
N/A |
male |
MNHN Th-379 |
|
|
Callichiridae |
Callichirus garthi |
130 |
35 |
male |
MZUC-UCCC (holotype) |
|
|
Callichiridae |
Callichirus major |
150 |
N/A |
female |
N/A |
|
|
Callichiridae |
Callichirus seilacheri |
117 |
25 |
male |
SMF 4941 |
|
|
Callichiridae |
Corallianassa intesi |
102 |
28 |
female |
MNHN Th-368 |
|
|
Callichiridae |
Glypturus acanthochirus |
140 |
31.5 |
female |
MNHN Th-1593 |
this study |
|
Callichiridae |
Glypturus armatus |
147 |
35 |
male |
ZMH K 38197 |
this study |
|
Callichiridae |
Glypturus armatus |
175 |
50 |
N/A |
NHMW 25460 |
Dworschak 2018; based on a regression |
|
Callichiridae |
Glypturus laurae |
153 |
33.4 |
female |
NHMW 21940 |
|
|
Callichiridae |
Karumballichirus karumba |
131 |
30 |
female |
ZMB 3353 |
|
|
Callichiridae |
Lepidophthalmus bocourti |
N/A |
25 |
male |
ULLZ 4639 |
|
|
Callichiridae |
Lepidophthalmus eiseni |
N/A |
26 |
female |
MCZH 4370 |
|
|
Callichiridae |
Lepidophthalmus tridentatus |
100 |
18.1 |
female |
NHML 2739 |
|
|
Callichiridae |
Lepidophthalmus turneranus |
162 |
32 |
male |
ZMH K 26371 |
|
|
Callichiridae |
Neocallichirus darwinensis |
110 |
24 |
female |
NTM CR.000090 (holotype) |
|
|
Callichiridae |
Neocallichirus grandimana |
134 |
N/A |
male |
MCZH 12873 |
|
|
Callichiridae |
Neocallichirus guassutinga |
136 |
N/A |
female |
N/A |
|
|
Callichiridae |
Neocallichirus mericeae |
115.2 |
31.1 |
male |
USNM 268686 |
|
|
Callichiridae |
Neocallichirus natalensis |
117 |
24.4 |
female |
NHMW 24900 |
|
|
Callichiridae |
Neocallichirus vaugelasi |
139 |
29.8 |
female |
MNHN Th-651 |
|
|
Callichiridae |
Neocallichirus vigilax |
124 |
32.4 |
N/A |
N/A |
Dworschak 2011b; based on a regression |
|
Ctenochelidae |
Ctenocheles collini |
120 |
N/A |
N/A |
QM (holotype) |
|
|
Ctenochelidae |
Ctenocheles maorianus |
122 |
N/A |
N/A |
AMNZ (paratype) |
Institutional abbreviations.—AMNZ, Auckland Museum, Auckland, New Zealand; CPAG, Centre for Pure and Applied Geology, University of Sindh, Jamshoro, Pakistan; GA, GeoSphere Austria, Vienna, Austria; MCZ, Museo Civico “G. Zannato”, Montecchio Maggiore, Italy; MCZH, Museum of Comparative Zoology, Harvard University, Cambridge, USA; MBFSZ, Mining and Geological Survey of Hungary, Budapest, Hungary; MSNM, Museo Civico di Storia Naturale di Milano, Italy; MNHN.F, Collection de Paléontologie, Muséum national dʼHistoire naturellein Paris, France; MZUC-UCCC, Museo de Zoología de la Universidad de Concepción, Chile; NHML, Natural History Museum London, UK; NHMW, Naturhistorisches Museum Wien, Austria; NMCR, National Museum of the Philippines, Manila, Philippines; NTM, Northern Territory Museum, Darwin, Australia; QM, Queensland Museum, Brisbane, Australia; RMNH, National Museum of Natural History, Leiden, the Netherlands; SMF, Forschungsinstitut Senckenberg, Frankfurt am Main, Germany; ULLZ, Zoological collections of the University of Louisiana, Lafayette, USA; USNM, United States National Museum, Smithsonian Institution, Washington, D.C., USA; ZMB, Zoologisches Museum Berlin, Germany; ZMH, Zoologisches Museum Hamburg, Germany.
Material and methods
Measurements on extant ghost shrimps were conducted on individuals collected in the field (Sulawesi) by one of us (DK), and on specimens found in museum collections. Measuring ghost shrimps can be influenced by numerous factors, including the method of measuring and how well preserved the respective specimen is. In museum collections, wet specimens often curl up. Measured length values of curled animals slightly differ from those measured if animals are stretched out. Generally, the latter method gives lower values because when curled, the measuring process may include also membranes between pleonal segments; the membranes are hidden when the animals are stretched out and do not contribute to the measured value. However, if parts of the exoskeleton are very soft and transparent (e.g., due to some degree of decay), it is easy to stretch the animal out to become unnaturally long. Additionally, in some species, e.g., Lepidophthalmus turneranus White, 1861, there is a narrow plate fringing the posterior margin of the dorsal carapace. In well-preserved animals, this plate preserves the same pigmentation as the rest of the dorsal carapace and will likely be included in the measurement of the carapace length. In not-so-well-preserved animals, it assumes the same pigmentation as the soft tissue part connecting the dorsal carapace to the first pleonal somite, potentially leading to it not being included in the measurement of the carapace length. Including or not including this plate in the measurement of the carapace length can make a difference of 1 or 2 mm. One of the methods for measuring museum specimens is by using a thread; this method was used for specimens measured by us.
An overview of the largest modern ghost shrimps collected to date was compiled via screening the literature as well as adding new data on previously unpublished specimens, and some of the previously published specimens were re-measured (Table 1; SOM, Supplementary Online Material available at http://app.pan.pl/SOM/app70-Hyzny_etal_SOM.pdf). All the fossil material was studied first-hand by one of us (MH). Some specimens were coated with ammonium chloride prior to photography (see figure captions for details). Fossil samples consisted of isolated and/or fragmentary cheliped elements. Estimates of the total length are based on extrapolations of the regression growth curve of extant congeneric specimens of Karumballichirus karumba (Poore & Griffin, 1979) as published by Dworschak (2008); the following regressions (Peter Dworschak, personal communication 2015 and 2024) were used: CL (carapace length) = 0.2313 TL (total length) + 1.6722; P1Ma (pereiopod 1 manus) = 0.9528 CL -4.6965 (for males). Specimens of K. karumba, deposited in the NHMW (published previously by Dworschak 2008), were examined for comparative purposes.
Systematic palaeontology
Order Decapoda Latreille, 1802
Infraorder Axiidea Saint Laurent, 1979
Family Callichiridae Manning & Felder, 1991
Genus Karumballichirus Poore et al., 2019
Type species: Callianassa karumba Poore & Griffin, 1979, by original designation; Recent, Australia (Queensland, Karumba).
Remarks.—The systematic overview of (sub)fossil large-sized Karumballichirus presented below focuses on taxa personally studied by one of the authors (MH). It is not meant to be exhaustive by any means, but rather to demonstrate the geographic and stratigraphic distribution of the genus.
Karumballichirus khadroensis (Hyžný & Charbonnier in Hyžný et al., 2016a) comb. nov.
Fig. 2.
2016a Neocallichirus khadroensis Hyžný & Charbonnier; Hyžný et al. 2016a: 344, figs. 2, 5A3, C2, D3, 6I.
Material.—Holotype: CPAG.RAN.I.55 (cast MNHN.F. A52405), left propodus with articulated dactylus; paratypes: 8 specimens, CPAG.RAN.I.56–I.63 (casts MNHN.F.A52406–A52413), isolated cheliped elements; from Gawar Band section (Khadro Formation, Danian), Ranikot, Sindh, Pakistan.
Description.—Detailed description of the species was provided by Hyžný et al. (2016a) and is therefore not repeated here.
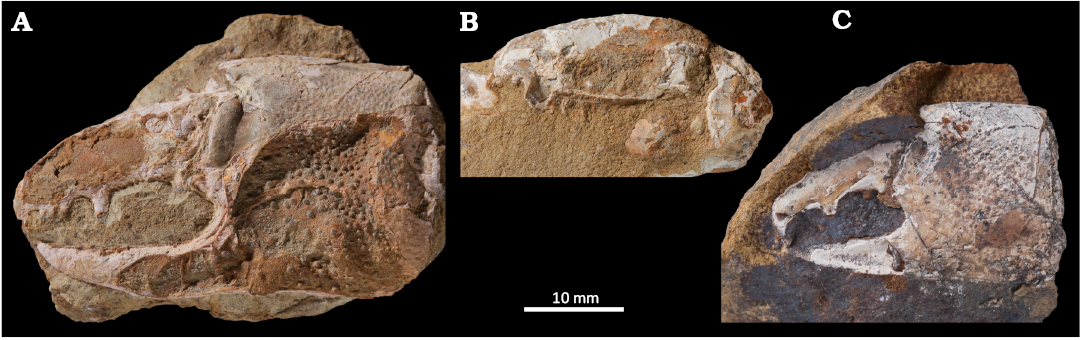
Fig. 2. Ghost shrimp Karumballichirus khadroensis (Hyžný & Charbonnier in Hyžný et al., 2016a) comb. nov., Danian (Paleocene) of Ranikot, Sindh, Pakistan. A. CPAG.RAN.I.55 (holotype), left major chela (propodus and dactylus) in outer lateral view. B. CPAG.RAN.I.63 (paratype), right major cheliped merus in outer lateral view. C. CPAG.RAN.I.60 (paratype), left major chela (propodus and dactylus) in outer lateral view. All specimens are shown to the same scale.
Remarks.—Hyžný et al. (2016a) assumed that the growth rate of Neocallichirus khadroensis was largely the same as the one of Neocallichirus karumba; the assumption was based on striking morphological similarities between both taxa as shown by Hyžný et al. (2016a: fig. 5). Based on an extrapolation of data on the growth of N. karumba (Dworschak 2008), Hyžný et al. (2016a) estimated the total length of the largest individuals of N. khadroensis to be 120 mm. The establishment of Karumballichirus (with the type species N. karumba) allows re-assignment of N. khadroensis to the respective genus.
Stratigraphic and geographic range.—Danian (Paleocene) of Pakistan (Hyžný et al. 2016a).
Karumballichirus lakhraensis (Hyžný & Charbonnier in Hyžný et al., 2016a) comb. nov.
Figs. 3, 4.
2013 Calliax sp.; Charbonnier et al. 2013: 106, fig. 2A, B.
2016a Neocallichirus lakhraensis Hyžný and Charbonnier in Hyžný et al., 2016a: 346, figs. 4, 5A2, B2, C3, D2, E2, 6H.
