

A new attachment trace of a verrucid barnacle on Pliocene bivalve shells, Santa Maria Island, Azores
ALFRED UCHMAN, MAX WISSHAK, PATRÍCIA MADEIRA, CARLOS S. MELO, CLAUDIA SACCHETTI, GONÇALO CASTELA ÁVILA, and SÉRGIO P. ÁVILA
Uchman, A., Wisshak, M., Madeira, P., Melo, C.S., Sacchetti, C., Ávila, G.C., and Ávila, S.P. 2025. A new attachment trace of a verrucid barnacle on Pliocene bivalve shells, Santa Maria Island, Azores. Acta Palaeontologica Polonica 70 (1): 143–157.
A new attachment trace belonging to the ichnogenus Centrichnus has been recognized on bivalve shells in a Pliocene coquina of the Pedra-que-Pica section in Santa Maria Island (Azores Archipelago). The new ichnospecies Centrichnus dentatus isp. nov. is characterized by an elliptical outline, bounded by a groove and/or a series of pits, and by having a more or less pronounced central to off-center depression surrounded by a flat area. Based on these new findings, the diagnosis of the ichnogenus Centrichnus is emended, as is the diagnosis of the ichnofamily Centrichnidae. The new trace fossil was produced by the barnacle Verruca spengleri, which was found in direct association with the trace. Some specimens of Centrichnus dentatus isp. nov. were found cross-cut by phoronid borings (Talpina isp.) or clionaid sponge borings (Entobia isp.), and they co-occur with polychaete borings (Maeandropolydora isp.) and bivalve borings (Gastrochaenolites isp.). The traces belong to the Gnathichnus ichnofacies, which refers to the early colonization of hard substrates taking place within months, even though the recorded ichnocoenoses suggest longer exposure and colonization by several generations of cirripeds, lasting several years rather than months.
Key words: Cirripedia, Centrichnus, bioerosion, ichnotaxonomy, etching trace, Azores, Portugal.
Alfred Uchman [alfred.uchman@uj.edu.pl; ORCID: https://orcid.org/0000-0002-0591-777X ] (corresponding author), Institute of Geological Sciences, Jagiellonian University, Gronostajowa 3a, PL-30-387 Kraków, Poland.
Max Wisshak [max.wisshak@senckenberg.de; ORCID: https://orcid.org/0000-0001-7531-3317 ], Senckenberg am Meer, Marine Research Department, Südstrand 40, 26382 Wilhelmshaven, Germany.
Patrícia Madeira [patricia.ga.madeira@uac.pt; ORCID: https://orcid.org/0000-0003-4356-9769 ] and Carlos S. Melo [csmelo@ciencias.ulisboa.pt; ORCID: http://orcid.org/0000-0001-7825-3858 ], CIBIO-Açores, Research Centre in Biodiversity and Genetic Resources, InBio Associate Laboratory, MPB-Marine PalaeoBiogeography Lab, University of the Azores; Faculty of Sciences and Technology, Rua da Mãe de Deus, 9500 321 Ponta Delgada, Portugal; UNESCO Chair – Land Within Sea: Biodiversity & Sustainability in Atlantic Islands, University of the Azores, Rua da Mãe de Deus, 9500 321 Ponta Delgada, Portugal.
Claudia Sachcetti [claudia.sacchetti@cibio.up.pt; ORCID: https://orcid.org/0000-0002-3225-3139 ] and Gonçalo Castela Ávila [goncalorenteavila@gmail.com; ORCID: https://orcid.org/0009-0009-5361-3015 ], CIBIO-Açores, Research Centre in Biodiversity and Genetic Resources, InBio Associate Laboratory, MPB-Marine PalaeoBiogeography Lab, University of the Azores; Faculty of Sciences and Technology, Rua da Mãe de Deus, 9500 321 Ponta Delgada, Portugal.
Sérgio P. Ávila [avila@uac.pt; ORCID: http://orcid.org/0000-0003-4317-3051 ], CIBIO-Açores, Research Centre in Biodiversity and Genetic Resources, InBio Associate Laboratory, BIOPOLIS Program in Genomics, Biodiversity and Land Planning; UNESCO Chair – Land Within Sea: Biodiversity & Sustainability in Atlantic Islands, University of the Azores; MPB-Marine PalaeoBiogeography Lab, University of the Azores; and Faculty of Sciences and Technology, Rua da Mãe de Deus, 9500 321 Ponta Delgada, Portugal.
Received 31 October 2024, accepted 15 January 2025, published online 19 March 2025.
Copyright © 2025 A. Uchman et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
In oceanic settings, volcanic islands offer a relief from the deep seafloor where remote rocky shores are first established when seamounts rise above the sea (Ramalho et al. 2013). Insular systems are viewed as key, yet dynamic and discrete settings for the study of the processes and factors shaping biological communities. In recent years, a large volume of research has been published targeting biota associated with these remote nearshore hard-substrate habitats (e.g., Wisshak et al. 2011, 2015a). Despite hard substrates covering a relatively minor proportion of the marine benthic environment globally (Gutiérrez et al. 2022), biodiversity within this realm could be considered an important component of island biota and the first witness of the early stages of colonization in these remote settings.
Among hard substrate dwellers, many benthic organisms attach themselves to the substrate in order to stabilise their body position and protect against waves and predators. In the fossil record, hard substrate assemblages can offer a snapshot into the geologic past (though incomplete) as they preserve mostly the sessile components of the original communities, particularly encrusting and boring organisms which tend to retain their original positions on the substrate after fossilisation (Taylor and Wilson 2003). However, in oceanic islands, particularly those without reefs, erosion tends to occur rapidly once eruption has ceased (Woodroffe 2014). In these nearshore hard-substrate environments exposed to high energy conditions, biological communities can be regarded as having a low preservation potential, as the presence of past communities is often reduced to traces of their biologic activity.
Among encrusting organisms, some etch the substrate and leave characteristic traces of a recurrent morphology. These traces are called attachment scars or traces (e.g., Radwański 1977) and have ichnotaxonomic names (e.g., Bromley and Heinberg 2006; Neumann et al. 2015; Wisshak et al. 2019, and references therein). They belong to the ethological category fixichnia (Gibert et al. 2004). Their list is not exhaustive, and several new ichnotaxa have been added in the last two decades, including Spirolites radwanskii Uchman et al., 2018, from the Miocene of central Poland, Solealites ovalis Uchman & Rattazzi, 2018, from the Oligocene of NW Italy, or Santichnus mayorali Verde et al., 2022, from the Miocene–Pliocene of Uruguay. Most of the attachment traces belong to the ichnofamilies Centrichnidae Wisshak et al., 2019, and Renichnidae Wisshak et al., 2019.
In this paper, a new attachment trace produced by a verrucid barnacle on bivalve shells is described from the palaeosite Pedra-que-Pica on Santa Maria Island, Azores (Fig. 1). Its distinct morphology, association with the skeletal remains of the producer, not previously known to the island’s fossil record, and well-determined palaeoenvironmental context, pose an opportunity for its accurate interpretation. This is also an opportunity to discuss the ichnofamily Centrichnidae and its type ichnogenus Centrichnus, whose diagnoses and content of ichnotaxa require updates. In this backdrop, the present study offers an important tool for the study of hard substrate communities whether in a geologic past or recent setting.
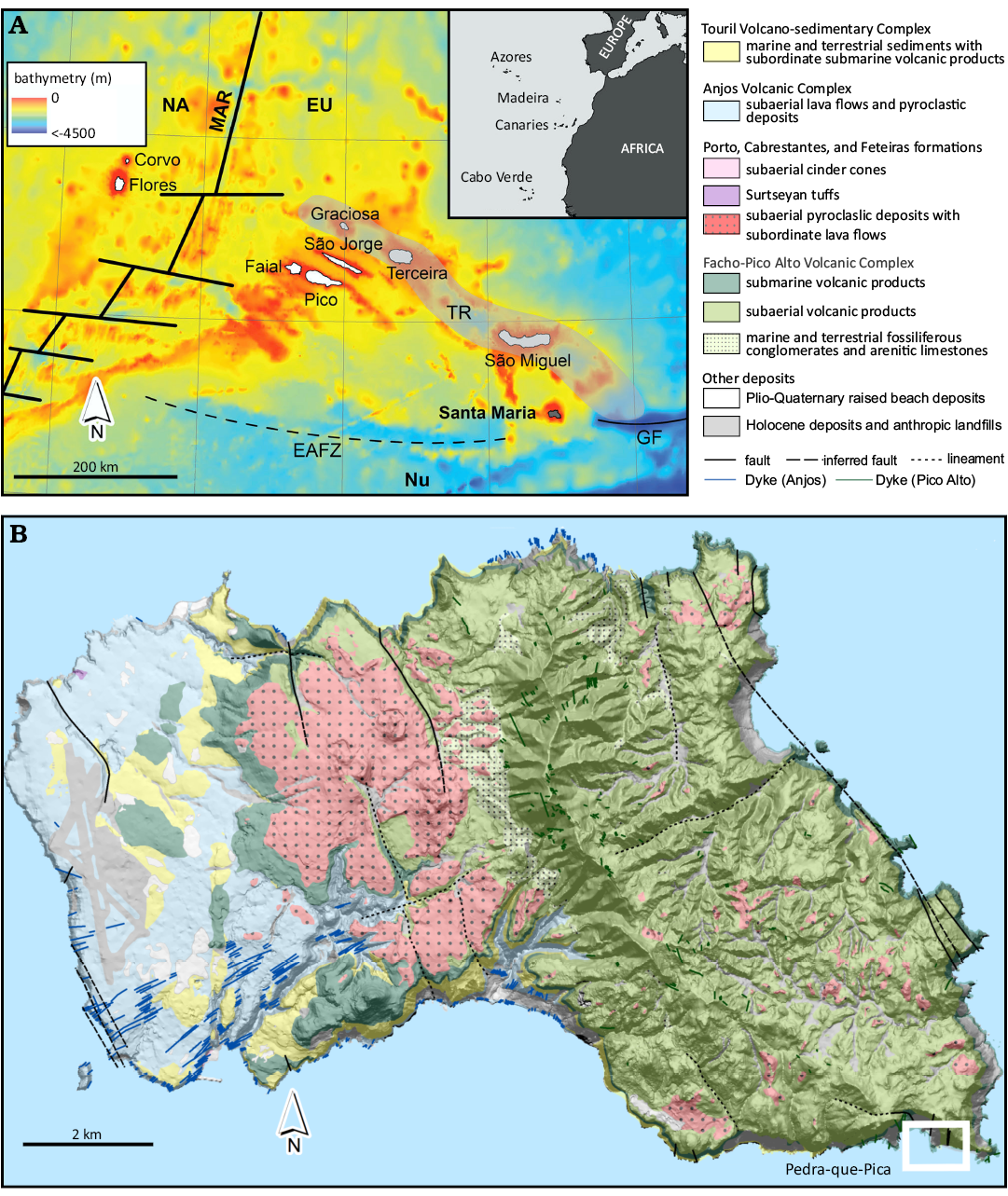
Fig. 1. A. Location of the Azores Archipelago within the NE Atlantic (insert) and Santa Maria within the Azores Archipelago. NA, North American plate; EU, Eurasian plate; Nu, Nubian (African) plate; MAR, Mid‐Atlantic Ridge; TR, Terceira Rift; EAFZ, East Azores Fracture Zone; GF, Gloria Fault. Bathymetry extracted from GEBCO 2019 (https://www.gebco.net/data_and_products/gridded_bathymetry_data/); coastline delimitation from the Portuguese Hydrographic Institute free data (https://www.hidrografico.pt/op/33). B. Simplified geological map of Santa Maria Island (adapted from Serralheiro et al. 1987; Serralheiro 2003) with location of Pedra-que-Pica outcrop (rectangle).
Institutional abbreviations.—DBUA-F, fossil collection of the Department of Biology of the University of the Azores, Ponta Delgada, Portugal; INGUJ, Institute of Geological Sciences, Jagiellonian University (collection in the Nature Education Centre of the Jagiellonian University-Museum of Geology), Kraków, Poland.
Nomenclatural acts.—This published work and the nomenclatural acts it contains have been registered in ZooBank: urn:lsid:zoobank.org:pub:2B3446B6-477E-42EC-9A7F-8B874523F6E5.
Geological setting
The remote Azores Archipelago is in the Central Atlantic Ocean (36–43°N, 25–31°W), in a complex geotectonic setting where three tectonic plates interact: the North American plate to the west of the Mid-Atlantic Ridge, the Eurasian plate to the north, and the Nubian (African) plate to the south (Fig. 1A). The archipelago is composed of nine islands that spread for about 600 km. Santa Maria Island is the oldest (6.0 Ma; Ramalho et al. 2017) and the only with a relevant marine fossil record (for a review see Ávila et al. 2018 and references therein; Fig. 1B).
Pedra-que-Pica (Fig. 2A) is one of the most important palaeosites in Santa Maria Island. Classified as an international relevant geosite by Ávila et al. (2016) and Raposo et al. (2018), this early Pliocene coquina (Fig. 2B) with a Zanclean age ranging from 4.78±0.13 to 4.13±0.19 Ma (Sibrant et al. 2015; Ramalho et al. 2017) was described in detail by Ávila et al. (2015b, 2022). Located near the SE tip of Santa Maria, the shell bed of Pedra-que-Pica consists of a 10–11 m thick sedimentary sequence of which the uppermost 3–4 m are exposed above present sea level. Including its underwater section, the coquina occupies a total area of about 23,450 m2 (Ávila et al. 2015b, 2018). Two spurs located to the West of Pedra-que-Pica (Fig. 2A) acted as natural barriers against currents, protecting the sediments from erosion. The shells and deposits that form the coquina accumulated in a large, natural, depression that was preserved by the later deposition of the volcano-sedimentary sequence above (Fig. 2G; Ávila et al. 2015b, 2022). Three events were involved in this process: the first shell bed and its fossiliferous deposits (Fig. 2G) rest on top of a basalt pillow lava (Fig. 2G) that was covered by a discontinuous 30–60 cm thick-bed of subangular to subrounded basaltic cobbles and boulders (Fig. 2G), that, by its turn, was covered by a second massive layer of coquina up to 2.6 m thick (Fig. 2G), that was finally covered by volcanic tuffs (Fig. 2G) (Ávila et al. 2015b, 2018, 2022). This topmost coquina layer is poorly sorted and contains large, disarticulated valves of bivalves (Fig. 2C), barnacles (Winkelmann et al. 2010; Fig. 2D), gastropods (Janssen et al. 2008; Sacchetti et al. 2023), echinoids (Madeira et al. 2011; Fig. 2E), brachiopods (Kroh et al. 2008), ostracods (Meireles et al. 2012), bryozoans, decapod crustaceans (Hyžný et al. 2021), calcareous algae forming rhodoliths (Rebelo et al. 2016), small stony-corals and, more rarely, teeth of bony fishes (Ávila et al. 2020) and sharks (Ávila et al. 2012, 2015a; Fig. 2F), as well as ribs of cetaceans (Estevens and Ávila 2007; Ávila et al. 2015a). The described trace fossil occurs here as well.

Fig. 2. The Pedra-que-Pica outcrop, a unique geosite with Zanclean (lower Pliocene) coquina in Santa Maria Island, Azores. A. Aerial view of Pedra-que-Pica outcrop (in dashed ellipse) in the southern coast of Santa Maria Island. B. General view of the outcrop. C. Bivalve shell of Gigantopecten latissimus (Brocchi, 1814), field photograph. D. The balanid Zullobalanus santamariaensis Buckeridge & Winkelmann in Wilkenmann et al., 2010, photographed at the surface of the Pedra-que-Pica outcrop, Santa Maria Island, Azores (Winkelmann et al. 2010). E. Fragment of a test of the echinoderm Eucidaris tribuloides (Lamarck, 1816), DBUA-F 455 (Madeira et al. 2011). F. Tooth of the shark Carcharodon hastalis (Agassiz, 1838), DBUA-F 275 in labial view (Ávila et al. 2012). G. Stratigraphic section at Pedra-que-Pica representing the main lithologies, sedimentary structures, contacts and fossiliferous content present in the Pliocene sedimentary and volcanic successions of Anjos, Touril and Pico Alto Volcanic Complexes (sensu Serralheiro et al. 1987; Serralheiro 2003; and Ramalho et al. 2017). Numbers correspond to the depositional units as described in Ávila et al. (2015b, 2018).
In the topmost section of the coquina, within a matrix of faintly bedded calcarenite, several types of ichnofossils are visible, the most conspicuous being Asterosoma isp. and Bichordites isp. (Ávila et al. 2022). Then, a sudden change occurs in the succession, with the calcarenite passing into a thick unit of 36 m water-settled lapilli tuffs resulting from a Surtseyan eruption (Ávila et al. 2015b, 2018a). This layer of tuffs (Fig. 2G) belongs to the Pico Alto Complex (Ávila et al. 2015a, 2018, 2022) and is capped by a conglomerate (Fig. 2G) that is overlain by a lava delta sequence constituted of a series of pillow lavas and hyaloclastite, as well as a set of subaerial flows (Ávila et al. 2015b). The boundary between the subaerial and submarine flows traces the coeval sea level, nowadays located at about 50 m above present mean sea level (Ávila et al. 2022).
Systematic palaeoichnology
Ichnofamily Centrichnidae Wisshak et al., 2019
Type ichnogenus: Centrichnus Bromley & Martinell, 1991.
Original diagnosis.—Single to multiple, roughly circular depressions on the surface of hard substrates, shallower than wide, with individual grooves often arranged concentrically or excentrically (from Wisshak et al. 2019).
Emended diagnosis.—Roughly circular, elliptical, or tear-shaped bioerosion structures on the surface of hard substrates, shallower than wide.
Remarks.—The original diagnosis includes only the “roughly circular depressions”, while Centrichnus eccentricus Bromley & Martinell, 1991, the type ichnospecies of Centrichnus Bromley & Martinell, 1991, being representative of Centrichnidae, is tier-shaped (as is Lacrimichnus Santos et al., 2003). Reinvestigation of C. eccentricus has furthermore shown that the complete morphology includes not only that tier-shaped byssus attachment scar but also abrasion marks of the anomiid trace maker’s shell margin and hinge area (Neumann et al. 2015). This fact was originally accounted for by the, now deleted, “Single to multiple”, which was misleading because it would have included clusters of several attachment scars produced by several individuals. Instead, the more complex morphology of C. eccentricus is now accommodated by using the term “structures” instead of “depressions”. The reference to the concentric/eccentric grooves has been deleted to use a broader definition while retaining a clear distinction to the related ichnofamily Renichnidae (Wisshak et al. 2019).
The family Centrichnidae includes Augoichnus Arendt, 2012, Centrichnus Bromley & Martinell, 1991, Patellichnus Dragastan in Dragastan et al., 1998 (see also Brustur 2020), Lacrimichnus Santos et al., 2003, Ophthalmichnus Wisshak et al., 2014, Solealites Uchman et al., 2018, and Tremichnus Brett, 1985 (for ichnotaxonomic history of Tremichnus see Wisshak et al. 2015b and Buatois et al. 2017), according to a primary assignment (Wisshak et al. 2019). The ichnotaxonomic status of Thatchtelithichnus Zonneveld et al., 2015, known from the turtle plastrons and bones (see Collareta et al. 2021), is still under debate. It was initially established as a trace fossil but later considered a bioclaustration structure (Wisshak et al. 2019) and recently defended again as a trace fossil (Zonneveld and Bartels 2020), in which case it should be included in the Centrichnidae.