Material.—Holotype: CPAG.RAN.I.64 (cast MNHN.F. A52414), right propodus with articulated dactylus; paratypes: CPAG.RAN.I.65–I.72 (casts MNHN.F.A52415–A52422), 4 additional specimens: CPAG.RAN.I.85–I.87 (casts MNHN.F. A91718–A91720), largely articulated chelipeds consisting of dactylus, propodus, carpus, merus, and ischium; all from Rhob Nala section (Lakhra Formation, Ypresian), Thatta District, Sindh, Pakistan; MNHN.F.A47685, right major propodus, Lakhra Dome coal mine field (Bara Formation, Paleocene, Thanetian?), Thatta District, Sindh, Pakistan.
Description.—Detailed description of the species was provided by Hyžný et al. (2016a) and is therefore not repeated here.

Fig. 3. Ghost shrimp Karumballichirus lakhraensis (Hyžný & Charbonnier in Hyžný et al., 2016a) comb. nov., Ypresian (Eocene) of Thatta District, Sindh, Pakistan. A. CPAG.RAN.I.64 (holotype), right major chela (propodus and dactylus) in outer lateral (A1) and inner lateral (A2) views. B. CPAG.RAN.I.85 (cast MNHN.F.A91718), left major chela (incomplete propodus and dactylus). C. CPAG.RAN.I.86 (cast MNHN.F.A91719), left major chela in inner lateral (C1) and outer lateral (C2) views. D. CPAG.RAN.I.87 (cast MNHN.F.A91720), right major dactylus in outer lateral view. E. CPAG.RAN.I.70 (paratype), right major cheliped (ischium, merus, incomplete carpus) in outer lateral view. All specimens are shown to the same scale. Specimens in B–D were covered with ammonium chloride prior to photography.
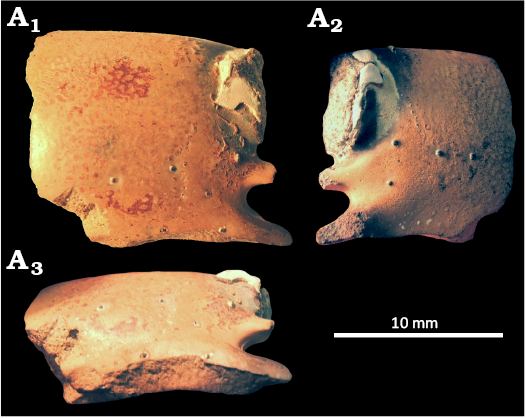
Fig. 4. Ghost shrimp Karumballichirus lakhraensis (Hyžný & Charbonnier in Hyžný et al., 2016a) comb. nov., Paleocene of Lakhra Dome coal mine field (Bara Formation, Paleocene, Thanetian?), Pakistan. MNHN.F.A47685, right major propodus in outer lateral (A1), inner lateral (A2), and ventral (A3) views.
Remarks.—Hyžný et al. (2016a) assumed that the growth rate of Neocallichirus lakhraensis was largely the same as the one of Neocallichirus karumba; the assumption was based on striking morphological similarities between both taxa as shown by Hyžný et al. (2016a: fig. 5). Based on an extrapolation of data on the growth of N. karumba (Dworschak 2008), Hyžný et al. (2016a) estimated the total length of the largest individuals of N. lakhraensis to be 110 mm. The establishment of Karumballichirus allows the re-assignment of N. lakhraensis to the respective genus.
Charbonnier et al. (2013) reported a single isolated propodus from the ?Thanetian (upper Paleocene) of southern Pakistan. They interpreted it tentatively as a minor chela propodus of Calliax sp. Personal re-examination of the specimen by one of us (MH) revealed that the specimen is not complete and what appeared to be a short fixed finger is actually part of a broken fixed finger, much larger than anticipated previously (Fig. 4).
Stratigraphic and geographic range.—?Thanetian (upper Paleocene)–Ypresian (lower Eocene) of Pakistan (Charbonnier et al. 2013; Merle et al. 2014; Hyžný et al. 2016a).
Karumballichirus maximus (A. Milne-Edwards, 1870) comb. nov.
Fig. 5.
1870 Callianassa maxima; Milne-Edwards 1870: 97, pl. 2: 5.
non 1915 Callianassa maxima Milne-Edwards, 1870; Kemp 1915: 252, pl. 13: 1–5.
non 1954 Callianassa maxima Milne-Edwards, 1870; Pillai 1954: 23, figs. 1–5.
non 1981 Callianassa (Callichirus) maxima Milne-Edwards, 1870; Daniel 1981: 193, pl. 6: 1A–I.
Material.—Lectotype: MNHN.F.A74264, left major chela (propodus and dactylus) (Fig. 5A; Milne-Edwards 1870: pl. 2: 5); paralectotype: MNHN.F.A74265, right major chela (propodus and dactylus) (Fig. 5B). The type material was originally composed of several syntypes, the two of which were recently rediscovered by one of us (SC) in the palaeontological collections at the MNHN, Paris.
Description.—Major cheliped manus subquadrate; upper and lower margins keeled and distinctly serrated, especially the lower one; lower margin concave at junction with fixed finger; distal margin with large, sharp tooth at articulation with dactylus. Inner and outer lateral surfaces densely covered with evenly spaced tubercles. Major cheliped fixed finger approximately as long as manus; lateral surface with faint ridge with row of seven large tubercles running onto manus; occlusal surface unarmed. Major cheliped dactylus long and slender, approximately four times longer than high; upper margin proximally adorned with tubercles; lateral surfaces tuberculated proximally and adorned with complex setal pores; occlusal margin armed with complex molariform protuberance proximally with two blunt teeth and followed by a gap, one peg-like blunt tooth, another gap and a saw-like series of teeth ending with a sharp curved fingertip.

Fig. 5. Ghost shrimp Karumballichirus maximus (Milne-Edwards, 1870) comb. nov. from the Holocene (subfossil strata) of Thailand. A. MNHN.F.A74264 (lectotype), left major chela (propodus and dactylus) in outer lateral (A1), proximal (A2), inner lateral (A3), ventral (A4), and dorsal (A5) views, the occlusal view of dactylus (A6). B. MNHN.F.A74265 (paralectotype), right major chela (propodus and dactylus) in outer lateral (B1) and inner lateral (B2) views, occlusal view of dactylus (B3).
Remarks.—Callianassa maxima was described based on several subfossil chelae found during the construction of a channel in the territory of present-day Thailand (Milne-Edwards 1870). Kemp (1915), Pillai (1954) and Daniel (1981) provided a more detailed description based on more complete material from India, including larval stages (Daniel 1981). Sakai (1999), however, argued that the material of Kemp (1915) differs from C. maxima and erected a new species for it, Neocallichirus kempi. Dworschak (2008) re-investigated the specimens attributed at that time to C. maxima (except its type material) and N. kempi, and considered them conspecific with Callianassa karumba; Callianassa maxima was considered a separate species by Dworschak (2008). The type material of Callianassa maxima was considered lost (Sakai 1999: 103) and found again in the collection of MNHN.F; photographs of it are provided herein for the very first time. The material has been suggested to conform to the intraspecific variation of K. karumba (Peter C. Dworschak, personal communication 2019). However, we have not studied the type material of C. karumba and N. kempi first-hand, so we are reluctant to synonymise these taxa with C. maxima at present.
It should be noted that C. maxima was described based on more than one specimen, as is clearly mentioned by Milne-Edwards (1870: 97–98). However, all subsequent authors erroneously stated that it was described based on a single subfossil chela (Kemp 1915: 252; Pillai 1954: 23; Daniel 1981: 193; Sakai 1999: 103; Dworschak 2008: 75).
Schweitzer et al. (2010) incorrectly attributed the authorship of C. maxima to A. Milne-Edwards (1860) instead of Milne-Edwards (1870).
Stratigraphic and geographic range.—Holocene (subfossil strata) of Thailand (A. Milne-Edwards 1870).
Karumballichirus tuberculatus (Lőrenthey in Lőrenthey and Beurlen, 1929)
Fig. 6.
1929 Calianassa [sic!] tuberculata Lőrenthey; Lőrenthey and Beurlen 1929: 51, pl. 1: 9.
2006 Neocallichirus borensis; Beschin et al. 2006: 97, fig. 2, pl. 1: 4–6.
2016 Neocallichirus tuberculatus (Lőrenthey in Lőrenthey and Beurlen, 1929); Hyžný et al. 2016a: 350, fig. 6D–F.
2020 Karumballichirus tuberculatus (Lőrenthey in Lőrenthey and Beurlen, 1929); Hyžný and Zorn 2020: 21, pl. 5: 3–5. [cum. syn.]
Material.—Callianassa tuberculata syntypes: MBFSZ E9465 (16 specimens with collective repository number), isolated major cheliped elements (carpus, propodus, dactylus) from the middle Eocene of Kosd, Hungary; Neocallichirus borensis holotype: MCZ 2423, isolated left major propodus from the Eocene (Priabonian) of Priabona, Italy; additional specimens: GA 2010/035/0021, isolated left major propodus from the Eocene (Guttaring Group) of Guttaring, Austria; GA 2010/266/0001, isolated right major propodus with broken fixed finger from the Eocene of Meledo Basso, Italy; GA 2010/029/0008, incomplete right major propodus from the Eocene (Priabonian) of Mortisa, Belluno, Italy.
Description.—A detailed description of the species was provided by Lőrenthey and Beurlen (1929) and is therefore not repeated here.
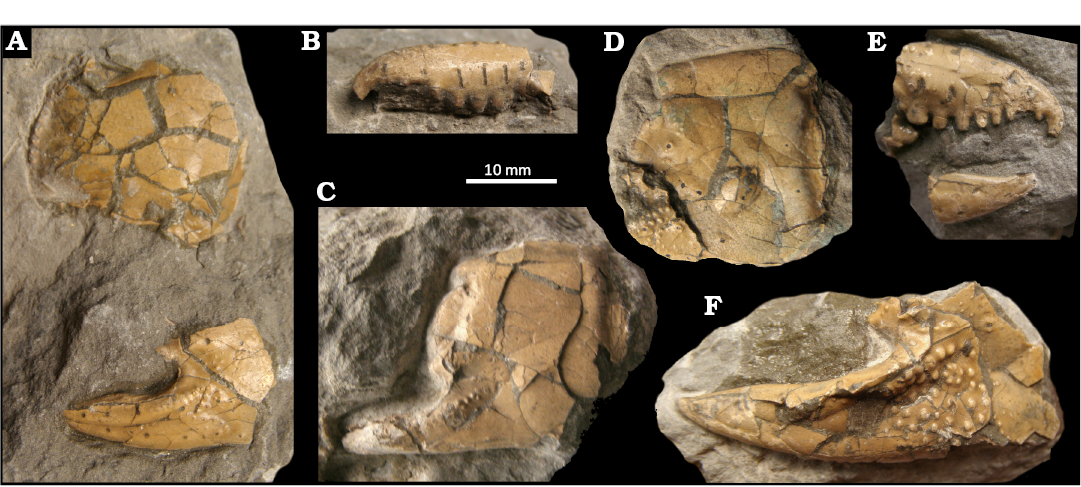
Fig. 6. Ghost shrimp Karumballichirus tuberculatus (Lőrenthey in Lőrenthey and Beurlen, 1929), middle Eocene of Kosd, Hungary. A–F. MBFSZ E9465 (syntype collection of Callianassa tuberculata), consisting of isolated major cheliped elements: carpus (A), propodus (C, D), fixed finger (A, F), and dactylus (B, E).