Ichnogenus Centrichnus Bromley & Martinell, 1991
Type ichnospecies: Centrichnus eccentricus Bromley & Martinell, 1991, Pleistocene deposits (Würmian glacial period), Palamós, Girona, Catalonia, Spain.
Original diagnosis.—Shallow biogenic etching traces on carbonate lithic or skeletal substrates comprising centrically arranged arcuate or ring-shaped grooves (from Bromley and Martinell 1991).
Emended diagnosis.—Roughly circular to elliptical, or tear-shaped bioerosion structures on the surface of carbonate lithic or skeletal substrates, shallower than wide, comprising centrically arranged arcuate or ring-shaped grooves, surrounded or bounded by a deeper groove and/or a series of pits.
Remarks.—The original diagnosis is emended to better cover all morphologies under this ichnogenus, including the more complex morphology of the type ichnospecies C. eccentricus. The variance of the outline and the shallow nature of the depression have been added (see also remarks to the ichnofamily Centrichnidae) and the trace margin has been characterised as “surrounded or bounded by a deeper groove and/or a series of pits”. The latter addition also suits the inclusion of the new ichnospecies described herein. Furthermore, it better accommodates the morphology of the former ichnospecies of Anellusichnus Santos et al., 2005, regarded by Wisshak et al. (2019) as a subjective junior synonym of Centrichnus Bromley & Martinell, 1991.
Centrichnus dentatus isp. nov.
Figs. 3–8.
Zoobank LSID: urn:lsid:zoobank.org:act:2B3446B6-477E-42EC-9A 7F- 8B874523F6E5.
Etymology: From Latin dentatus, toothed; in reference to the marginal series of pits formed by the verrucids anchoring structures.
Type material: Holotype INGUJ249P204, a distinct two-step depression (marked “h” in Fig. 3A1, A2, A5, A6) in an oyster shell with well-expressed collar. Paratypes all other specimens in INGUJ249P204 (Figs. 3A1–A4, 4A, B) and INGUJ249P115 (Fig. 4C), isolated or partly overlapping depressions showing different depths and more or less developed bounding grooves or series of pits.
Type locality: Pedra-que-Pica palaeosite, Santa Maria Island, Azores, Portugal.
Type horizon: Zanclean, lower Pliocene, coquina of the Touril Complex.
Material.—Type material, 19 small bivalve shells or valve fragments (DBUA-F 95-12, 99-15, 18, 20, 30, 180-1, 185-2, 10, 17, 24, 25, 186-4, 10, 25, 187-2, 4, 15, 19, 193-2) with a total of about 70 attachment traces, and 33 small bivalve shells or valve fragments (collective number DBUA-F 1545: INGUJ249P115, 116, 119–123, 125–138, 145, 150, 156, 157, 186, 192, 201–205a, b, 206), with a total of about 400 attachment traces, and one thin section (INGUJ249P207) crossing a trace vertically.
Diagnosis.—Centrichnus circular or elliptical in outline, bounded by a groove and/or a series of pits and having a more or less pronounced central to decentral depression surrounded by a flat area.
Description.—Regular to irregular circular or oval, smooth bowl-like depression on bivalve shells, usually 2–3 mm and occasionally up to 4 mm in diameter, encircled by a narrow groove and small pits piercing the bottom of the groove (Figs. 3–7). The marginal groove is ca. 0.1 mm wide. The pits are trapezoid with very rounded corners in outline, ca. 0.1 mm wide (longer axis), and ca. 0.1 mm apart (Fig. 5A) with the longer axis commonly oriented perpendicular to the groove. The number of pits varies between individual borings and ranges from 25 to 70.
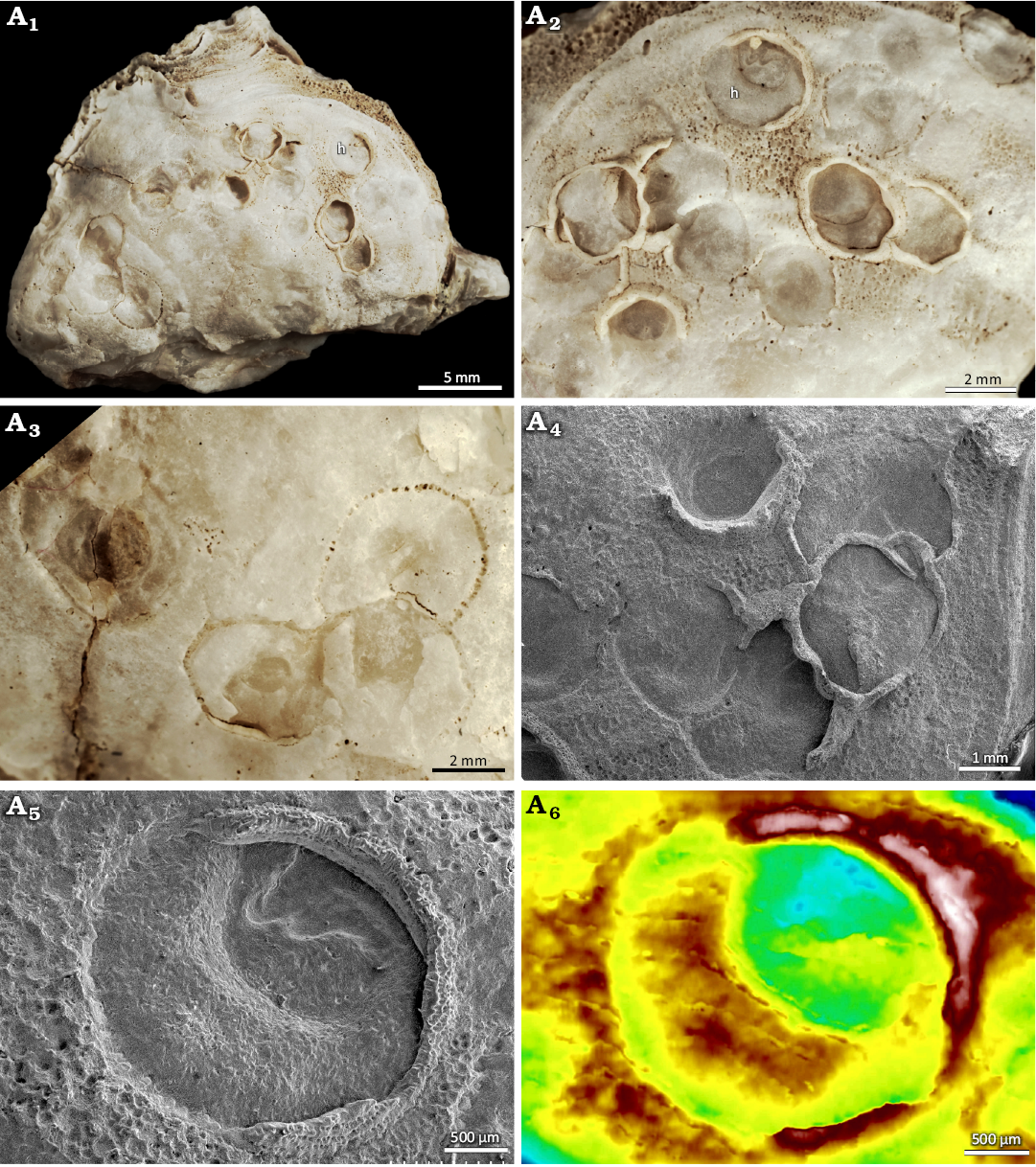
Fig. 3. The verrucid barnacle attachment trace Centrichnus dentatus isp. nov. on an oyster host shell (probably Ostrea stentina Payraudeau, 1826 sensu lato, DBUA-F 1545: INGUJ249P204) from Pedra-que-Pica, lower Pliocene, Santa Maria, Azores. General view (A1) and close ups (A2, A3) of the shell with the holotype (h) and paratypes. SEM image showing a few paratypes (A4). SEM image of the holotype (A5). Colour-coded topography of the holotype (A6).
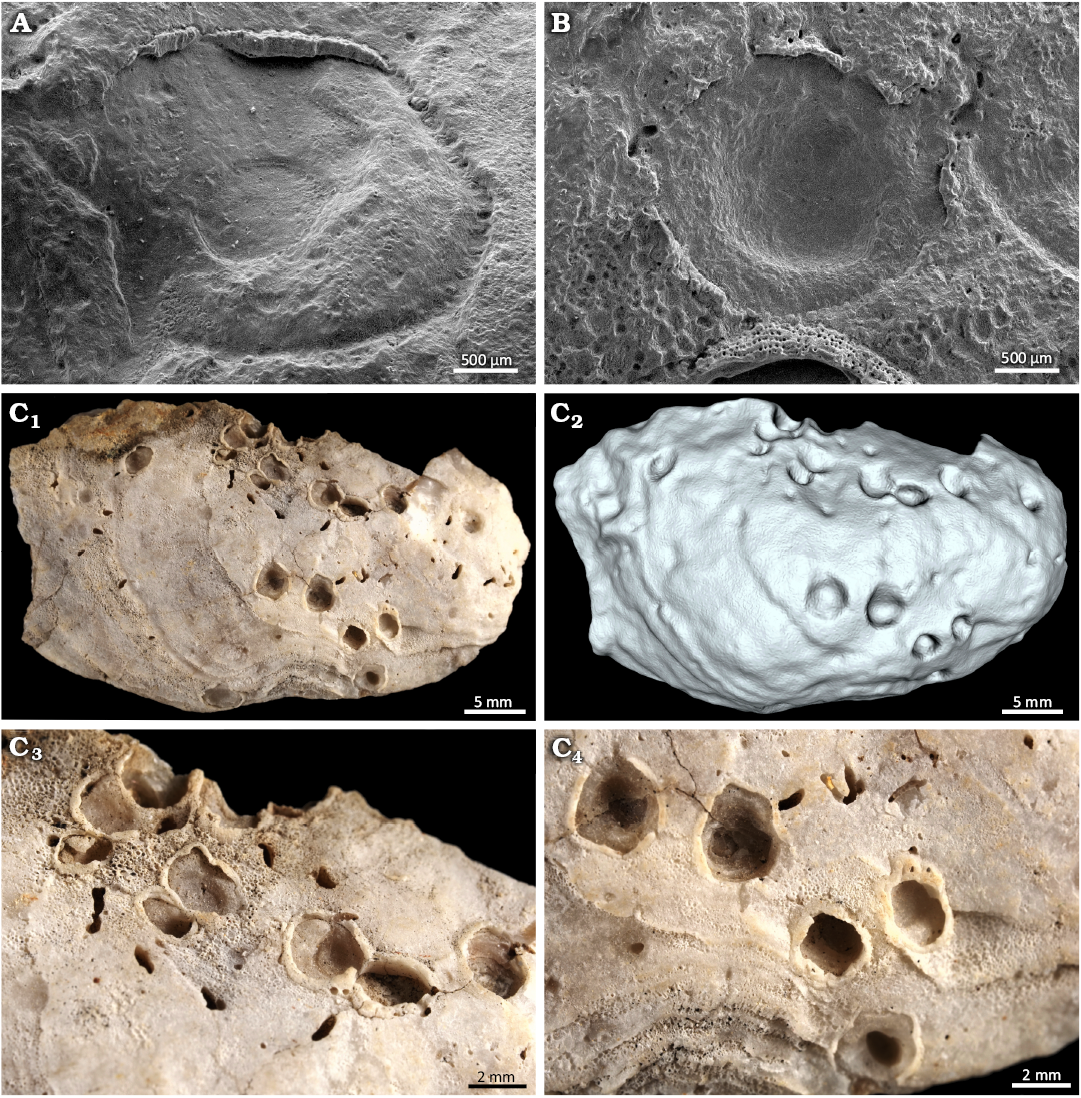
Fig. 4. The verrucid barnacle attachment traces Centrichnus dentatus isp. nov. from Pedra-que-Pica, lower Pliocene, Santa Maria, Azores. A, B. SEM images of two paratypes DBUA-F 1545: INGUJ249P204. C. Paratypes in another oyster shell (probably Ostrea stentina Payraudeau, 1826 sensu lato, DBUA-F 1545: NGUJ249P125), a general view of the oyster shell with the paratypes (C1), surface scan of the shell with paratypes (C2), details (C3, C4).
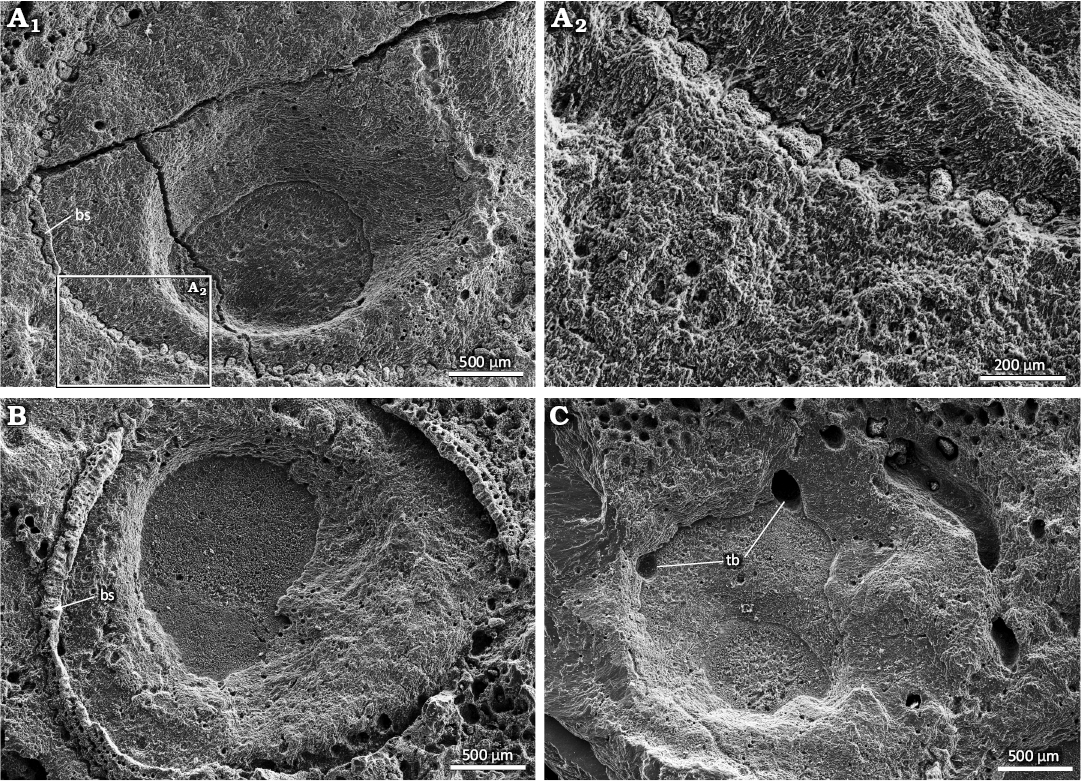
Fig. 5. SEM images of verrucid barnacle attachment traces Centrichnus dentatus isp. nov. on an oyster shell (DBUA-F 1545: INGUJ249P206) from Pedra-que-Pica, lower Pliocene, Santa Maria, Azores. The slopes of the depressions show terraces related to layers in the host shell. A. Centrichnus dentatus with remnants of the producer (barnacle) shell (bs) preserved in the marginal groove and pits (A1), detail (A2). B. Specimen showing the basal part of the producer (barnacle) shell (bs) still in situ. C. Specimen cross-cutting tubular borings (tb).
The bowl-like, central to decentral depressions are up to 1.5 mm deep, but never deeper than the trace is wide. The deeper that depression, the wider it is, and the smaller the flat area between the depression and the marginal groove/pits becomes during ichnogeny sensu Belaústegui et al. (2016), i.e., changes of the trace during ontogenetic development of the tracemaker (Fig. 5A, B). On the slopes of the deeper depressions, intersections of shell layers can be visible, and, in some cases, differential dissolution has terraced these layers, which then appear as concentric rings, but with no grooves or ridges (Figs. 4C4, 5, 6B, 7B).
In many specimens, the marginal groove and pits still contain remnants of the skeletal elements of the trace-making verrucid barnacle. These skeletons are at least as wide as the groove and reach 0.25 mm in thickness. Where the barnacle is not preserved, the marginal groove with pits becomes visible in its virtual prolongation (Fig. 5A, B). The thin section perpendicular to the depression shows that the shell of the barnacle is attached to the slope of the depression in its upper marginal part, the cross-cut shell layers of the bivalve, and is indented in the bivalve shells by tooth-like protrusions, which are ca. 0.05 mm wide and deep (Fig. 8). In any case, shell remains are part of the basal part of the barnacle shell, which, with its dental protrusions, is anchored in the shell of the bivalve host. SEM images clearly confirm this interpretation. Therefore, these structures are not a part of the trace fossil but a part of the tracemaker.
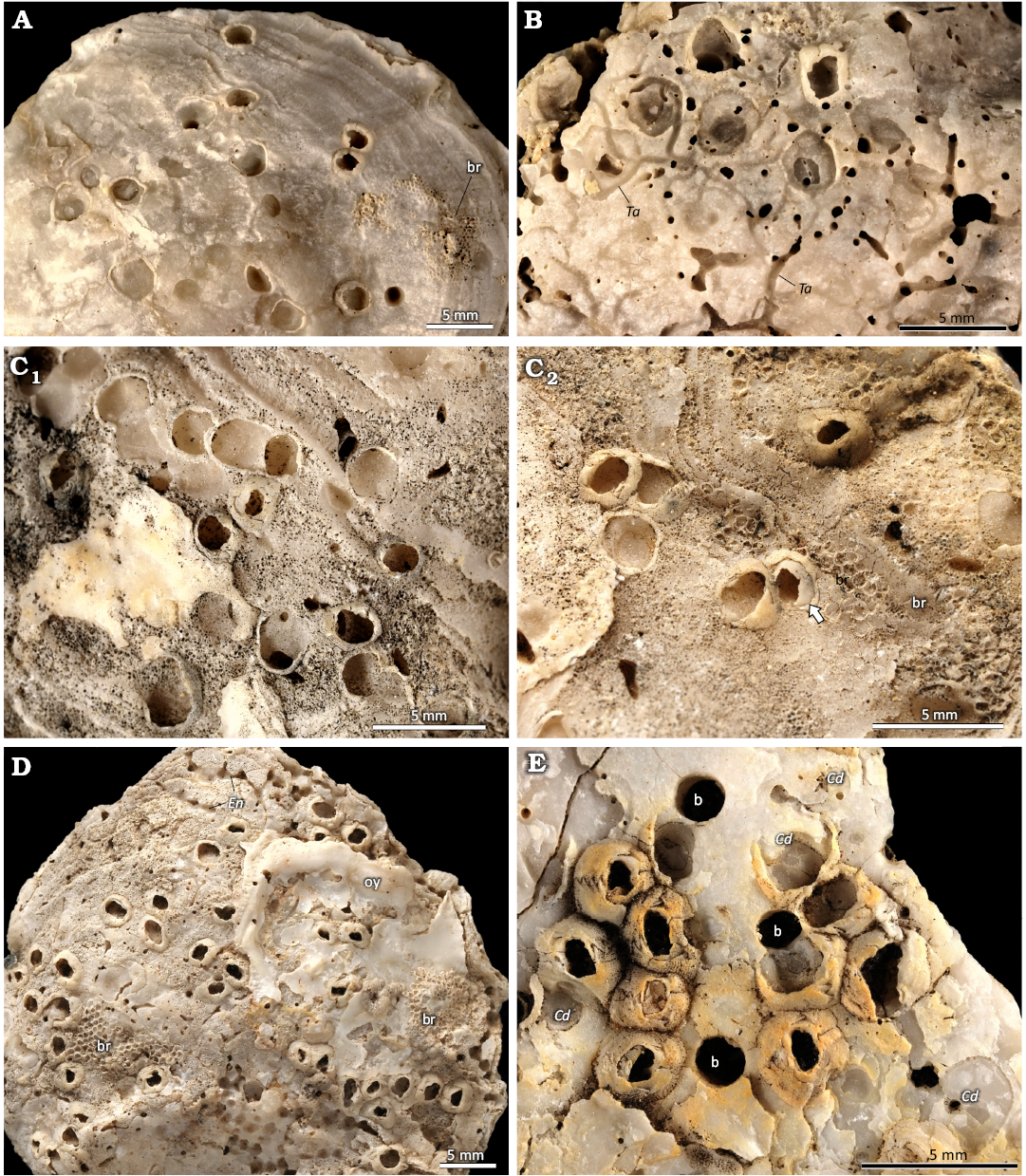
Fig. 6. The verrucid barnacle attachment trace Centrichnus dentatus isp. nov. from Pedra-que-Pica, lower Pliocene, Santa Maria, Azores. Note: some with remnants of barnacle shells on bivalve shells. A. Several traces of variable morphology. Bryozoans (br) cover a barnacle, DBUA-F 1545: INGUJ249P203. B. Several traces, some crossed by the boring Talpina isp. (Ta), DBUA-F 1545: INGUJ249P202. C. Several traces, DBUA-F 1545: INGUJ249P115; a row of partly overlapping traces (C1), a few partly overlapping traces (C2) shell of a barnacle embedded in C. dentatus with a partly preserved shell on the margins (arrow); bryozoans (br) cover a part of C. dentatus in the lower right corner. D. Oyster (oy) covering barnacles and their traces, bryozoan colonies (br), and clionaid sponge boring Entobia isp. (En), DBUA-F 1545: INGUJ249P116. E. Barnacle shells on a fragment of Gigantopecten latissimus (Brocchi, 1814) shell, C. dentatus (Cd), and circular, probably bivalve borings (b), DBUA-F 1545: INGUJ249P121.