Remarks.—Lőrenthey in Lőrenthey and Beurlen (1929) described Callianassa tuberculata based on a number of fragmentary specimens from the middle Eocene of Hungary. It is important to note that his figure (Lőrenthey in Lőrenthey and Beurlen 1929: pl. 1: 9) is a reconstruction and does not represent any particular specimen from the studied sample. Nevertheless, the largest fragment probably belonged to an animal with a total length exceeding 180 mm, as already suggested by Hyžný et al. (2016a). Neocallichirus borensis, originally described from the upper Eocene of Italy (Beschin et al. 2006), has recently been considered a junior subjective synonym of Callianassa tuberculata by Hyžný and Zorn (2020). They also reassigned the species to the genus Karumballichirus.
Stratigraphic and geographic range.—Eocene of Hungary (Lőrenthey and Beurlen 1929), Italy (Beschin et al. 2006; Hyžný and Zorn 2020), and Austria (Hyžný and Zorn 2020).
Karumballichirus sp.
Fig. 7.
Material.—MSNM i28030 (collective number), one entire right major cheliped dactylus and two fingertips of left major cheliped dactyli. It was collected in the surrounding of the village of Berivotra, ca. 50 km S of the city of Mahajanga, NW Madagascar where the Berivotra Formation of the late Maastrichtian age crops out (Rogers et al. 2000). Brief overview of the accompanying fauna (including other decapods) was presented by Garassino and Pasini (2003).
Description.—Major cheliped dactylus long and slender, approximately seven times longer than tall. Upper margin proximally adorned with tubercles and with five complex pores accommodating tufts of setae during life. Lateral surfaces tuberculated proximally and adorned with complex setal pores; outer lateral surface with two distinct rows of tubercles positioned in the upper half and five large elongated setal pores perpendicular to the dactylus-longitudinal axis; inner lateral surface with tubercles arranged in less-distinct rows and adorned with setal pores with oval outline. Occlusal margin armed with a number of teeth; complex molariform protuberance positioned proximally with four blunt teeth and followed by a gap, two peg-like blunt teeth of unequal length, another gap and a saw-like series of 10+ teeth ending with a sharp curved fingertip.
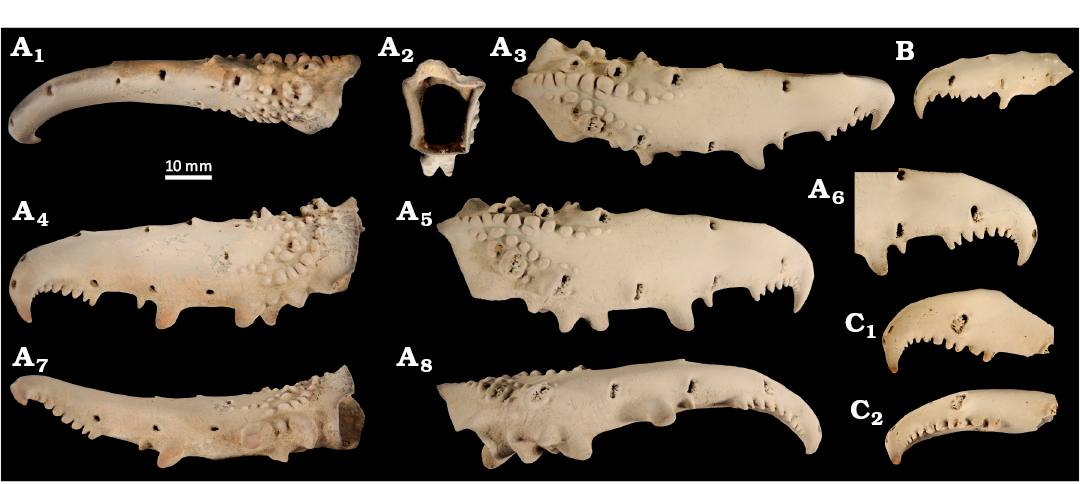
Fig. 7. Ghost shrimp Karumballichirus sp. (MSNM i28030), Maastrichtian of Berivotra, Madagascar. A. Right major cheliped dactylus in dorsal (A1), proximal (A2), dorso-lateral (A3), inner lateral (A4), outer lateral (A5, A6), and occlusal (A7, A8) views. B. Incomplete left major cheliped dactylus in outer lateral view. C. Incomplete left major cheliped dactylus in outer lateral (C1) and occlusal (C2) views. Specimens are shown to the same scale. All specimens were covered with ammonium chloride prior to photography.
Remarks.—The studied material is fragmentary, no propodus is preserved. Identification based on isolated dactyli of decapod crustaceans may prove difficult; in the present case, however, we argue for assignment to ghost shrimps, and the genus Karumballichirus in particular, based on the following characters:
(i) arrangement of setal pores; ghost shrimps often have large setal pores on the dactylus of the major cheliped, especially on the upper margin and outer lateral surface. In representatives of Karumballichirus (e.g., Dworschak 2008: figs. 4b, 5k), Lepidophthalmus (e.g., Felder and Manning 1997: figs. 1b, 3a, 4d, f) and Callianopsis Saint Laurent, 1973 (Schweitzer Hopkins and Feldmann 1997: fig. 4A, B), to name a few examples, they are elongated and oriented perpendicularly to the longitudinal axis of the dactylus. This character is also present in many ghost shrimp fossils (e.g., Schweitzer Hopkins and Feldmann 1997: fig. 7D; Hyžný and Klompmaker 2015: fig. 4D; Hyžný et al. 2016a: figs. 5, 6D). Moreover, dactyli from Madagascar show rather complex setal pores which, during life, accommodated tufts of setae, a condition which is common in extant ghost shrimps (e.g., Felder and Manning 1997; Dworschak 2008, 2011a, b). Similar setal pores can be found in some hermit crabs (Komai and Rahayu 2014; Fraaije et al. 2015; Hyžný et al. 2016b), their chelae, however, have a completely different general morphology, usually having robust, suboval propodi with short fingers (McLaughlin 2003);
(ii) curvature of the dactylus; ghost shrimps have major cheliped dactyli curved inward in a specific manner; the proximal portion is relatively straight whereas curvature is present in the distal portion close to the tip (Klompmaker et al. 2016a: figs. 14R, 14T, 15C–D);
(iii) dentition; decapod crustaceans are highly variable in the shape of chelipeds and development of their dentition (Schäfer 1954; Glaessner 1969). Often, dentition is regular along the occlusal margin of the fingers. Many ghost shrimps have highly irregular “tooth formulae”, often with large blunt molariform teeth positioned proximally and many near-equal teeth positioned close to the tip (e.g., Pillai 1954; Sakai 1969; Hyžný 2012; Hyžný and Hudáčková 2012; Hyžný and Muñiz 2012). Additionally, a strongly hooked dactylus is rather typical for ghost shrimps (e.g., Sakai 1969; Manning and Felder 1991; Hyžný and Hudáčková 2012), whereas it is fairly uncommon among brachyurans (see some representative figures in Ng et al. 2008 and in Poore and Ahyong 2023). The dentition of the studied dactyli from Madagascar is similar to several fossil and extant ghost shrimps (see discussion below).
The material from Madagascar exhibits a strong resemblance to ghost shrimps of the genus Karumballichirus (as discussed above). Although it most probably belongs to a species yet unknown to science, the material is simply not sufficient to justify the erection of a new taxon. In ghost shrimps, intraspecific variation and sexual dimorphism is commonly expressed in the morphology of the major pereiopod 1 dactylus (as discussed in detail by Hyžný and Klompmaker 2015). Therefore, assignment of the dactyli from Madagascar to the species level must await the discovery of more complete material.
Discussion
Taxonomic evaluation of isolated cheliped elements.—The decapod cheliped is a multifunctional organ, often quite characteristic for respective groups (Schäfer 1954; Lee 1995; Mariappan et al. 2000). Taxonomic evaluation of isolated fossil cheliped elements and their fragments can be challenging.
In brachyuran crabs, for example, the taxonomic evaluation of cheliped fragments can be troublesome due to intraspecific variation and use-induced changes (e.g., Smith and Palmer 1994; Schenk and Wainwright 2001; Silva et al. 2017), even though there have been many attempts to identify them at least to the family level (e.g., Förster 1979a, b; Müller 1984; Kato and Karasawa 1998; Ando et al. 2015, 2016). Therefore, brachyuran claws seldom are in the focus of alpha-taxonomists, but paguroid hermit crabs (e.g., McLaughlin 2003; Komai and Rahayu 2014; Fraaije et al. 2015; Hyžný et al. 2016b) and axiidean shrimps (e.g., Sakai 1969; Schweitzer Hopkins and Feldmann 1997; Hyžný and Klompmaker 2015; Klompmaker et al. 2016a), to name at least two major clades, are commonly classified based on cheliped elements. These are often the only parts that are preserved, and they can be used successfully to assign to low taxonomic ranks. There are even extreme cases where isolated fossil cheliped fingers were used for the erection of new species (e.g., Rathbun 1935), although this has been criticised by Hyžný and Klompmaker (2015). In some ghost shrimps, however, attribution to the genus level is possible based only on isolated major cheliped dactyli. Such is the case of Karumballichirus sp. presented herein.
Careful comparison with extant taxa is always necessary to evaluate fossil remains to some degree of confidence. In this respect, it can be stated that the more specialized the studied animal is, the easier and more straightforward the identification of its fossil remains may be. The cheliped dentition of large ghost shrimps, especially of the callichirid genera discussed above, i.e., Glypturus, Karumballichirus, Lepidopthalmus, and Neocallichirus, exhibits a great array of characters (e.g., tubercles, occlusal teeth and setal pores) which can be evaluated taxonomically and identified in the fossil record.
Size estimates of ghost shrimps based on isolated cheliped elements.—Based on the reported occurrences of modern taxa, the size, i.e., total length of an adult ghost shrimp from the tip of the rostrum to the end of the telson, ranges from about 15 mm to 175 mm (Dworschak 2015, 2018). Of 265 species in 74 genera (Robles et al. 2020), 28 species in 10 genera within the four families Anacalliacidae, Callianassidae, Callichiridae, and Ctenochelidae reach or exceed a total length of 100 mm (Table 1). Within the family Callianopsidae, Lin et al. (2007) reported a male individual of Callianopsis goniophthalma Rathbun, 1902 (USNM 28354) with a carapace length of 27.5 mm. Based on the comparison with other representatives of this species as well as other ghost shrimp taxa, the total length of the respective individual probably reached 100 mm. The largest specimens, reaching at least 150 mm, were documented in one genus (Neotrypaea) within the family Callianassidae and three genera (Callichirus, Glypturus, Lepidophthalmus) within the family Callichiridae.