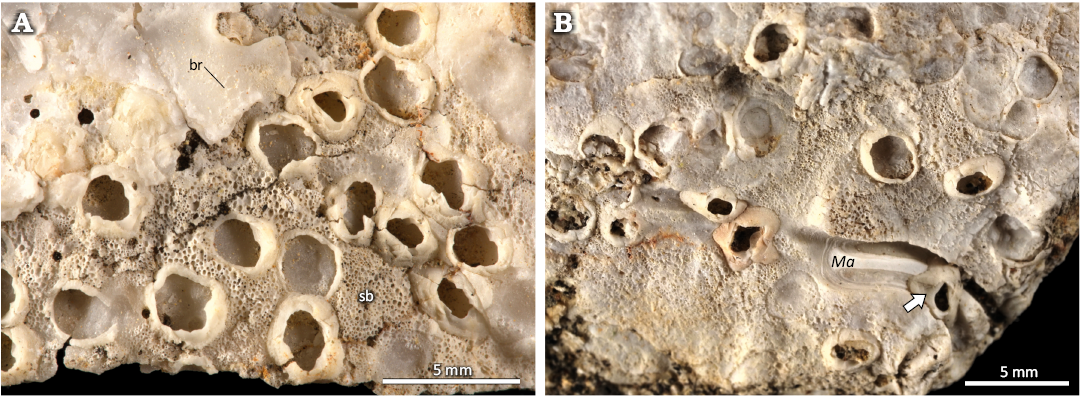
Fig. 7. The verrucid barnacle attachment traces Centrichnus dentatus isp. nov. from Pedra-que-Pica, lower Pliocene, Santa Maria, Azores. Note: some with remnants of barnacle shells (producer). A. Barnacles (producers) on a fragment of Gigantopecten latissimus (Brocchi, 1814) shell, bryozoans (br), and spongy layer of the oyster (probably Ostrea stentina Payraudeau, 1826 sensu lato) shell (sb), DBUA-F 1545: INGUJ249P126. B. Several barnacles (producer) and their traces. One barnacle (arrow) settling in the polychaete boring Maeandropolydora isp. (Ma), DBUA-F 1545: INGUJ249P128.

Fig. 8. Photograph of a thin section crossing vertically a verrucid barnacle attachment trace Centrichnus dentatus isp. nov. (INGUJ249P207) from Pedra-que-Pica, lower Pliocene, Santa Maria, Azores. Abbreviations: oy, oyster shell, ba, barnacle shell; t, grey tooth-like indentation of the lighter barnacle shell into the oyster shell.
The traces are isolated or can be fused in clusters or rows to form composite traces (Figs. 6C1, 7A). One depression can truncate the neighbouring one (Figs. 3A1, A2, 4C1, C3, 6C, 7B). The number of depressions in one shell may attain nearly forty.
Remarks.—The circular or oval outline of Centrichnus dentatus is similar to that of Centrichnus concentricus Bromley & Martinell, 1991, Centrichnus circularis (Santos et al., 2005), and Centrichnus ellipticus (Buckeridge et al., 2019), but C. concentricus and C. ellipticus have distinct concentric ridges and crenulated margins, and C. circularis lacks the central to decentral depression. While shallow morphotypes of C. dentatus are very close in shape to those of C. circularis, the latter does not show the central depression, and it does not have pits in the marginal groove. Hence, these pits are the most relevant diagnostic feature of C. dentatus. Centrichnus undulatus differs by its flabellate margin and no distinct depression (Santos et al. 2005) and C. eccentricus Bromley & Martinell, 1991, is a more complex Centrichnus with a tier-shaped central etching and eccentric grooves, surrounded by abrasive imprints of the trace maker shell margin and hinge area (see Neumann et al. 2015 for a revision of this type ichnospecies).
Stratigraphic and geographic range.—The Touril Complex (Zanclean, lower Pliocene), Santa Maria, Azores.
Relation to epibionts and other trace fossils
Centrichnus dentatus is cross-cut by some tubular borings (Fig. 5C). Some of them are identified as Talpina isp., which is produced by phoronids (Gaaloul et al. 2023). Some C. dentatus are cross-cut by and co-occur with clionaid sponge borings of the ichnogenus Entobia (Fig. 6D). In some cases, C. dentatus with attached remnants of the barnacle shell is surrounded by the porous vesicular shell microstructure of the oyster (Fig. 7A). In some host shells, C. dentatus co-occurs with the polychaete boring Maeandropolydora isp. In one case, the barnacle is nested in the unroofed gallery of a Maeandropolydora and adjusted to its margins (Fig. 7B). Some circular holes perforating the host shell (Fig. 7F) are related to bivalve borings of the ichnogenus Gastrochaenolites, that are commonly found also in other bioclasts in the studied locality.
Among epibionts on the shells bearing C. dentatus, colonies of cheilostome bryozoans are the most common. Some of them cover the barnacle shells (Fig. 6A), or grow on their flanks (Fig. 6D), or cover a part of the C. dentatus (Fig. 6B). Figure 6D shows an upper side of the oyster valve with another oyster attached (most probable Ostrea stentina Payraudeau, 1826 sensu lato) that covers the barnacles. The attached shell itself is partly overgrown by bryozoans. Sparsely, coiled tubes of the polychaete genus Spirorbis co-occur.
Producer
The fossil material is overall abraded, with very few complete specimens, arranged either isolated or in sheets on bivalve (oyster) shells (Figs. 6, 7). A single specimen presents opercular plates, which are completely crushed (Fig. 6E). The barnacle shells are small (about 3 mm maximum diameter), with an overall low-conic shape. The wall is formed by four plates of different sizes (carina, rostrum, fixed scutum, and fixed tergum), which are attached directly to the host shell, forming a circular to hexagonal (in more densely packed areas) cross-section. Overall, asymmetrical appearance with a relatively small operculum (maximum diameter about 2.4 times the maximum opercular length), parallel to the base, forming a D-shape (maximum length about 1.5 times the maximum width) slightly off-center (to the left, towards the fixed tergum). Shell plates present an overall smooth surface, apart from the growth of transverse striae and the presence of pores arranged concentrically, along growth lines. Interlocking ridges between plates are present, exhibiting a chevron pattern which is particularly evident between the rostral and carinal wall plates (Fig. 9). Despite the fragmentary nature of the studied material, the barnacle shells present clear diagnostic features as described above, identifying them with confidence to Verruca spengleri Darwin, 1854 (see Young 1998; Young et al. 2003).
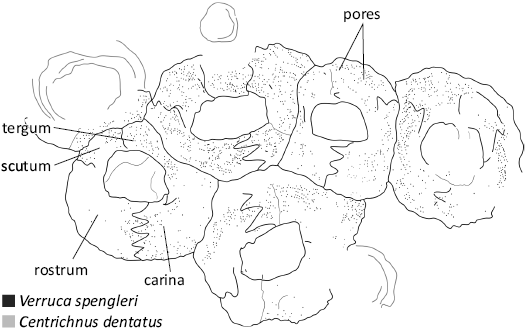
Fig. 9. Drawing of verrucid barnacle Verruca spengleri Darwin, 1854, and its attachment trace Centrichnus dentatus isp. nov., based on Fig. 6E.
Verrucid barnacles are rarely preserved in the fossil record, as most tend to rapidly disarticulate upon death (Buckeridge and Finger 2001; Buckeridge 2011). Verrucomorphs, with their characteristic asymmetrical shells, are known to occur since the Cretaceous (Chan et al. 2021), whereas the earliest Verruca species is reported from the upper Maastrichtian of the Netherlands (Gale 2014; Gale and Vidovic 2023). Verruca spengleri is only known from the more recent fossil record, being solely reported from the Pleistocene of Sicily and Calabria (Ruggieri 1977). In the Azores fossil record, only balanids from the lower Pliocene (Zbyszewski and Ferreira 1962; Winkelmann et al. 2010) and upper Pleistocene of Santa Maria Island were previously reported (Callapez and Soares 2000). However, fossil cirripeds from the Azores are in desperate need of a throughout review. Thus, the present Pliocene record not only represents the first report of a verrucid in Santa Maria fossil record, but also the oldest material for the species.
Verruca spengleri is the only verrucomorph known to presently occur in the shallow-waters of the Azores (Young 1998, 2002), Madeira (type area; Darwin 1854), and Canaries archipelagos (González et al. 2012). In the extant shores of the nearest continents, the distribution of this species is restricted to the warmer waters of southern Atlantic coast of Spain and throughout the Mediterranean Sea (Young et al. 2003). The presence of this species in the fossil record of Pedra-que-Pica is in line with what is known from the Pliocene fauna of Santa Maria, that could be characterized, in general, as composed by shallow-water elements with strong tropical to warm-temperate affinities towards the eastern continental and insular coasts (Ávila et al. 2016, 2018; Sacchetti et al. 2023).
Discussion
Darwin (1854: pl. 21: 6) first noticed that verrucid barnacles, such as Verruca stroemia (Müller, 1776), V. laevigata (Sowerby, 1827), or V. spengleri Darwin, 1854, produce attachment scars and illustrated them. The presence of the verrucid barnacle in intimate relation to Centrichnus dentatus leaves no doubt as to the tracemaker. Verrucid barnacles are also producers of C. concentricus (see Bromley and Martinell 1991), and C. ellipticus (see Buckeridge et al. 2019), while C. circularis and C. undulates are produced by balanid barnacles (Santos et al. 2005). Centrichnus eccentricus, in contrast, is not the work of cirripeds but of anomiid bivalves (see Bromley and Martinell 1991; Neumann et al. 2015).
Today, in the Azores, Centrichnus concentricus is also produced by the verrucid barnacle Verruca spengleri (Fig. 10), as reported from settlement experiments deployed at 15 and 60 m water depth by Wisshak et al. (2011). These traces show the typical morphology of C. concentricus and are closely reminiscent of the type material on a Chlamys islandica (Müller, 1776) shell from the Pleistocene of Palamós, Spain (Bromley and Martinell 1991: fig. 7). That type material, however, was found in association with V. stroemia (Bromley and Martinell 1991: fig. 8), indicating that C. concentricus does not have a monospecific trace maker but can be formed by both V. spengleri and V. stroemia. Verruca spengleri, in turn, appears not to produce monoichnospecific traces, as both C. concentricus and C. dentatus have now been addressed to this trace maker species. This circumstance is puzzling, as the two ichnospecies rather fundamentally differ in their ichnogeny, implying that V. spengleri may exhibit two different underlying behavioural patterns of trace formation. However, both examples are in line with basic ichnological principles that stress that there is not necessarily a one-to-one relationship between a trace fossil ichnotaxon and a trace making biotaxon.
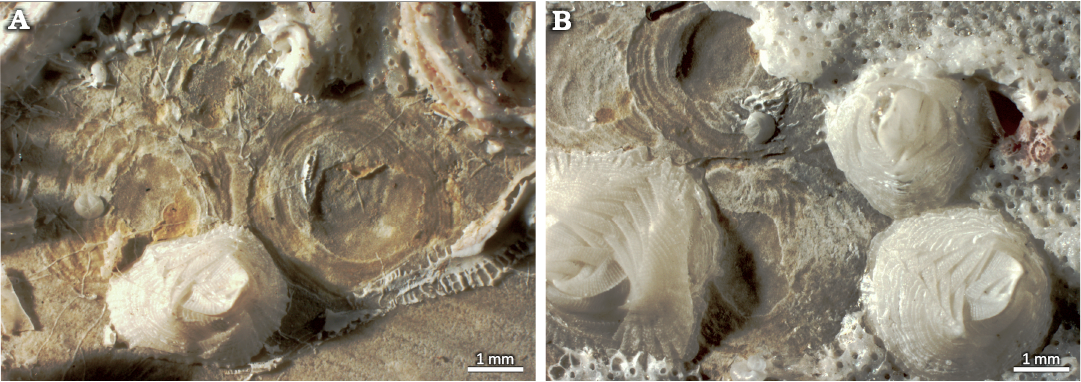
Fig. 10. Recent Centrichnus concentricus isp. nov. and its trace maker verrucid barnacle Verruca spengleri Darwin, 1854, on a settlement experiment deployed at 15 m water depth in the Azores. A. Overlapping traces and attached shells of the tracemaker. B. Traces and tracemaker shells partially covered by bryozoan colonies.
At least 90% of the valves of small oysters of the species Cubitostrea frondosa (de Serres, 1829) show Centrichnus dentatus, which also occurs occasionally on valves of Gigantopecten latissimus (Brocchi, 1814), Ostrea cf. edulis Linnaeus, 1758, or Spondylus sp. The colonization of these shells was possible when they were stabilized for some time. Oyster shells are relatively stable as they are commonly attached to the substrate, including neighbouring shells. Gigantopecten latissimus was also relatively stable due to its size and weight. The presence of borings cross-cutting C. dentatus and the presence of epibionts growing on barnacles point to further stabilization and exposure of the shells on the sea floor. The pervasive presence of the depressions shows that the barnacle tracemaker was well adapted to the bioerosion of shells by etching. Likely the depression supported the anchoring of the barnacle on the host shell, fostered by means of the tooth-like indentations. It is possible that the dissolved calcium carbonate was used for the biomineralization of the barnacle shell. In general, the ability of barnacles to etch hard substrates is enormous; they can etch even stainless steel through their protein cement (Sangeetha et al. 2010).
Centrichnus dentatus and other ichnospecies of Centrichnus represent the Gnathichnus ichnofacies (Mayoral and Muñiz 1996), which refers to the early colonization of hard substrates taking place within months (Bromley and Asgaard 1993; Radley 2006; Gibert et al. 2007). The presence of the polychaete borings Maeandropolydora, and the bivalve borings suggests that some of the bioclasts bearing C. dentatus were subjected to longer exposure and colonization, which lasted several years rather than months (cf. Bromley and Asgaard 1993). Diverse preservation of the barnacle shells and overgrowth of each other suggests that the hosting shells were colonized by several generations of barnacles in an environment, where abrasion took place. This corroborates with the origin of the Pedra-que-Pica coquina, which contains a mixture of shells and shell debris accumulated in a topographically protected area below the fair-weather wave base at around 50 m depth and originating from shallower environments (Ávila et al. 2015b, 2018).
Conclusions
Centrichnus dentatus isp. nov. is a new attachment trace produced by the verrucid barnacle Verruca spengleri on bivalve shells in a Pliocene coquina of the Pedra-que-Pica section in Santa Maria Island (Azores Archipelago). It shows an elliptical outline, bounded by a groove and/or a series of pits, and by having a more or less pronounced central to off-center depression surrounded by a flat area. The diagnosis of the ichnogenus Centrichnus is emended, and the ichnofamily Centrichnidae is revised. Its members belong to the Gnathichnus ichnofacies, which refers to the early colonization of hard substrates.
Acknowledgements
Mark A. Wilson (The College of Wooster, USA) and Radek Mikuláš (Institute of Geology, Czech Academy of Sciences, Prague, Czech Republic) provided useful reviews. SPA acknowledges his contracts through the project M1.1.A/INFRAEST CIENT/A/001/2021—Base de Dados da PaleoBiodiversidade da Macaronésia, funded by Direção Regional da Ciência e Tecnologia, Governo Regional dos Açores, and FCT/2023.07418 CEEECIND research contract. PM acknowledges her contract through the project M1.1.A/INFRAEST CIENT/A/001/2021—Base de Dados da PaleoBiodiversidade da Macaronésia. CSM benefited from a PhD grant M3.1.a/F/100/2015 funded by the Regional Government of the Azores. This work also benefited from FEDER funds, through the Operational Program for Competitiveness Factors—COMPETE, and from National Funds, through FCT UIDB/50027/2020 (CIBIO/INBIO), and POCI-01-0145-FEDER-006821 and LA/P/0048/2020 (CIBIO/INBIO), as well as through the Regional Government of the Azores (M1.1.a/005/Funcionamento-C-/2016, CIBIO-A; M3.3.B/ORG.R.C./005/2021).
Editor: Andrzej Kaim.