The maximum size of ghost shrimps remains a mere estimate because it is difficult to catch ghost shrimps, particularly the large-sized tropical representatives (Shinn 1968; Vaugelas 1985; Garcia et al. 2003; Kneer et al. 2013; Dworschak 2015). Because of these constraints, the fossil record could be helpful in providing additional clues, especially considering the suggestion that larger specimens have a greater fossilisation potential (Hyžný and Klompmaker 2015). However, the fossil record is biased towards isolated cheliped elements (Hyžný and Klompmaker 2015) and the size estimate of a once living animal is not straightforward.
A strong correlation between the size of ghost shrimp chelipeds and carapace length has been observed. Therefore, the total length of the animal may be estimated with some certainty using chelipeds. Such estimates must be done carefully, as in some genera allometric growth has been identified in sexually mature animals, including differences in the growth of males and females (e.g., Callichirus, Hernáez and Wehrtmann 2007; Eucalliaxiopsis, Dworschak 2006; Lepidophthalmus, Felder and Lovett 1989; Nates and Felder 1999; Neotrypaea, Labadie and Palmer 1996; Nihonotrypaea, Shimoda et al. 2005). Moreover, the slope of increase can differ greatly between and within species of the same genus (e.g., Paratrypaea, Dworschak 2012: fig. 3), although this apparently does not apply universally to all ghost shrimps.
As for reliable estimates, based on careful examination of numerous specimens, one may refer to Dworschak (2011b, 2018). Dworschak (2011b) reported remains of Neocallichirus vigilax (Man, 1916) with a total length estimated as 124 mm; in this case the size was calculated from regressions of propodus length/carapace length and carapace length/total length. Based on an isolated major cheliped ischium of Glypturus armatus (Milne-Edwards, 1870) collected at Panglao Island, Philippines (Fig. 1C), Dworschak (2018) estimated the total length of the animal as reaching at least 175 mm, making it the largest documented extant ghost shrimp known to date.
To name another aspect that may hinder the correct total length estimate based on isolated fossil chelipeds, and hence evolutionary change, Klompmaker et al. (2016a) demonstrated that the major propodus length/height ratios increased faster throughout growth in geologically older assemblages based on three assemblages of Glypturus spp. from the Late Miocene, Holo-Pleistocene, and modern times. This could be a case of heterochrony, although more data are needed to test this rigorously.
The (sub)fossil taxa discussed briefly in the systematic section above are based on close morphological similarities, considered to represent a single lineage with the modern Karumballichirus karumba. Assuming the growth rate was the same for all species of this lineage, the total length of various animals can be calculated based on the data from their extant representatives. These estimates are summarised in Table 2 and graphically presented in Fig. 8. They demonstrate that a total length exceeding 150 mm was reached by ghost shrimp individuals throughout a stratigraphic span of 70 million years, ranging from Karumballichirus sp. from the Maastrichtian of Madagascar over Karumballichirus tuberculatus from the Eocene of Hungary to the subfossil Karumballichirus maximus. The material of Karumballichirus sp. from Madagascar represents remains of an animal with a total length of approximately 200 mm. Interestingly, within representatives of Glypturus, this length apparently is reached but not exceeded (Table 1, Fig. 1).
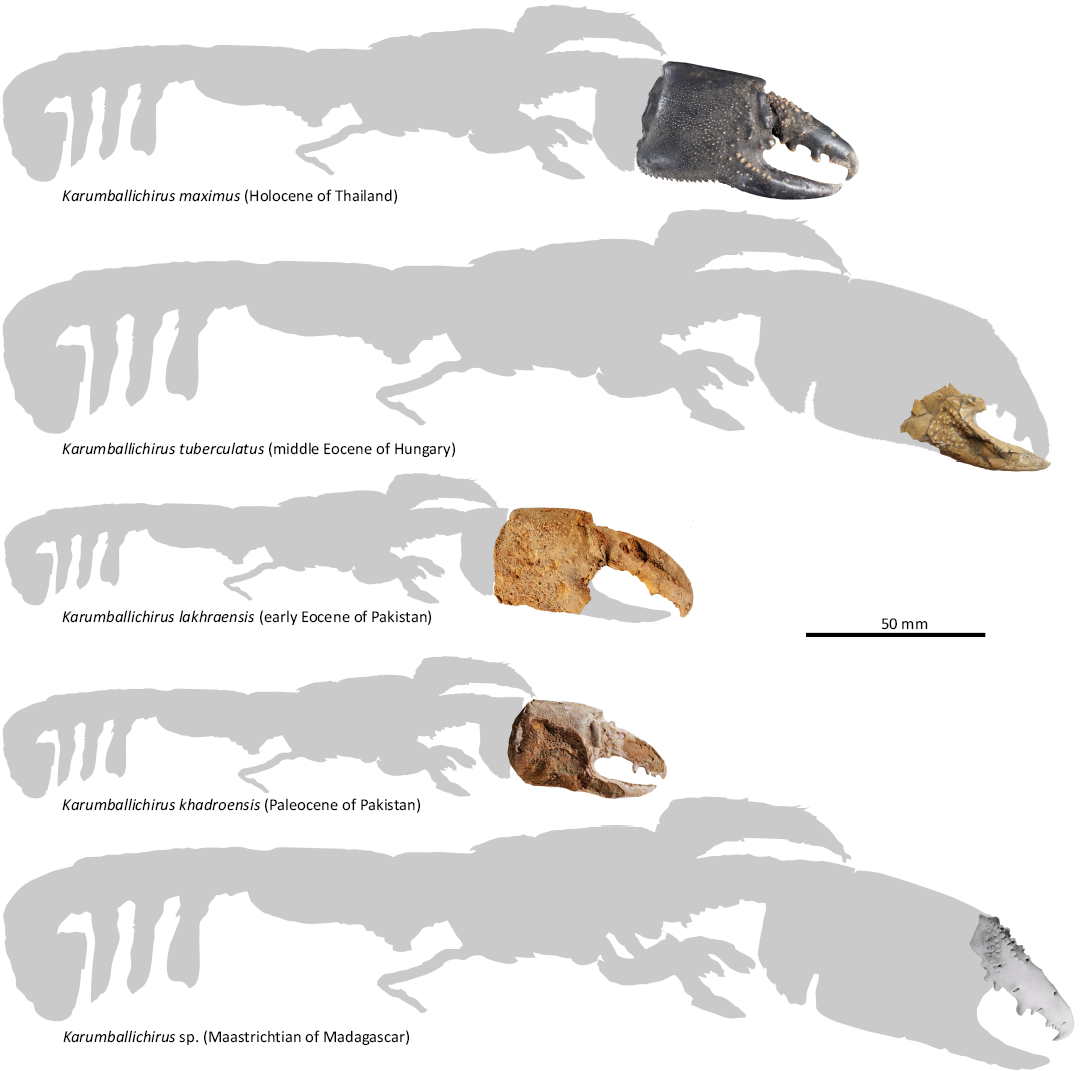
Fig. 8. Graphical representation of the total length estimate for fossil Karumballichirus species (compare with Table 2). The base outline represents extant Karumballichirus karumba (Poore & Griffin, 1979).
How big can ghost shrimps grow?—Other decapods with a comparable (not crab-like) general body shape, e.g., nephropid lobsters (Homarus spp.) and palinurid spiny lobsters can attain a length of up to 600 mm or more (Holthuis 1991). As discussed above, we know there are ghost shrimps with a total length of at least 175 mm, and we also know that this size was attained by some ghost shrimps in the distant past, going back to at least 70 million years ago as documented by the Maastrichtian occurrence reported herein. Is this the size limit of ghost shrimps? So far, no remains of larger ghost shrimp individuals have been found. Interestingly, ghost shrimp with a total length of approximately 200 mm are documented from various ages, including the Maastrichtian, Eocene, and Holocene (Table 2). Even though these data come from a single ghost shrimp lineage (i.e., species of Karumballichirus within the family Callichiridae), they are considered representative herein because there are only a few extant ghost shrimp species known to reach more than 150 mm in length. It appears that indeed the representatives of the family Callichiridae may have been the largest ghost shrimps ever.
Table 2. Fossil callichirid ghost shrimp species exceeding a total length of 100 mm (as based on size estimation discussed in the text).
|
Taxon |
Age |
Occurrence |
Total length |
|
Karumballichirus maximus (Milne-Edwards, 1870) |
Holocene |
Thailand |
~150 mm |
|
Karumballichirus tuberculatus (Lőrenthey in Lőrenthey and Beurlen, 1929) |
middle Eocene |
Hungary |
>180 mm |
|
Karumballichirus lakhraensis (Hyžný & Charbonnier in Hyžný et al., 2016) |
early Eocene |
Pakistan |
~110 mm |
|
Karumballichirus khadroensis (Hyžný & Charbonnier in Hyžný et al., 2016) |
Paleocene |
Pakistan |
~120 mm |
|
Karumballichirus sp. |
Maastrichtian |
Madagascar |
>180 mm |
Ghost shrimp burrows may be indicative of the size of their architects. Although in many ghost shrimps, there is a tight fit between their body width and height and the burrow diameter (Dworschak et al. 2012), the size estimate of the burrow inhabitant can be difficult. Based on burrow casts of extant Neocallichirus vigilax and Glypturus armatus from Sulawesi (Kneer et al. 2013), some sections of the burrows (vertical parts connecting galleries) show a consistent diameter which only permits the passage of a single animal and does not allow it to turn around (presumably this is also where shrimp will position themselves in order to efficiently ventilate their burrows), whereas others (horizontal galleries) can be two- or threefold wider and higher to allow turning around, passing other shrimps and relocating larger shell/coral fragments (Kneer et al. 2013; DK unpublished data). Fossil burrows pose additional problems due to the fact that it may be hard to distinguish between overlapping burrows constructed by different individuals at different times (Bromley and Ekdale 1986; Tedesco and Wanless 1991), and the creators of the burrows are rarely found within their burrows (Hyžný and Klompmaker 2015; Hyžný and Summesberger 2019). Also, the attribution of various ichnogenera to ghost shrimps is not always straightforward, because the ichnotaxa do not relate to taxonomic groups but are based on burrow shape representing behaviour (Bertling et al. 2006).
Both determinate and indeterminate growth occurs in a wide range of decapod groups, and both can occur within the same higher taxon (Hartnoll 1983). Unfortunately, we do not know what the case is in ghost shrimps. However, the fact that growth is indeterminate in many decapod crustaceans, in the sense that moulting does not stop, does not mean that size is unrestricted (Hartnoll 1983). For decapods in general, the percentage of the moult increment (i.e., the percentage of size increase with each moult) declines and the intermoult period increases with size, thereby limiting growth (Hartnoll 1983). A growth rate decrease has not been determined for any ghost shrimp known to the authors. If only hard evidence is considered, all we can say is that the maximum ghost shrimp length is around 200 mm and that this has not changed at least since the Maastrichtian.