References
Agassiz, L. 1833–1843. Recherches sur les poissons fossiles. Vol. 3. 390 pp. Imprimerie de Petitpierre, Neuchâtel. Crossref
Arendt, Y.A. 2012. Traces of Augoichnus dituberculatus gen. et sp. nov. on Hypermorphocrinus magnospinosus from the Lower Permian of Cisuralia. Paleontological Journal 46: 886–893. Crossref
Ávila, S.P., Azevedo, J.M.N., Madeira, P., Cordeiro, R., Melo, C.S., Baptista, L., Torres, P., Johnson, M.E., and Vullo, R. 2020. Pliocene and Late Pleistocene actinopterygian fishes from Santa Maria Island (Azores: NE Atlantic Ocean): systematics, palaeoecology and palaeobiogeography. Geological Magazine 157: 1526–1542. Crossref
Ávila, S.P., Cachão, M., Ramalho, R.S., Botelho, A.Z., Madeira, P., Rebelo, A.C., Cordeiro, R., Melo, C., Hipólito A., Ventura M.A., and Lipps, J.H. 2016. The palaeontological heritage of Santa Maria Island (Azores: NE Atlantic): a re-evaluation of geosites in GeoPark Azores and their use in geotourism. Geoheritage 8: 155–171. Crossref
Ávila, S.P., Cordeiro, R., Rodrigues, A.R., Rebelo, A.C., Melo, C., Madeira, P., and Pyenson, N.D. 2015a. Fossil Mysticeti from the Pleistocene of Santa Maria Island, Azores (NE Atlantic Ocean), and the prevalence of fossil cetaceans on oceanic islands. Palaeontologia Electronica 18.2.27: 1–12. Crossref
Ávila, S.P., Ramalho, R., and Vullo, R. 2012. Systematics, palaeoecology and palaeobiogeography of the Neogene fossil sharks from the Azores (Northeast Atlantic). Annales de Paléontologie 98: 167–189. Crossref
Ávila S.P., Ramalho, R.S., Da Silva, C.M., Johnson, M.E., Uchman, A., Berning, B., Quartau, R., Madeira, P., Melo, C.S., Rebelo, A.C., Baptista, L., Arruda, S., González, E., Rasser, M.W., Hipólito, A., Cordeiro, R., Meireles, R., Raposo, V., Pombo, J., Câmara, R., Kirby, M.X., Titschack, J., Habermann, J.M., Vullo, R., Kroh, A., Lipps, J.H., Cachão, M., and Madeira, J. 2022. Os fósseis de Santa Maria (Açores). 2. Pedra-que-Pica: uma história com 5 milhões de anos. 160 pp. OVGA-Observatório Vulcanológico e Geotérmico dos Açores, Lagoa.
Ávila, S.P., Ramalho, R.S., Habermann, J.M., and Titschack, J. 2018. The marine fossil record at Santa Maria Island (Azores). In: U. Kueppers and C. Beier (eds), Volcanoes of the Azores. Revealing the Geological Secrets of the Central Northern Atlantic Islands. Active Volcanoes of the World, 155–196. Springer, Berlin, Heidelberg. Crossref
Ávila, S.P., Ramalho, R.S., Habermann, J.M., Quartau, R., Kroh, A., Berning, B., Johnson, M., Kirby, M.X., Zanon, V., Titschack, J., Goss, A., Rebelo, A.C., Melo, C., Madeira, P., Cordeiro, R., Meireles, R.P., Bagaço, L., Hipólito, A., Uchman, A., Silva, C.M., Cachão, M., and Madeira, J. 2015b. Palaeoecology, taphonomy, and preservation of a lower Pliocene shell bed (coquina) from a volcanic oceanic island (Santa Maria Island, Azores, NE Atlantic Ocean). Palaeogeography, Palaeoecology, Palaeoclimatology 430: 57–73. Crossref
Belaústegui, Z., Muñiz, F., Mángano, M.G., Buatois, L A., Domènech, R., and Martinell, J. 2016. Lepeichnus giberti igen. nov. isp. nov. from the Upper Miocene of Lepe (Huelva, SW Spain): Evidence for its origin and development with proposal of a new concept, ichnogeny. Palaeogeography, Palaeoclimatology, Palaeoecology 452: 80–89. Crossref
Brett, C.E. 1985. Tremichnus: a new ichnogenus of circular-parabolic pits in fossil echinoderms. Journal of Paleontology 59: 625–635.
Brocchi, G.B. 1814. Conchiologia fossile subapennina con osservazioni geologiche sugli Apennini e sul suolo adiacente. Tomo Secondo. 241–712 pp. Stamperia Reale, Milano. Crossref
Bromley, R.G. and Asgaard, U. 1993. Two bioerosion ichnofacies produced by early and late burial associated with sea-level change. Geologische Rundschau 82: 276–280. Crossref
Bromley, R.G. and Heinberg, C. 2006. Attachment strategies of organisms on hard substrates: A palaeontological view. Palaeogeography, Palaeoclimatology, Palaeoecology 232: 429–453. Crossref
Bromley, R.G. and Martinell, J. 1991. Centrichnus, new ichnogenus for centrically patterned attachment scars on skeletal substrates. Bulletin Geological Society of Denmark 38: 243–252. Crossref
Brustur, T. 2020. Modern limpet home scar on the Devonian cliffs, North coast Central and West Cornwall (United Kingdom), and Lower Cretaceous Patellichnus from Romania. Geo-Eco-Marina 26: 195–209.
Buatois, L.A., Wisshak, M., Wilson, M.A., and Mángano, M.G. 2017. Categories of architectural designs in trace fossils: A measure of ichnodisparity. Earth-Science Reviews 164: 102–181. Crossref
Buckeridge, J.S. 2011. Taphonomy and systematics of a new late Cretaceous verrucid barnacle (Cirripedia, Thoracica) from Canterbury, New Zealand. Palaeontology 54: 365–372. Crossref
Buckeridge, J.S. and Finger, K.L. 2001. First record of a fossil verrucid barnacle in California—Verruca digitali sp. nov. (Cirripedia: Thoracica) from the Late Miocene. Journal of Crustacean Biology 21: 443–449. Crossref
Buckeridge, J., Kočí, T., Schlögl, J., Tomašových, A., and Kočová Veselská, M. 2019. Deep-water cirripedes colonizing dead shells of the cephalopod Nautilus macromphalus from New Caledonian waters. Integrative Zoology 14: 561–575. Crossref
Callapez, P. and Soares, A.F. 2000. Late Quaternary warm marine mollusks from Santa Maria (Azores) paleoecologic and paleobiogeographic considerations. Ciências da Terra/Earth Sciences Journal 14: 313–322.
Chan, B.K., Dreyer, N., Gale, A.S., Glenner, H., Ewers-Saucedo, C., Pérez-Losada, M., Kolbasov, G.A., Crandall, K.A., and Høeg, J.T. 2021. The evolutionary diversity of barnacles, with an updated classification of fossil and living forms. Zoological Journal of the Linnean Society 193: 789–846. Crossref
Collareta, A., Tsai, C.-H., Coletti, G., and Bosselaers, M. 2021. Thatchtelithichnus on a Pliocene grey whale mandible and barnacles as possible tracemakers. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen 302: 53–61. Crossref
Darwin, C. 1854. A Monograph on the Cirripedia with Figures of All the Species. The Balanidae (or Sessile Cirripedes), The Verrucidae, etc. 684 pp. R. Society, London.
Dragastan, O., Neagu, T., Bărbulescu, A., and Pană, I. 1998. Jurasicul și Cretacicul din Dobrogea centrală și de sud. (Paleontologie și stratigrafie). 249 pp. Supergraph, Cluj-Napoca.
Estevens, M. and Ávila, S.P. 2007. Fossil whales from the Azores. Açoreana Suplement 5: 140–161.
Gaaloul, N., Uchman, A., Ben Ali, S., Janiszewska, K., Stolarski, J., Kołodziej, B., and Riahi, S. 2023. In vivo and post-mortem bioerosion traces in solitary corals from the upper Pliocene deposits of Tunisia. Acta Palaeontologica Polonica 68: 659–681. Crossref
Gale, A.S. 2014. Origin and phylogeny of verrucomorph barnacles (Crustacea, Cirripedia, Thoracica). Journal of Systematic Palaeontology 13: 753–789. Crossref
Gale, A.S. and Vidovic, S.U. 2023. The origins of major sessile cirripede groups; a revision of Cretaceous Brachylepadomorpha and Verrucomorpha. Journal of Systematic Palaeontology 21 (2258370): 1–60. Crossref
Gibert, J.M. de, Domènech, R., and Martinell, J. 2004. An ethological framework for animal bioerosion trace fossils upon mineral substrates with proposal of a new class, fixichnia. Lethaia 37: 429–437. Crossref
Gibert, J.M. de, Domènech, R., and Martinell, J. 2007. Bioerosion in shell beds from the Pliocene Roussillon Basin, France: Implications for the (macro)bioerosion ichnofacies model. Acta Palaeontologica Polonica 52: 783–798.
González, J.A., Martín, L., Herrera, R., González-Lorenzo, G., Espino, F., Barquín-Diez, J. and Southward, A.J. 2012. Cirripedia of the Canary Islands: distribution and ecological notes. Journal of the Marine Biological Association of the United Kingdom 92: 129–141. Crossref
Gutiérrez, J.L., Hacker, S.D., Coombes, M.A., Wild, C., Pereira-Filho, G.H., and Palomo, M.G. 2022. Marine hard substrate communities. In: P. Pan and P. Pratolongo (eds.), Marine Biology: A Functional Approach to the Oceans and their Organisms, 232–273. CRC Press, Boca Raton. Crossref
Hyžný, M., Melo, C.S., Ramalho, R.S., Cordeiro, R., Madeira, P., Baptista, L., Rebelo, A.C., Gómez, C., Torres, P., Uchman, A., Johnson, M.E., Berning B., and Ávila, S.P. 2021. Pliocene and Late Pleistocene (MIS 5e) decapod crustacean crabs from Santa Maria Island (Azores Archipelago: NE Atlantic): systematics, palaeoecology and palaeobiogeography. Journal of Quaternary Science 36: 91–109. Crossref
Janssen, A.W., Kroh A., and Ávila, S.P. 2008. Early Pliocene heteropods and pteropods (Mollusca, Gastropoda) from Santa Maria (Azores, Portugal): systematics and biostratigraphic implications. Acta Geologica Polonica 58: 355–369.
Kroh, A., Bitner M.A., and Ávila, S.P. 2008. Novocrania turbinata (Brachiopoda) from the Early Pliocene of the Azores (Portugal). Acta Geologica Polonica 58: 473–478.
Lamarck, J.-B.M.de. 1816. Histoire naturelle des animaux sans vertèbres, Tome troisième. 586 pp. Verdière, Paris.