Why do ghost shrimps not grow larger?—Two hundred millimetres may be the upper limit to which an animal with a specialized burrowing lifestyle such as a ghost shrimp can grow, because not only does the volume of the animal grow relatively faster than its surface area with increasing size, but so does the volume of the water that needs to be moved to keep the extensive burrow system ventilated. For such specialized burrowers living in often dysoxic/anoxic sediments (Dworschak et al. 2012 and references therein), growing any larger might simply make respiration too energetically costly without bringing any significant other advantages: as deposit feeders, ghost shrimps do not need to grow big to be able to overpower larger prey, and because they are already well protected by their burrows, there is less pressure to grow large to avoid predation than there would be if they spend extended periods of time on the sediment surface.
Today, most ghost shrimp species occur in very shallow (less than 2 m depth) water, and the highest diversity is found in the tropical latitudes (Dworschak 2000, 2005). The largest representatives reaching a total length of 150 mm are known not only from the tropics, as documented by the Callichiridae (Glypturus, Karumballichirus, Lepidophthalmus, and Neocallichirus spp.) (Sakai 1999, 2005; Wicksten 2011; Kneer et al. 2013), but also from temperate regions, as documented in the Anacalliacidae (Anacalliax) and in the Callianassidae (Neotrypaea) (Biffar 1971a; Sakai 1999, 2005). A particularly large size may help to fend off competitors and/or play role in both intrasexual and intersexual fighting; in most documented cases of Neotrypaea spp. fighting, the winners were larger than the losers (Shimoda et al. 2005). In decapods, there is a positive correlation between the body size of females and their fecundity (Corey and Reid 1991; Hines 1991; Reid and Corey 1991a, b), which has been demonstrated also for ghost shrimps (Botter-Carvalho et al. 2007; Hernáez et al. 2008; Rosa-Filho et al. 2013; Peiró et al. 2014; Hernáez and Araujo João 2018). In fact, females of decapods (including ghost shrimps) attain, on average, a larger body size than males (Hernáez 2018; Hernáez et al. 2008). The overview of the largest documented ghost shrimp specimens presented herein (Table 1; SOM) confirms this observation.
Among extant decapods, other large burrowing representatives are the gebiidean Thalassina anomala (Herbst, 1804), reaching a length of up to 300 mm (Holthuis 1991) or even 350 mm (Dworschak 2015), and the astacideans Nephrops norvegicus (Linnaeus, 1758), attaining a length of 240 mm (Holthuis 1991); Acanthacaris caeca (Milne-Edwards, 1881), reaching a length of up to 400 mm (Holthuis 1991); Homarus gammarus (Linnaeus, 1758) and Homarus americanus (Milne Edwards, 1837) which are able to grow up to a length of 600 and 640 mm, respectively (Holthuis 1991). Their burrowing strategy differs from the one employed by ghost shrimps in being much simpler; Thalassina is moving around clumps of mud, with the excavated mud resulting from the burrowing activity forming a chimney or mound over the opening of the burrows (Sankolli 1963; Holthuis 1991; Ngoc-Ho and Saint Laurent 2009), Nephrops and young Homarus digs simple U-shaped tubes (Rice and Chapman 1971; Lawton and Lavalli 1995), and adult Homarus lobsters create hollow spaces under e.g., large stones (Atema and Voigt 1995). The burrows of Acanthacaris are 100 to 200 mm deep and contain near-vertical walls (Correa et al. 2012). Ventilation may also be simpler for Thalassina, Nephrops, and Homarus, as the burrow of Thalassina can be found far above high-tide level and is therefore filled with air during low tides (Sankolli 1963), and the burrows of Nephrops and Homarus are relatively short and unlined, meaning the animals are always relatively close to sources of oxygen due to water circulation; severe hypoxia has not been detected in burrows of Nephrops (Atkinson and Taylor 1988). Outside the Decapoda there is Lysiosquilla maculata (Fabricius, 1793) (Stomatopoda) with a maximum total length of 380 mm (Manning 1998), which is digging relatively straight and short burrows and then stays close to the entrance most of the time waiting to ambush prey, which again makes ventilation relatively simple. More comparative research is needed to explain the advantages of growing large, particularly in malacostracan taxa.
Conclusions
Ghost shrimps are mostly small animals with a total length not exceeding a few centimetres; however, representatives of some tropical taxa can grow to relatively large sizes. The collection and/or literature survey presented herein has yielded a number of ghost shrimp individuals with a total length reaching or exceeding 100 mm, belonging to 10 genera in four families, namely Anacalliacidae (Anacalliax), Callianassidae (Neotrypaea), Callichiridae (Audacallichirus, Callichirus, Corallianassa, Glypturus, Karumballichirus, Lepidophthalmus, Neocallichirus), and Ctenochelidae (Ctenocheles), and possibly also in the family Callianopsidae (Callianopsis). It should be noted that the maximum size of ghost shrimps often remains a mere estimate because it is difficult to catch ghost shrimps, particularly the large-sized tropical representatives. However, large individuals have a greater fossilization potential, so the ghost shrimp fossil record may provide relevant information on the maximum size of these animals. The largest extant ghost shrimp individual reported to date is an individual of Glypturus armatus from Panglao Island, Philippines, with an estimated total length of 175 mm (Dworschak 2018). The existence of even larger animals reaching a total length exceeding 180 mm is documented herein from the Maastrichtian of Madagascar and the middle Eocene of Hungary, with both belonging to the genus Karumballichirus. The overview of both extant and fossil ghost shrimp occurrences suggests that a total length of 200 mm is rarely, if ever, reached or exceeded by these animals. We therefore assume that physiological limits imposed by the specialized burrowing lifestyle might prevent ghost shrimp from growing any larger. In burrowing animals, not only does the volume of the animal grow relatively faster than its surface area with increasing size, but so does the volume of the water that needs to be moved to keep the extensive burrow system, which is typical for ghost shrimps, ventilated. Growing any larger might simply make respiration too energetically costly without bringing any significant other advantages. Large ghost shrimps are mostly deposit feeders, they do not need to grow exceedingly large to be able to overpower big prey, and because they are already well protected by their burrows, there is less pressure to grow large to avoid predation than there would be if they spend extended periods of time on the sediment surface.
Acknowledgements
We thank Emese Bodor (MBFSZ), Viviana Frisone (MCZ), and Alessandro Garassino (MSNM) for the access to the material deposited in respective institutions. Peter C. Dworschak (NHMW) is thanked for discussions on the maximum size of ghost shrimps and for providing some previously unpublished measurements of Glypturus specimens. Jean de Vaugelas (Nice, France) is acknowledged for sharing his observations during his studies of large tropical ghost shrimps. Nancy F. Mercado Salas and Petra Wagner (both ZMH) are thanked for additional information about the specimens housed in their collection. Ni Luh Ulansari Manikan Widayani helped to measure some of the specimens at ZMH. Oliver Coleman, Kristina von Rintelen, and Antje Schwiering (all ZMB) provided access to the material at ZMB, and Karsten Reise (Alfred-Wegener-institute for Polar and Marine Research, Bremerhaven, Germany) and Nils Volkenborn (School of Marine and Atmospheric Sciences, Stony Brook University, USA) are thanked for discussions about ecological aspects of size and body shape. Philippe Loubry and Lilian Cazes (both MNHN) are thanked for photos of N. khadroensis and N. lakhraensis. Denis Audo (MNHN) and an anonymous reviewer are thanked for their constructive criticism which improved the final manuscript. The research of MH was supported by VEGA-2/0169/19, VEGA-2/0106/23 and the Slovak Research and Development Agency under contracts nos. APVV-20-0079 and APVV-22-0523. Funding of the research of DK was granted as part of the German-Indonesian research project “Science for the Protection of Indonesian Coastal Marine Ecosystems II” (SPICE II) by the German Federal Ministry of Education and Research (BMBF, grant 03F0472C). Additional financial support was provided by the Alfred-Wegener-Institute for Polar and Marine Research Bremerhaven (AWI). The permission to carry out fieldwork in Indonesia was granted by the Indonesian Ministry of Research and Technology (RisTek) (permits No. 353/FRP/SM/III/08, 2424/FRP/SM/X/08, 0249/FRP/SM/X/09 and 0023/EXT/FRP/SM/V/2010).
Editor: Andrzej Kaim.
References
Ando, Y., Kawano, S., and Ugai, H. 2015. Fossil stomatopods and decapods from the upper Pleistocene Ogushi Formation, Kyushu, Japan. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen 276: 303–313. Crossref
Ando, Y., Kawano, S., Komatsu, T., and Niitani, M. 2016. Decapod crustaceans from the Pleistocene Oe Formation in Minamishimabara City, Nagasaki Prefecture, Japan. Journal of Fossil Research 48: 16–25 [in Japanese, with English abstract].
Anker, A. and Dworschak, P.C., 2007. Jengalpheops rufus gen. nov., sp. nov., a new commensal alpheid shrimp from the Philippines (Crustacea: Decapoda). Zoological Studies 46: 290–302.
Atema, J. and Voigt, R. 1995. Behavior and sensory biology. In: J.R. Factor (ed.), Biology of the Lobster Homarus americanus, 313–348. Academic Press, New York. Crossref
Atkinson, R.J.A. and Taylor, A.C. 1988. Physiological ecology of burrowing decapods. In: P.S. Meadows and A. Meadows (eds.), The Environmental Impact of Burrowing Animals and Animal Burrows. Symposium of the Zoological Society of London 59: 201–226. Clarendon Press, Oxford.
Berkenbusch, K. and Rowden, A.A. 2003. Ecosystem engineering—moving away from ʽjust-soʼ stories. New Zealand Journal of Ecology 27: 67–73.
Bertling, M., Braddy, S.J., Bromley, R.G., Demathieu, G.R., Genise, J., Mikuláš, R., Nielsen, J.K., Nielsen, K.S.S., Rindsberg, A.K., Schlirf, M., and Uchman, A. 2006. Names for trace fossils: a uniform approach. Lethaia 39: 265–286. Crossref
Beschin, C., De Angeli, A., Checchi, A., and Mietto, P. 2006. Crostacei del Priaboniano di Priabona (Vicenza – Italia settentrionale). Lavori Società Veneziana di Scienze Naturali 31: 95–112.
Biffar, T.A. 1971a. New species of Callianassa (Decapoda, Thalassinidea) from the Western Atlantic. Crustaceana 21: 225–236. Crossref
Biffar, T.A. 1971b. The genus Callianassa (Crustacea, Decapoda, Thalassinidea) in south Florida, with keys to the Western Atlantic species. Bulletin of Marine Science 21: 637–715. Crossref
Bishop, G.A. and Bishop, E.C. 1992. Distribution of ghost shrimp: North Beach, St. Catherines Island, Georgia. American Museum Novitates 3042: 1–17.
Bishop, G.A. and Williams, A.B. 2005. Taphonomy and preservation of burrowing thalassinidean shrimps. Proceedings of the Biological Society of Washington 118: 218–236. Crossref
Bonner, J.T. 2006. Why Size Matters: From Bacteria to Blue Whales. 176 pp. Princeton University Press, Princeton. Crossref
Botter-Carvalho, M.L., Santos, P.J.P., and Carvalho, P.V.V.C. 2007. Population dynamics of Callichirus major (Say, 1818) (Crustacea, Thalassinidea) on a beach in northeastern Brazil. Estuarine, Coastal and Shelf Science 71: 508–516. Crossref
Bromley, R.G. and Ekdale, A.A. 1986. Composite ichnofabrics and tiering of burrows. Geological Magazine 123: 59–65. Crossref
Charbonnier, S., Garassino, A., Pasini, G., Métais, G., Merle, D., Bartolini, A., Brohi, I.A., Solangi, S.H., Lashari, R.A., Welcomme, J.-L., and Marivaux, L. 2013. Early Paleogene decapod crustaceans from the Sulaiman and Kirthar Ranges, Pakistan. Annales de Paléontologie 99: 101–117. Crossref
Corey, S. and Reid, D.M. 1991. Comparative fecundity of decapod crustaceans, I. The fecundity of thirty-three species of nine families of caridean shrimp. Crustaceana 60: 270–294. Crossref
Correa, T.B.S., Eberli, G.P., Grasmueck, M., Reed, J.K., and Correa, A.M.S. 2012. Genesis and morphology of cold-water coral ridges in a unidirectional current regime. Marine Geology 326–328: 14–27. Crossref
Curran, H.A. and Martin, A.J. 2003. Complex decapod burrows and ecological relationships in modern and Pleistocene intertidal carbonate environments, San Salvador Island, Bahamas. Palaeogeography, Palaeoclimatology, Palaeoecology 192: 229–245. Crossref
Daniel, A. 1981. The thalassinid burrowing shrimp, Callianassa (Callichirus) maxima M. Edwards, 1870 (Crustacea: Decapoda: Callianassidae) as a pest in the salt factories in Voyalur in Chingleput District of Tamil Nadu and in Manginapudi in Krshna District of Andhra Pradesh. Bulletin of the Zoological Survey of India 3 (3): 191–204.
Dworschak, P.C. 2000. Global diversity in the Thalassinidea (Decapoda). Journal of Crustacean Biology 20: 238–245. Crossref
Dworschak, P.C. 2005. Global diversity in the Thalassinidea (Decapoda): an update (1998–2004). Nauplius 13: 57–63.
Dworschak, P.C. 2006. A new species of Eucalliax Manning & Felder, 1991 (Decapoda: Callianassidae) from the Philippines. The Raffles Bulletin of Zoology 54: 349–359.
Dworschak, P.C. 2008. Neocallichirus kempi Sakai, 1999, a junior synonym of Callianassa karumba Poore & Griffin, 1979 (Decapoda: Callianassidae). Raffles Bulletin of Zoology 56: 75–84.
Dworschak, P.C. 2011a. Redescription of Callianassa jousseaumei Nobili, 1904, a junior subjective synonym of Callianassa indica de Man, 1905 with description of a new species of Neocallichirus (Decapoda: Axiidea: Callianassidae). Zootaxa 2746: 1–19. Crossref
Dworschak, P.C. 2011b. Redescription of Callianassa vigilax de Man, 1916, a subjective senior synonym of Neocallichirus denticulatus Ngoc-Ho, 1994 (Crustacea: Decapoda: Callianassidae). Annalen des Naturhistorischen Museums in Wien B 112: 137–151.
Dworschak, P.C. 2012. On the identities of Callianassa bouvieri Nobili, 1904, C. maldivensis Borradaile, 1904, and C. gravieri Nobili, 1905 (Crustacea: Decapoda: Callianassidae): a morphometric approach. Zootaxa 3149: 39–56. Crossref
Dworschak, P.C. 2015. Methods collecting Axiidea and Gebiidea (Decapoda): a review. Annalen des Naturhistorischen Museums in Wien B 117: 415–428.
Dworschak, P.C. 2018. Axiidea of Panglao, the Philippines: families Callianideidae, Eucalliacidae and Callichiridae, with a redescription of Callianassa calmani Nobili, 1904. Annalen des Naturhistorischen Museums in Wien B 120: 15–40.
Dworschak, P.C. 2022. On a collection of axiidean shrimp (Decapoda: Callianassidae, Callichiridae and Callianideidae) from the Gulf of Aqaba, Red Sea. Annalen des Naturhistorischen Museums in Wien B 124: 249–264.
Dworschak, P.C., Felder, D.L., and Tudge, C.C. 2012. Infraorders Axiidea de Saint Laurent, 1979 and Gebiidea de Saint Laurent, 1979 (formerly known collectively as Thalassinidea). In: F.R. Schram, J.C. von Vaupel Klein, M. Charmantier-Daures, and J. Forest (eds.), Treatise on Zoology—Anatomy, Taxonomy, Biology. The Crustacea. Vol. 9 Part B, 109–219. Brill, Leiden. Crossref
Fabricius, J.C. 1793. Entomologia systematica emandata et aucta. Secundum classes, ordines, genera, species adjectis synonimis, locis, observationibus descriptionibus. 519 pp. Proft, Christian Gottlob, Hafniae. Crossref
Felder, D.L. 2001. Diversity and ecological significance of deep-burrowing macrocrustaceans in coastal tropical waters of the Americas (Decaoida: Thalassinidea). Intersciencia 26: 440–449.
Felder, D.L. 2003. Ventrally sclerotized members of Lepidophthalmus (Crustacea: Decapoda: Callianassidae) from the Eastern Pacific. Annalen des Naturhistorischen Museums in Wien B 104: 429–442.
Felder, D.L. and Lovett, D.L. 1989. Relative growth and sexual maturation in the estuarine ghost shrimp Callianassa louisianensis Schmitt, 1935. Journal of Crustacean Biology 4: 540–553. Crossref
Felder, D.L. and Manning, R.B. 1997. Ghost shrimps of the genus Lepidophthalmus from the Caribbean region, with description of L. richardi, new species, from Belize (Decapoda: Thalassinidea: Callianassidae). Journal of Crustacean Biology 17: 309–331. Crossref
Felder, D.L. and Manning, R.B. 1998. A new ghost shrimp of the genus Lepidophthalmus from the Pacific coast of Columbia (Decapoda: Thalassinidea: Callianassidae). Proceedings of the Biological Society of Washington 111: 398–408.
Förster, R. 1979a. Decapod crustaceans from the Korytnica basin (Middle Miocene; Holy Cross Mountains, Central Poland). Acta Geologica Polonica 29: 253–268.
Förster, R. 1979b. Decapod crustaceans from the Middle Miocene (Badenian) deposits of Southern Poland. Acta Geologica Polonica 29: 89–106.
Fraaije, R.H.B., van Bakel, B.W.M., and Jagt, J.W.M. 2015. A new Albian hermit crab (Anomura, Paguroidea) from France—another example of capsulated setae in an extinct form. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen 277: 353–359. Crossref
Garassino, A. and Pasini, G. 2003. First record of Calappoidea, Portunoidea and Dromioidea in the Upper Cretaceous (Upper Maastrichtian) of NW Madagascar. Bulletin of the Mizunami Fossil Museum 30: 121–135. Crossref
Garcia, K.E., Embry, S.J., Grossblat, D., Holbrook, A.-M., McLaren, W.M., Reed, S.K., Wildey, H.C., and Shuster, S.M. 2003. A comparison of two methods for sampling the Gulf of California mud shrimp, Neotrypaea uncinata (Crustacea: Thalassinidea). Journal of Natural History 37: 1847–1854. Crossref
Glaessner, M.F. 1969. Decapoda. In: R.C. Moore (ed.), Treatise on Invertebrate Paleontology, Part R. Arthropoda 4 (2), R399–R533. Geological Society of America, Boulder, and University of Kansas Press, Lawrence.
Hartnoll, R.G. 1983. Strategies of crustacean growth. In: J.K. Lowry (ed.), Papers From the Conference on the Biology and Evolution of Crustacea, Held at the Australian Museum Sydney, 1980. Australian Museum Memoir 18: 121–131. Crossref
Herbst, J.F.W. 1782–1804. Versuch einer Naturgeschichte der Krabben und Krebse, nebst einer systematischen Beschereibung ihrer verschiedenen Arten. Vol. 1 (1782–1790): 1–274; Vol. 2 (1791–1796): i–viii+1–226; Vol. 3 (1799–1804): 1–66, 1–46, 1–54, 1–49. Bei Gottlieb August Lange, Berlin.
Hernáez, P. 2018. An Update on Reproduction in Ghost Shrimps (Decapoda: Axiidea) and Mud Lobsters (Decapoda: Gebiidea). In: M. Türkoğlu, U. Onal, and A. Ismen (eds.), Marine Ecology—Biotic and Abiotic Interactions, 231–253. Intechopen, London. Crossref
Hernáez, P. and Araujo João, M.C. 2018. Social structure, sexual dimorphism and relative growth in the ghost shrimp Callichirus seilacheri (Bott, 1955) (Decapoda, Axiidea, Callianassidae) from the tropical eastern Pacific. Marine Biology Research 14: 856–867. Crossref
Hernáez, P. and Wehrtmann, I.S. 2007. Population biology of the burrowing shrimp Callichirus seilacheri (Decapoda: Callianassidae) in northern Chile. Revista de Biología Tropical (International Journal of Tropical Biology and Concervation 55 (Supplement 1): 141–152. Crossref
Hernáez, P., Palma, S., and Wehrtmann, I.S. 2008. Egg production of the burrowing shrimp Callichirus seilacheri (Bott, 1955) (Decapoda, Callianassidae) in northern Chile. Helgoland Marine Research 62: 351–356. Crossref
Hines, A.H. 1991. Fecundity and reproductive output in nine species of Cancer crabs (Crustacea, Brachyura, Cancridae). Canadian Journal of Fisheries and Aquatic Sciences 48: 267–275. Crossref
Holmes, S.J. 1904. On some new or imperfectly known species of West American Crustacea. Proceedings of the California Academy of Sciences (Series 3) 3: 307–328.
Holthuis, L.B. 1991. FAO Species Catalog. Vol. 13. Marine Lobsters of the World. An Annotated and Illustrated Catalogue of Species of Interest to Fisheries Known to Date. FAO Fisheries Synopsis. No. 125, Vol. 13. 292 pp. FAO, Rome.