Linnaeus, C. 1758. Systema naturæ per regna tria naturæ, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Tomus I. Editio decima, reformata. 824 pp. Laurentius Salvius, Stockholm. Crossref
Madeira, P., Kroh, A., Cordeiro, R., Meireles, R., and Avila, S.P. 2011. The fossil echinoids of Santa Maria Island, Azores (Northern Atlantic Ocean). Acta Geologica Polonica 61: 243–264.
Mayoral, E. and Muñiz, F. 1996. Icnofacies de Gnathichnus en el sector Suroccidental de la Cuenca del Guadalquivir. Coloquios de Paleontología, COL PA 48: 87–102.
Meireles, R.P., Faranda, C., Gliozzi, E., Pimentel, A., Zanon V., and Ávila, S.P. 2012. Late Miocene marine ostracods from Santa Maria Island, Azores (NE Atlantic): Systematics, palaeoecology and palaeobiogeography. Révue de Micropaléontologie 55: 133–148. Crossref
Müller, O.F. 1776. Zoologiae Danicae prodromus, seu Animalium Daniae et Norvegiae indigenarum: characteres, nomina, et synonyma imprimis popularium. 282 pp. Typis Hallagerii, Havni, Copenhagen. Crossref
Neumann, C., Wisshak, M., Aberhan, M., Girod, P., Rösner, T., and Bromley, R.G. 2015. Centrichnus eccentricus revisited: A new view on anomiid bivalve bioerosion. Acta Palaeontologica Polonica 60: 539–549. Crossref
Payraudeau, B.C. 1826. Catalogue descriptif et méthodique des annelides et des mollusques de l’Ile de Corse; avec huit planches représentant quatre-vingt-huit espèces, dont soixante-huit nouvelles. 218 pp. Imprimerie de J. Tastu, Paris. Crossref
Radley, J.D. 2006. Grazing bioerosion on oyster concentrations (Lower and Middle Jurassic, England). Ichnos 13: 47–50. Crossref
Radwański, A. 1977. Present-day types of trace in the Neogene sequence; their problems of nomenclature and preservation. In: T.P. Crimes and J.H. Harper (eds.), Trace fossils 2. Geological Journal, Special Issue 9: 227–264.
Raposo, V.B., Melo, C.S., Silva, L., Ventura, M.A., Câmara, R., Pombo, J., Johnson, M.E., and Ávila, S.P. 2018. Comparing methods of evaluation of geosites: the fossiliferous outcrops of Santa Maria Island (Azores: NE Atlantic) as a case-study for sustainable Island tourism. Sustainability 10: 3596. Crossref
Ramalho, R.S., Helffrich, G., Madeira, J., Cosca, M., Thomas, C., Quartau, R., Hipólito, A., Rovere, A., Hearty, P.J., and Ávila, S.P. 2017. The emergence and evolution of Santa Maria Island (Azores)—the conundrum of uplifted islands revisited. Bulletin of the Geological Society of America 129: 372–390. Crossref
Ramalho, R.S., Quartau, R., Trenhaile, A.S., Mitchell, N.C., Woodroffe, C.D., and Ávila, S.P. 2013. Coastal evolution on volcanic oceanic islands: A complex interplay between volcanism, erosion, sedimentation, sea-level change and biogenic production. Earth-Science Reviews 127: 140–170. Crossref
Rebelo, A.C., Meireles, R.P., Barbin, V., Neto, A.I., Melo, C., and Ávila, S.P. 2016. Diagenetic history of lower Pliocene rhodoliths of the Azores archipelago (NE Atlantic): application of cathodoluminescence techniques. Micron 80: 112–121. Crossref
Ruggieri, G. 1977. Le Verruca del Pleistocene neritico della Sicilia (Cirripedia, Thoracica, Verrucomorpha). Il Naturalista Sicicliano 4: 67–74.
Sacchetti, C., Landau B., and Ávila, S.P. 2023. The Lower Pliocene marine gastropods of Santa Maria Island, Azores: Taxonomy and palaeobiogeographic implications. Zootaxa 5295: 1–150. Crossref
Sangeetha, R., Kumar, R., Doble, M., and Venkatesan, R. 2010. Barnacle cement: An etchant for stainless steel 316L? Colloids and Surfaces B: Biointerfaces 79: 524–530. Crossref
Santos, A., Mayoral, E., and Muñiz, F. 2003. New trace fossils produced by etching molluscs from the Upper Neogene of the southwestern Iberian Peninsula. Acta Geologica Polonica 53: 181–188.
Santos, A., Mayoral, E., and Muñiz, F. 2005. Bioerosion scars of acorn barnacles from the southwestern Iberian Peninsula, Upper Neogene. Rivista Italiana di Paleontologia e Stratigrafia 111: 181–189.
Serralheiro, A. 2003. A geologia da ilha de Santa Maria, Açores. Açoreana 10: 141–192.
Serralheiro, A., Alves, C.M., Forjaz, V.H., and Rodrigues, B. 1987. Carta Vulcanológica dos Açores, Ilha de Santa Maria. Centro de Vulcanologia INIC, Ponta Delgada.
Serres, M. de 1829. Géognosie des terrains Tertiaires, ou tableau des principaux animaux invertébrés des terrains marins Tertiaires du midi de la France. 275 pp. Pomathio-Durville, Paris. Crossref
Sibrant, A.L.R., Hildenbrand, A., Marques, F.O., and Costa, A.C.G. 2015. Volcano-tectonic evolution of the Santa Maria Island (Azores): Implications for paleostress evolution at the western Eurasia-Nubia plate boundary. Journal of Volcanology and Geothermal Research 291: 49–62. Crossref
Sowerby, G.B. 1827. Genera of Recent and Fossil Shells, for the Use of Students, in Conchology and Geology, Volume 2. [unpganinatd]. G.B. Sowerby, London.
Taylor, P.D. and Wilson, M.A. 2003. Palaeoecology and evolution of marine hard substrate communities. Earth-Science Reviews 62: 1–103. Crossref
Uchman, A. and Rattazzi, B. 2018. A new etching trace from the Savignone Conglomerate (Oligocene), NW Italy, probably produced by limpet gastropods. Acta Geologica Polonica 68: 651–662.
Uchman, A., Stachacz, M., and Salamon, K. 2018. Spirolites radwanskii n. igen. n. isp.—a vermetid gastropod attachment etching trace from the middle Miocene rocky coast of the Paratethys, Poland. Journal of Paleontology 92: 883–895 Crossref
Verde, M., Castillo, C., Martín-González, E., Cruzado-Caballero, P., Mayoral, E., and Santos, A. 2022. A new Miocene–Pliocene ichnotaxon for vermetid anchoring bioerosion structures. Frontiers in Earth Sciences 10: 906493. Crossref
Winkelmann, K., Buckeridge, J.S., Costa, A.C., Dionísio, M.A.M., Medeiros, A., Cachão, M., and Ávila, S.P. 2010. Zullobalanus santamariaensis sp. nov. a new late Miocene barnacle species of the family Archeobalanidae (Cirripedia: Thoracica), from the Azores. Zootaxa 2680: 33–44. Crossref
Wisshak, M., Alexandrakis, E., and Hoppenrath, M. 2014. The diatom attachment scar Ophthalmichnus lyolithon igen. et isp. n. Ichnos 21: 111–118. Crossref
Wisshak, M., Berning, B., Jakobsen, J., and Freiwald, A. 2015a. Temperate carbonate production: Biodiversity of calcareous epiliths from intertidal to bathyal depths (Azores). Marine Biodiversity 45: 87–112. Crossref
Wisshak, M., Knaust, D., and Bertling, M. 2019. Bioerosion ichnotaxa: review and annotated list. Facies 65: 24. Crossref
Wisshak, M., Kroh, A., Bertling, M., Knaust, D., Nielsen, J.K., Jagt, J.W.M., Neumann, C., and Nielsen, K.S.S. 2015b. In defence of an iconic ichnogenus—Oichnus Bromley, 1981. Annales Societatis Geologorum Poloniae 85: 445–451. Crossref
Wisshak, M., Tribollet, A., Golubic, S., Jakobsen, J., and Freiwald, A. 2011. Temperate bioerosion: Ichno- and biodiversity from intertidal to bathyal depths (Azores). Geobiology 9: 492–520. Crossref
Woodroffe, C.D. 2014. The rock coasts of oceanic islands. Geological Society, London, Memoirs 40: 247–261. Crossref
Young, P.S. 1998. Cirripedia (Crustacea) from the “Campagne Biaçores” in the Azores region, including a generic revision of Verrucidae. Zoosystema-Paris 20: 31–92. Crossref
Young, P.S. 2002. Revision of the Verrucidae (Crustacea, Cirripedia) from the Atlantic Ocean studied by Abel Gruvel (Travailleur and talisman scientific expeditions). Zoosystema 24: 771–798.
Young, P.S., Zibrowius, H., and Bitar, G. 2003. Verruca stroemia and Verruca spengleri (Crustacea: Cirripedia): distribution in the north-eastern Atlantic and the Mediterranean Sea. Journal of Marine Biology Association of the United Kingdom 83: 89–93. Crossref
Zbyszewski G. and Ferreira, O.V. 1962. La faune Miocène de l’île de Santa Maria (Açores). Comunicações dos Serviços Geológicos de Portugal 46: 247–289.
Zonneveld, J.-P. and Bartels, W.S. 2020. Ichnologic note: in defence of Thatchtelithichnus Zonneveld, Bartels, Gunnell and McHugh, 2015. Ichnos 27: 152–155. Crossref
Zonneveld, J.-P., Bartels, W.S., Gunnell, G.F., and McHugh, L.P. 2015. Borings in early Eocene turtle shell from the Wasatch Formation, South Pass, Wyoming. Journal of Paleontology 89: 802–820. Crossref
Acta Palaeontol. Pol. 70 (1): 143–157, 2025
https://doi.org/10.4202/app.01222.2024