Hyžný, M. 2012. Calliaxina chalmasii (Brocchi, 1883) comb. nov. (Decapoda: Axiidea: Callianassidae: Eucalliacinae), a ghost shrimp from the Middle Miocene of Europe, with reappraisal of the fossil record of Eucalliacinae. Zootaxa 3492: 49–64. Crossref
Hyžný, M. and Hudáčková, N. 2012. Redescription of two ghost shrimps (Decapoda: Axiidea: Callianassidae) from the Middle Miocene of the Central Paratethys: systematics, intraspecific variation, and in situ preservation. Zootaxa 3210: 1–25. Crossref
Hyžný, M. and Klompmaker, A.A. 2015. Systematics, phylogeny, and taphonomy of ghost shrimps (Decapoda): a perspective from the fossil record. Arthropod Systematics & Phylogeny 73: 401–437. Crossref
Hyžný, M. and Muñiz, F. 2012. Podocallichirus laepaensis, a new ghost shrimp (Crustacea, Decapoda, Callianassidae) from the Late Miocene of Southwest Spain. Journal of Paleontology 86: 616–625. Crossref
Hyžný, M. and Summesberger, H. 2019. A new species of Mesostylus (Decapoda, Axiidea, Callianassidae)—A peep into the private life of a Late Cretaceous burrowing shrimp. Cretaceous Research 101: 108–123. Crossref
Hyžný, M. and Zorn, I. 2020. A catalogue of the fossil decapod crustaceans in the collections of the Geological Survey of Austria in Vienna. Abhandlungen der Geologischen Bundesanstalt 74: 1–111.
Hyžný, M., Charbonnier, S., Merle, D., Lashari, A., Bartolini, A., and Métais, G. 2016a. New Early Cenozoic ghost shrimps (Decapoda, Axiidea, Callianassidae) from Pakistan and their palaeobiogeographic implications. Geodiversitas 38: 341–353. Crossref
Hyžný, M., Fraaije, R.H.B., Martin, J.E., Perrier, V., and Sarr, R. 2016b. Paracapsulapagurus poponguinensis, a new hermit crab (Decapoda: Anomura: Paguroidea) from the Maastrichtian of Senegal. Journal of Paleontology 90: 1133–1137. Crossref
Kato, H. and Karasawa, H. 1998. Pleistocene fossil decapod Crustacea from the Boso Peninsula, Japan. Natural History Research, Special Issue 5: 1–31.
Kemp, S. 1915. Fauna of the Chilka Lake No. 3. Crustacea Decapoda. Memoirs of the Indian Museum 5: 199–325. Crossref
Kishinouye, K. 1926. Two rare and remarkable forms of macrurous Crustacea from Japan. Annotationes Zoologicae Japonenses 11: 63–70.
Klompmaker, A.A., Hyžný, M., Portell, R.W., and Kowalewski, M. 2016a. Growth, inter- and intraspecific variation, palaeobiogeography, taphonomy, and systematics of the Cenozoic ghost shrimp Glypturus. Journal of Systematic Palaeontology 14: 99–126. Crossref
Klompmaker, A.A., Jakobsen, S.L., and Lauridsen, B.W. 2016b. Evolution of body size, vision, and biodiversity of coral-associated organisms: evidence from fossil crustaceans in cold-water and tropical coral ecosystems. BMC Evolutionary Biology 16: 132. Crossref
Klompmaker, A.A., Schweitzer, C.E., Feldmann, R.M., and Kowalewski, M. 2015. Environmental and scale-dependent evolutionary trends in the body size of crustaceans. Proceedings of the Royal Society B 282: 20150440. Crossref
Kneer, D., Asmus, H., and Jompa, J. 2013. Do burrowing callianassid shrimp control the lower boundary of tropical seagrass beds? Journal of Experimental Marine Biology and Ecology 446: 262–272. Crossref
Komai, T. and Rahayu, D.L. 2014. New records and new species of the hermit crab genus Pagurus Fabricius, 1775 (Crustacea: Decapoda: Anomura: Paguridae) from the Philippines. Raffles Bulletin of Zoology 62: 620–646. Crossref
Komai, T., Maenosono, T., and Osawa, M. 2015. Records of three species of callianassid ghost shrimp from the genera Glypturus Stimpson, 1866 and Corallianassa Manning, 1987 (Crustacea: Decapoda: Axiidea) from the Ryukyu Islands, Japan, with remarks on the taxonomic status of the two genera. Fauna Ryukyuana 27: 13–59.
Kornienko, E.S. 2013. Burrowing shrimp of the infraorders Gebiidea and Axiidea (Crustacea: Decapoda). Russian Journal of Marine Biology 39: 1–14. Crossref
Labadie, L.V. and Palmer, A.R. 1996. Pronounced heterochely in the ghost shrimp, Neotrypaea californiensis (Decapoda: Thalassinidea: Callianassidae): allometry, inferred function and development. Journal of Zoology 240: 659–675. Crossref
Latreille, P.A. 1802–1803. Histoire naturelle, générale et particulière des crustacés et des insectes. 468 pp. F. Dufart, Paris. Crossref
Lawton, P. and Lavalli, K.L. 1995. Postlarval, juvenile, adolescent, and adult ecology. In: J.R. Factor (ed.), Biology of the Lobster Homarus americanus, 47–88. Academic Press, New York. Crossref
Lee, S.Y. 1995. Cheliped size and structure: the evolution of a multifunctional decapod organ. Journal of Experimental Marine Biology and Ecology 193: 161–176. Crossref
Lin, F.-J., Komai, T., and Chan, T.-Y. 2007. First record of the thalassinidean genus Callianopsis de Saint Laurent, 1973 (Decapoda, Ctenochelidae) in the West Pacific, with the description of a new species from Taiwan. Crustaceana 80: 1193–1203. Crossref
Linnaeus, C. 1758. Systema naturæ per regna tria naturæ, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Tomus I. Editio decima, reformata. 824 pp. Laurentius Salvius, Stockholm. Crossref
Lőrenthey, E. and Beurlen, K. 1929. Die fossilen Dekapoden der Länder der Ungarischen Krone. Geologica Hungarica, Series Palaeontologica 3: 1–421.
Man, J.G. de 1916. Description of a new species of the genus Callianassa Leach and of a species of the genus Alpheus Fabr., both from the Indian archipelago. Zoologische Mededeelingen, Leiden 2: 57–61.
Manning, R.B. 1987. Notes on western Atlantic Callianassidae (Crustacea: Decapoda: Thalassinidea). Proceedings of the Biological Society of Washington 100: 386–401.
Manning, R.B. 1998. Stomatopods. In: K.E. Carpenter and V.H. Niem (eds.), FAO Species Identification Guide for Fishery Purposes. The Living Marine Resources of the Western Central Pacific. Vol. 2. Cephalopods, Crustaceans, Holothurians and Sharks, 827–849. FAO, Rome.
Manning, R.B. and Felder, D.L. 1991. Revision of the American Callianassidae (Crustacea: Decapoda: Thalassinidea). Proceedings of the Biological Society of Washington 104: 764–792.
Manning, R.B. and Felder, D.L. 1995. Description of the ghost shrimp Sergio mericeae, a new species from south Florida, with reexamination of S. guassutinga (Crustacea: Decapoda: Callianassidae). Proceedings of the Biological Society of Washington 108: 266–280.
Mariappan, P., Balasundaram, C., and Schmitz, B. 2000. Decapod crustacean chelipeds: an overview. Journal of Biosciences 25: 301–313. Crossref
Maszczyk, P. and Brzeziński, T. 2018. Body size, maturation size, and growth rate of crustaceans. In: G.A. Wellborn and M. Thiel (eds.), The Natural History of the Crustacea. Life Histories, Vol. 5, 35–67. Oxford University Press, Oxford. Crossref
McLaughlin, P.A. 2003. Illustrated keys to families and genera of the superfamily Paguroidea (Crustacea: Decapoda: Anomura), with diagnoses of genera of Paguridae. In: R. Lemaitre and C.C. Tudge (eds.), Biology of the Anomura. Proceedings of a symposium at the Fifth International Crustacean Congress, Melbourne, Australia, 9–13 July 2001. Memoirs of Museum Victoria 60: 111–144. Crossref
Merle, D., Pacaud J.-M., Métais, G., Bartolini, A., Lashari, R.A., Brohi, I.A., Solangi, S.H., Marivaux, L., and Welcomme, J.-L. 2014. Volutidae (Mollusca: Gastropoda) of the Lakhra Formation (Earliest Eocene, Sindh, Pakistan): systematics, biostratigraphy and paleobiogeography. Zootaxa 3826 (1): 101–138. Crossref
Milne Edwards, H. 1834–1840. Histoire Naturelle des Crustacés, Comprenant l´Anatomie, la Physiologie et la Classification de ces Animaux. Vol. 1 (1834): i–xxxv+1–468; Vol. 2 (1837): 1–532; Vol. 3 (1840): 1–638. Encyclopédique Roret, Paris.
Milne-Edwards, A. 1860. Monographie des décapodes macroures fossiles de la famille des Thalassiniens. Annales de Sciences Naturelles, Zoologie (série 4) 14: 294–357.
Milne-Edwards, A. 1870. Révision du genre Callianassa (Leach) et description de plusieurs espèces nouvelles de ce groupe faisant partie de la collection du Muséum. Nouvelles Archives du Muséum d’Histoire naturelle de Paris 6: 75–102. Crossref
Milne-Edwards, A. 1881. Description de quelques crustacés macroures provenant des grandes profondeurs de la Mer des Antilles. Annales des Sciences naturelles, Zoologie, sér. 6 11 (4): 1–16.
Müller, P. 1984. Decapod Crustacea of the Badenian. Geologica Hungarica, Series Palaeontologica 42: 3–317.
Nates, S.F. and Felder, D.L. 1999. Growth and maturation of the ghost shrimp Lepidophthalmus sinuensis Lemaitre and Rodrigues 1991 (Crustacea, Decapoda, Callianassidae), a burrowing pest in penaeid shrimp culture ponds. Fishery Bulletin 97: 526–541.
Ng, P.K.L., Guinot, D., and Davie, P.J.F. 2008. Systema Brachyurorum: Part I. An annotated checklist of extant brachyuran crabs of the world. The Raffles Bulletin of Zoology (Supplement 17): 1–286.
Ngoc-Ho, N. and Saint Laurent, M. de 2009. The genus Thalassina Latreille, 1806 (Crustacea: Thalassinidea: Thalassinidae). The Raffles Bulletin of Zoology (Supplement 20): 121–158.
Nickel, L.A. and Atkinson, R.J.A. 1995. Functional morphology of burrows and trophic modes of three thalassinidean shrimp species, and a new approach to the classification of thalassinidean burrow morphology. Marine Ecology Progress Series 128: 181–197. Crossref
Peiró, D.F., Wehrtmann, I.S., and Mantelatto, F.L. 2014. Reproductive strategy of the ghost shrimp Callichirus major (Crustacea: Axiidea: Callianassidae) from the southwestern Atlantic: sexual maturity of females, fecundity, egg features, and reproductive output. Invertebrate Reproduction & Development 58: 294–305. Crossref
Peters, R. 1983. The Ecological Implications of Body Size. 329 pp. Cambridge University Press, Cambridge. Crossref
Pillai, N.K. 1954. A note on Callianassa maxima M. Edwards (Decapoda). Bulletin of the Central Research Institute of the University of Travancore 30: 23–26.
Poore, G.C. B. and Ahyong, S.T. 2023. Marine Decapod Crustacea. A Guide to Families and Genera of the World. 916 pp. CSIRO Publishing and CRC Press, Clayton. Crossref
Poore, G.C.B. and Griffin, D.J.G. 1979. The Thalassinidea (Crustacea: Decapoda) of Australia. Records of the Australian Museum 32: 217–321. Crossref
Poore, G.C.B. and Suchanek, T.H. 1988. Glypturus motupore, a new callianassid shrimp (Crustacea: Decapoda) from Papua New Guinea with notes on its ecology. Records of the Australian Museum 40: 197–204. Crossref
Poore, G.C.B., Dworschak, P.C.D., Robles, R., Mantelatto, F.L., and Felder, D.L. 2019. A new classification of Callianassidae and related families (Crustacea: Decapoda: Axiidea) derived from a molecular phylogeny with morphological support. Memoirs of Museum Victoria 78: 73–146. Crossref
Powell, A.W.B. 1949. New species of Crustacea from New Zealand of the genera Scyllarus and Ctenocheles, with notes on Lyreidus fastigatus. Records of the Auckland Institute Museum 3: 368–371.
Rathbun, M.J. 1902. Descriptions of new decapod crustaceans from the west coast of North America. Proceedings of the United States National Museum 24: 885–905. Crossref
Rathbun, M.J. 1935. Fossil Crustacea of the Atlantic and Gulf Coastal Plain. Geological Society of America Special Papers 2: 1–viii + 1–160. Crossref
Reid, D.M. and Corey, S. 1991a. Comparative fecundity of decapod crustaceans, II. The fecundity of fifteen species of anomuran and brachyuran crabs. Crustaceana 61: 175–189. Crossref
Reid, D.M. and Corey, S. 1991b. Comparative fecundity of decapod crustaceans, III. The fecundity of fifty-three species of Decapoda from tropical, subtropical, and boreal waters. Crustaceana 61: 308–316. Crossref
Retamal, M.A. 1975. Descripción de una nueva especie del genero Callianassa y clave para reconocer las especies Chilenas. Boletín de la Sociedad de Biología de Concepción 49: 177–183.
Rice, A.L. and Chapman, C.J. 1971. Observations on the burrows and burrowing behaviour of two mud-dwelling decapod crustaceans, Nephrops norvegicus and Goneplax rhomboides. Marine Biology 10: 330–342. Crossref
Robles, R., Dworschak, P.C., Felder, D.L., Poore, G.C., and Mantelatto, F.L. 2020. A molecular phylogeny of Callianassidae and related families (Crustacea: Decapoda: Axiidea) with morphological support. Invertebrate Systematics 34: 113–132. Crossref
Rodrigues, S. de A. 1971. Mud shrimps of the genus Callianassa Leach from the Brazilian coast (Crustacea, Decapoda). Arquivos de Zoologia 20: 191–223. Crossref
Rogers, R.R., Hartman, J.H., and Krause, D.W. 2000. Stratigraphic analysis of Upper Cretaceous rocks in the Mahajanga Basin, northwestern Madagascar: implications for ancient and modern faunas. Journal of Geology 108: 275–301. Crossref
Rosa-Filho, J.S., Girard, T.C., and Frédou, F.L. 2013. Population dynamics of the burrowing shrimp Lepidophthalmus siriboia Felder and Rodrigues, 1993 (Reptantia: Axiidea: Callianassidae) on the Amazonian coast. Journal of Crustacean Biology 33: 503–511. Crossref
Rowden, A.A. and Jones, M.B. 1993. Critical evaluation of sediment turnover estimates for Callianassidae (Decapoda: Thalassinidea). Journal of Experimental Marine Biology and Ecology 173: 265–272. Crossref
Saint Laurent, M. de 1973. Sur la systématique et la phylogénie des Thalassinidea: définition des familles des Callianassidae et des Upogebiidae et diagnose de cinq genres nouveaux (Crustacea Decapoda). Comptes Rendus Hebdomadaires de Séances de l’Académie des Sciences (série D) 277: 513–516.
Saint Laurent, M. de 1979. Sur la classification et la phylogénie des Thalassinides: définitions de la superfamille des Axioidea, de la sous-famille des Thomassiniinae et de deux genres nouveaux (Crustacea Decapoda). Comptes Rendus Hebdomadaires de Séances de l’Académie des Sciences, Paris 288: 1395–1397.
Saint Laurent, M. de and Le Loeuff, P. 1979. Campagnes de la Calypso au large des côtes Atlantiques Africaines (1956 et 1959) (suite). 22. Crustacés Décapodes Thalassinidea. I. Upogebiidae et Callianassidae. In: J. Forest (ed.), Résultats Scientifiques des Campagnes de la Calypso. Fasc. 11 (22). Annales de l’Institut Océanographique, Monaco et Paris 55 (Supplement): 29–101.
Sakai, K. 1969. Revision of Japanese callianassids based on the variations of larger cheliped in Callianassa petalura Stimpson and C. japonica Ortmann (Decapoda: Anomura). Publications of the Seto Marine Biological Laboratory 17: 209–252. Crossref
Sakai, K. 1970. Supplementary description of Callianassa (Callichirus) tridentata von Martens (Crustacea, Thalassinidea). Publications of the Seto Marine Biological Laboratory 17 (6): 393–401. Crossref
Sakai, K. 1988. A new genus and five new species of Callianassidae (Crustacea: Decapoda: Thalassinidea) from northern Australia. The Beagle, Occasional Papers of the Northern Territory Museum of Arts and Sciences 5: 51–69. Crossref
Sakai, K. 1999. Synopsis of the family Callianassidae, with keys to subfamilies, genera and species, and the description of new taxa (Crustacea: Decapoda: Thalassinidea). Zoologische Verhandelingen, Leiden 326: 1–152.
Sakai, K. 2005. Callianassoidea of the world (Decapoda: Thalassinidea). Crustaceana Monographs 4: 1–285. Crossref
Sakai, K. 2011. Axioidea of the World and a reconsideration of the Callianassoidea (Decapoda, Thalassinidea, Callianassida). Crustaceana Monographs 13: 1–520. Crossref
Sakai, K. 2015. A revised list of all ghost shrimps (Callianassidea and Thalassinidea) (Decapoda, Pleocyemata) from the Red Sea area, with a new genus, Lepidophthalminus gen. nov. and two new species in the genera Gilvossius and Neocallichirus. Crustaceana 88: 422–448. Crossref
Sakai, K. and Türkay, M. 2014. A review of the collections of the infraorders Thalassinidea Latreille, 1831 and Callianassidea Dana, 1852 (Decapoda, Pleocyemata) lodged in three German museums, with revised keys to the genera and species. Crustaceana 87: 129–211. Crossref
Sankolli, K.N. 1963. On the occurrence of Thalassina anomala (Herbst), a burrowing crustacean, in Bombay waters, and its burrowing methods. Journal of the Bombay Natural History Society 60: 600–605.
Schäfer, W. 1954 Form und Funktion der Brachyuren-Schere. Abhandlungen der Senckenbergischen Naturforschenden Gesellschaft 489: 1–65.
Schenk, A.C. and Wainwright, P.C. 2001. Dimorphism and the functional basis of claw strength in six brachyuran crabs. Journal of Zoology 255: 105–119. Crossref
Schweitzer Hopkins, C. and Feldmann, R.M. 1997. Sexual dimorphism in fossil and extant species of Callianopsis de Saint Laurent. Journal of Crustacean Biology 17: 236–252. Crossref
Schweitzer, C.E., Feldmann, R.M., Garassino, A., Karasawa, H., and Schweigert, G. 2010. Systematic list of fossil decapod crustacean species. Crustaceana Monographs 10: 1–222. Crossref
Shimoda, K., Wardiatno, Y., Kubo, K., and Tamaki, A. 2005. Intraspecific behaviors and major cheliped sexual dimorphism in three congeneric callianassid shrimps. Marine Biology 146: 543–557. Crossref
Shinn, E.A. 1968. Burrowing in Recent lime sediments of Florida and the Bahamas. Journal of Paleontology 42: 879–894.
Silva, A.C.F., Shapouri, M., Cereja, R., Dissanayake, A., and Vinagre, C. 2017. Variations in crab claw morphology and diet across contrasting inter-tidal habitats. Marine Ecology 38: e12374. Crossref
Smith, L.D. and Palmer, A.R. 1994. Effects of manipulated diet on size and performance of brachyuran crab claws. Science 264: 710–712. Crossref
Stamhuis, E.J., Schreurs, C.E., and Videler, J.J. 1997. Burrow architecture and turbative activity of the thalassinid shrimp Callianassa subterranea from the central North Sea. Marine Ecology Progress Series 151: 155–163. Crossref
Stimpson, W. 1866. Descriptions of new genera and species of macrurous Crustacea from the coasts of North America. Proceedings of the Chicago Academy of Sciences 1: 46–48.
Tedesco, L.P. and Wanless, H.R. 1991. Generation of sedimentary fabrics and facies by repetitive excavation and storm infilling of burrow networks, Holocene of South Florida and Caicos Platform, B.W.I. Palaios 6: 326–343. Crossref
Türkay, M. 1988. Göttinger Crustaceensammlung als Dauerleihgabe bei Senckenberg. Natur und Museum 118: 253–254.
Vaugelas, J. de 1985. A new technique for collecting large-sized callianassid mud-shrimp (Decapoda, Thalassinidea). Crustaceana 49: 105–109. Crossref
Vaugelas, J. de and Saint Laurent, M. de 1984. Premières données sur l’écologie de Callichirus laurae de Saint Laurent sp. nov. (Crustaceé Décapode Callianassidae): son action bioturbatrice sur les formations sédimentaires du golfe d’Aqaba (Mer Rouge). Comptes Rendus Hebdomadaires de Séances de l’Académie des Sciences, Paris 298 (6): 147–152.
Ward, M. 1945. A new crustacean. Memoirs of the Queensland Museum 12: 134–135.
White, A. 1861. Descriptions of two species of Crustacea belonging to the families Callianassidae and Squillidae. Proceedings of the Scientific Meetings of the Zoological Society of London 1861: 42–44.
Wicksten, M.K. 2011. Decapod Crustacea of the Californian and Oregonian Zoogeographic Provinces. 413 pp. Scripps Institution of Oceanography, UC San Diego. Crossref
Ziebis, W., Förster, S., Huettel, M., and Jørgensen, B.B. 1996. Complex burrows of the mud shrimp Callianassa truncata and their geochemical impact in the sea bed. Nature 382: 619–622. Crossref
Acta Palaeontol. Pol. 70 (1): 97–113, 2025
https://doi.org/10.4202/app.01112.2023